A Novel Digital Platform for a Monitored Home-based Cardiac Rehabilitation Program
Summary
The aim is to promote a new approach to cardiac rehabilitation (CR), using a unique remote patient monitoring system that will enable healthcare providers to monitor CR patients at home at low cost, with the intention of making CR services more accessible and improving compliance. The study is currently underway.
Abstract
Despite the evidence that cardiac rehabilitation (CR) reduces the risk of recurrent cardiac events, only a minority of eligible patients are willing to join existing programs at cardiac rehabilitation centers. The unique remote patient monitoring system presented here enables healthcare providers to monitor CR patients at home in real-time and at low cost. The system combines mobile technology, artificial intelligence, and supportive services, expanding the delivery of medical care to the patient's home. The primary aim of the study is to increase the long-term adherence to physical activity in patients who participate in CR via the addition of a home-based digitally monitored CR component to the standard CR program in patients with ischemic heart disease (IHD), with the idea of forming new habitual health behaviors and increasing the long-term motivation for physical exercise (PE) habits at home. Secondary aims are to assess the program's impact on the physical activity level measured by average steps per day, minutes of exercise per week, blood pressure, metabolic parameters, body mass index, and waist-to-hip ratio, as well as a quality-of-life (QoL) questionnaire.The study has two arms: (1) home-based monitored exercise using a smart digital garment and wristband, in addition to motivation and reinforcement via text messages; (2) standard CR facility-based exercise. The study design is a randomized, controlled trial comparing standard CR to a home-based monitoring and reinforcement program. The study program is designed for 12 weeks.Clinical tests and anthropometric measurements are performed before and after the study, measuring height, weight, waist circumference, visceral fat and body mass index (BMI), blood pressure, and HbA1c and lipid profile. Patients have to complete a baseline survey including socio-demographic characteristics and QoL questionnaire SF-36. At the end of the study, patients complete a survey regarding the use of the smart digital garment's benefits and usability. The study is currently underway.
Introduction
Regular exercise is an important method to prevent and treat cardiovascular disease1,2,3,4,5,6. Exercise lowers the risk of progression of vascular disease and significantly lowers the risk of cardiovascular morbidity and mortality7,8,9,10. Physical activity (PA) also improves endothelial dysfunction and restores arterial plasticity6,11. For patients discharged from the hospital with a diagnosis of coronary heart disease, CR programs are an important tool to promote health1,9. However, despite the evidence that CR reduces the risk of recurrent events following acute coronary syndromes, only a minority of eligible patients are willing to join existing programs at cardiac rehabilitation centers12,13. The reasons for this are many-lack of time and lack of motivation, a long distance between home and the CR center, and inconvenient accessibility. Whatever the reason, these patients are at a higher risk of recurrent events and hospitalizations, leading to reduced survival chances and quality of life with high costs. In order to prevent this, it is essential to find an alternative program for patients who cannot participate in a traditional rehabilitation program. Recent advances in mobile technology enable novel approaches to remotely guide and support these patients on an ongoing basis at relatively low cost14,15,16,17,18,19. The hypothesis of the study is that the novel approach of using remote patient-monitoring devices will enable a more convenient method of increasing participation in CR while achieving results similar to those of traditional cardiac rehabilitation.
The study has two arms, namely (1) home-based monitored exercise using the smart digital garment and wristband,in addition to motivation and reinforcement via text messages; (2) standard CR facility-based exercise with standard instructions regarding home-based exercise, not using a garment or wristband.
The health technology on trial combines mobile technology, artificial intelligence, and supportive services to expand the delivery of medical care from cardiac rehabilitation to the patient's home, empowering patients to better manage their health. A complete digital framework in three layers was designed for CR needs, including a personal (patient) layer, a support (coach) layer, and an information (education and guidance, administrative) layer (Figure 1). The program registers clinicians, coaches, and patients immediately. It is tailored to address a range of needs, including physical activity, behavioral health, medication, and nutrition, via chat, video, and audio communication channels between patients and coaches. Patients wearing a special wristband and smart digital garment (see the Table of Materials) will have 24/7 reciprocal multichannel access to their coach via their mobile phone for a personal plan and video and audio guidance. The coach is able to track the patient's activity at all times, leading to more precisely followed care plans and healthier decisions (Figure 2 and Figure 3). Commercially available, wearable PE tracking technology, which can connect users' PA data to an online network (control center), has the potential to partially address the need for innovative methods20,21. A wearable advanced fitness tracker (see the Table of Materials) will track the patient's heart rate and measure footsteps taken, distance traveled, floors climbed, active minutes, exercise, calories burned, and also sleep quality. The bracelet in the study (see the Table of Materials) was chosen as it is commonly used in various studies22,23. Also, for a population with reduced functional capacity, it is better and more suitable as it is more sensitive to movement24. As for the sample frequency, the pulse samples are taken continuously during activity and every minute during the day. In addition, the smart digital garment will be worn at predetermined times to allow tracking of various cardiac electrical changes as recorded by the patient's ECG (Figure 4).
The system is built with three integrated components, namely the smart digital garment, which is a machine-washable platform 12-lead ECG garment, a continuous remote monitoring and recording ECG device, and a cloud artificial intelligence (AI) server, which is cloud/server-based software for patient management and vital signs event detection (see the Table of Materials). The wearable fabric electrodes are robust in withstanding interference so that they can be used with minimal impact during normal daily activities, including walking, jogging, sitting, driving, sleeping, and exercising. The anatomical locations of the electrodes in the garment are the same as for electrodes during a standard ECG measurement. The standard limb leads I, II, and III each record the differences in electrical potential between two limbs. An additional six unipolar leads record the electrical potential difference between an exploring electrode and an electrode located centrally on the chest and computed from the average of the limb recordings (Figure 4). The connector in the garment is an High-Definition Multimedia Interface (HDMI) connector that is connected to the ECG device or to other standard ECG devices using an external adaptor. The device is a miniature ECG with an embedded processor containing data acquisition, data storage, data processing, accelerometer, respiration, body temperature (IR), and Bluetooth (BT) capabilities. The device acts as an ECG loop recorder, containing patient trigger event transmitting, live ECG transmission upon demand by the physician for a defined time period, and future auto-event detection of cardiac arrhythmia and ischemia. The heart rate accuracy of the smart garment is between 10-300 ± 2 bpm, and the frequency of the ECG device is 250 Hz. The device transmits the data in real-time to a communication device (such as a smartphone or tablet computer) with the dedicated mobile application via BT communication, so that the application can forward the data to a server for professional review via wireless internet access (see the Table of Materials). The platform data is stored in accordance with the Health Insurance Probability and Accountability Act (HIPAA) Privacy Rule for healthcare providers. The patient's ECG data is transferred to the cloud and encrypted and cyber-secured according to HIPPA regulations.
The participant's maximal heart rate is determined during a baseline exercise test. Aerobic exercises are performed continually at 60%-70% of the participant's heart rate reserve, using the modified Borg scale to evaluate perceived exertion during and after each training session. Treadmill speed and incline or cycle resistance and cadence are adjusted as needed to ensure that the assigned heart rate is reached at each training session. Evaluation of the degree of the patient's adherence and persistence and of the extent of the patient's involvement is done according to the intensity of use of chat and the manual delivery of data such as blood pressure.
Protocol
Ethical approval for the trial was obtained from the ethics committee of Hadassah Medical Center, Ein Kerem, Jerusalem (0306-17-HMO, MOH 2017-10-31_00073 4).
1. Study design
- Set the target for recruitment at 60 participants and divide the participants into two groups for a 3 month program.
NOTE: Power analysis to demonstrate a difference of 2,000 steps/day in intervention vs. nonintervention patients, with a standard deviation of 2,500 steps/day, at 80% power and .05 probability, requires 50 participants. In order to accommodate potential dropouts, five more participants will be recruited in each group. - Ensure that the participants are adults above the age of 21, with a diagnosis of ischemic heart disease (acute coronary syndrome with or without revascularization). Note that eligible candidates must be clinically stable ambulatory patients who are able to perform physical exercises.
- Exclude the participants with any serious or terminal illness that would preclude CR eligibilityNew York Heart Association Functional Classification (NYHA) above 2 as determined by the assessing cardiologist at the time of the intake. Also exclude patients planning to leave the geographic locale or those who participate in another randomized controlled trials (RCT), and patients who are incapable of using the technology prescribed or do not have a Bluetooth/standardapplication programming interface (API, Apple, Android).
- Perform baseline assessment after informed consent and prior to the start of CR.
- Randomly assign the participants by lottery (sealed envelope method) to the different interventions, namely facility CR or home CR (HCR).
- Derive study variables from measurements and questionnaires obtained pre- and post-intervention. Assess measurements at four time points (baseline, 3 months, 6 months, 12 months) and compare the variables between intervention and control groups. Calculate between-group comparisons for continuous variables using an unpaired t-test or Wilcoxon rank sum test, while categorical variables can be compared using a chi-squared test or Fisher's exact test for small numbers.
- Conduct within-group comparisons of variables at the four time points using a paired t-test for continuous variables and McNemar's test for categorical variables. Perform all statistical analysis using SPSS.
2. Preparation and end of the study
- Do clinical tests and anthropometric measurements. Measure the height, weight, waist circumference, visceral fat and BMI, blood pressure, and HbA1c and lipid profile of the patient.
- Complete a sociodemographic survey (SF-36).
- Perform a graded exercise ECG stress test using the Bruce protocol.
- Design individualized exercise programs.
- Assess the exercise intensity per patient with the heart rate reserve method.
- Complete a survey regarding the benefits and usability of the smart digital garment at the end of the study.
3. Patients entering outpatient CR
- Facility-based participants
- Let the facility-based participants attend the facility twice/week for 1 h over a 3 month period.
- Ask the participants to exercise at home, as per the guidelines, for at least 150 min of exercise per week at 60% of their target heart rate, with a preference for daily exercise.
- HCR participants
- For the first 6 weeks, let HCR participants attend the facility twice/week for 1 h, wearing the wristband (at all times) and the smart digital garment (at predetermined times).
- Tell the participants that they should perform monitored exercise at their individual capacity for 5 days a week, following instructions found in the app.
- Provide support and assistance regarding interventions and the achievement of goals.
- For the next 6 weeks, ask the participants to perform monitored exercise following instructions found in the app and ensure that they exercise at their individual capacity for 5 days a week.
4. Exercise procedure
- Facility-based training protocol
- Combine resistance and aerobic exercises in each training session.
- Measure the patient's blood pressure 3x, namely before, during, and at the end of each exercise session.
- Have the participant warm up for 5 min.
- Aerobic exercises for 30 min.
- Have the participant bike for 10 min.
- Have the participant walk on the treadmill for 15 min.
- Have the participant paddle on a hand cycle for 5 min.
- Have the participant perform resistance training at seven interval stations with active rest: rowing, chest press, leg press, shoulder press, leg extension, lateral pull down, and leg flexion. Each exercise consists of one set of 15 repetitions.
- Have the participant gradually cool down for 5 min.
- Monitor the heart rate using ECG telemetry.
- Make the training progressively more intense by increasing it from light (30% of 1-repetition maximum [1RM]) to 50% of 1RM.
- Home-based training protocol
- Let the participant attend the CR facility biweekly for the first 6 weeks and encourage them to perform monitored exercises 5 days a week at home.
- Provide the wearable wristband and the smart digital garment to the participant. While the participant is at the facility, have face-to-face sessions for the introduction of the equipment and instructions on how to use it.
- Ask the participant to wear the wristband at all time and the smart digital garment at predetermined times.
- Transfer ECG measurements before, during, and after the patient walks on the treadmill.
- Have the participant work out at home according to the instructions given in the app or digitally via chat or face to face by the coordinator. Provide support and assistance regarding interventions and the achievement of goals.
- For the remaining 6 weeks, let the participant exercise only at home.
- Tell the participant to wear the smart garment twice a week, and transfer ECG measurements before, during, and after performing proactive walking.
- Have the participant send manual blood pressure measurements via the app, taken before and after performing proactive walking.
- Confirm that the participant has taken his medications daily.
Representative Results
At present, 20 participants have been recruited to the study. For the participants in the study group, the smart digital garment and wristband are used as monitored tracking devices for most of the variables measured. Some variables, such as food intake, sugar levels, and weight, are entered manually by the patient. The patients in the study group have to wear the wristband most of the day and the smart digital garment twice a week for 30 min for 6 weeks while they are in the CR facility and 6 more weeks at home.
To perform the study, the wristband is used to measure all the physiological variables needed, and the smart digital garment is used for performing ECG. Both devices transmit the measured data to a smartphone that operates an application designed to collect data and transmit it directly to the system. The system is programmed to analyze and then execute the material analyzed (Figure 1). A dashboard facilitates the collection and visualization of the raw and analyzed data (Figure 2).
A combination of commercially available wearable tools is used with a system capable of evaluating and quantifying the various variables designed especially for the assessment of heart patients who use these devices. To this end, changes in physiological signals such as heart rate, sugar, and sleep, as well as nutrition data and more, are measured. In the course of the various activities, it is possible to assess the activity or inactivity of the patient on a daily basis, to know the patient's condition in real-time. After analyzing the data by the system, an immediate evaluation can be done by the medical team at the center who observes the results that appear on the dashboard (Figure 2 and Figure 3). The team has the ability to react immediately to any deviation from normal. The patient is under observation all day, and the coordinator is in contact with the patient to encourage them to continue keeping up their activity levels or to check why there is no activity by sending the patient daily to monthly graphic reports, and this is done throughout the 3 months' program (Figure 5 and Figure 6).
At this stage, initial observations, comments, and responses from participants show a clear preference for home digital telemedicine. Everyday contact via chat and the ability to practice throughout the day rather than being on a rigid schedule gives them an incentive to practice and be rehabilitated. Patients report a decrease in anxiety, and they are more confident and relaxed and sleep better at night for more time. The various indices measured also indicate an improvement; a decrease in general and abdominal fat levels and increased muscle mass was also observed, but this cannot yet be assessed statistically.
The results obtained from this study should support an alternative, home-based approach to enhance the long-term therapeutic efficacy of cardiac rehabilitation, especially for those patients who are unwilling or unable to participate in traditional rehabilitation programs.
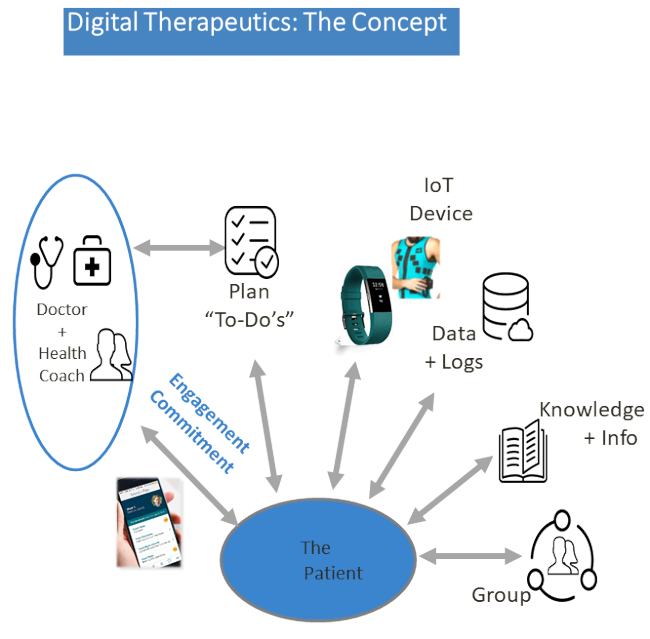
Figure 1: The digital program concept. The program registers the clinicians, as well as coaches and onboard patients, immediately. It is tailored to address the range of the medical team's needs concerning physical activity, behavioral health, medication, and nutrition via the application video and audio communication channels between patients and coaches.
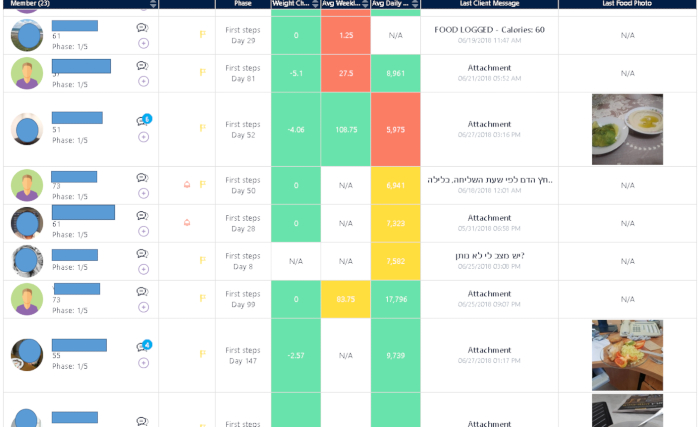
Figure 2: The platform dashboard. Patients, wearing a special wristband and garment, will have reciprocal multichannel access to their coach via their mobile phone for a personal plan and video and audio guidance. The coach is able to track the patient's activity at all times, leading to more precisely followed care plans and healthier decisions. Please click here to view a larger version of this figure.
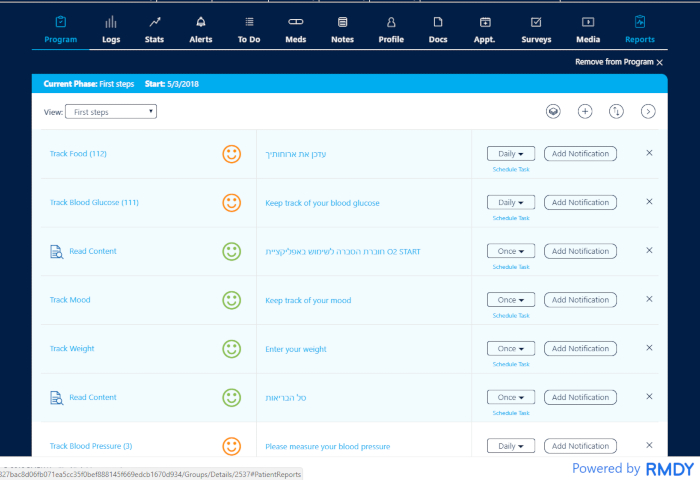
Figure 3: One patient's program. The patient is in observation all day long. The coordinator/coach is able to track the patient's activity at all times and is in contact with the patient to encourage them to continue their activity or to check why there is no activity. The multidisciplinary team has the ability to react immediately to any deviation from normality. Please click here to view a larger version of this figure.
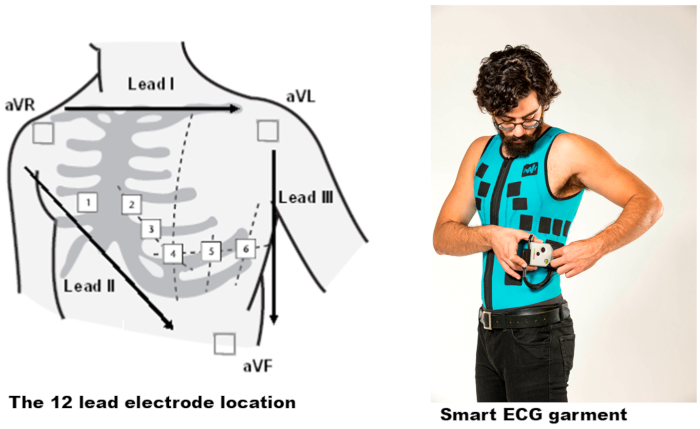
Figure 4: The Master Caution garment and the electrodes' locations. The standard limb leads I, II, and III each record the differences in electrical potential between two limbs. An additional six unipolar leads record the electrical potential difference between an exploring electrode and an indifferent electrode located centrally in the chest and computed from the average of the limb recordings. Please click here to view a larger version of this figure.
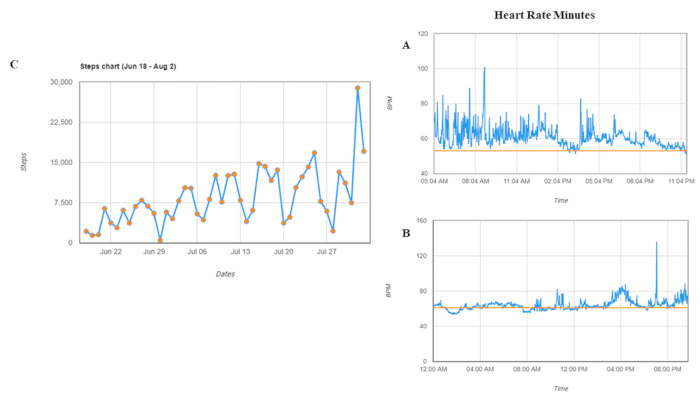
Figure 5: Graph reports for one patient. (A) A day of an active person. (B) A day of a sedentary person. (C) Average resting heart rate. Patients can be followed per day and per month. Heart rate = heart rate per minute. Please click here to view a larger version of this figure.
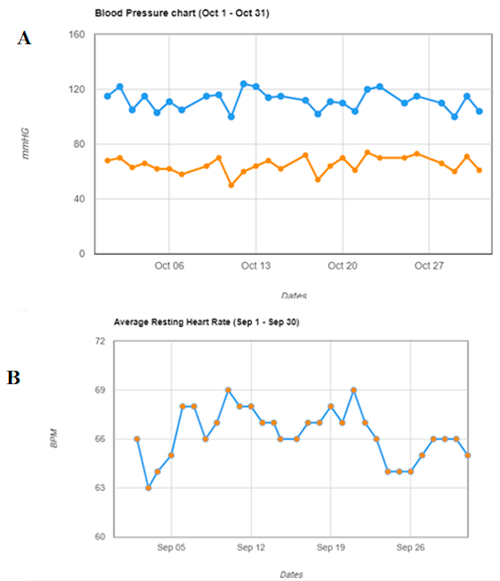
Figure 6: Graph reports for one patient. (A) Blood pressure. (B) Steps chart. Patients can be followed per day and per month.
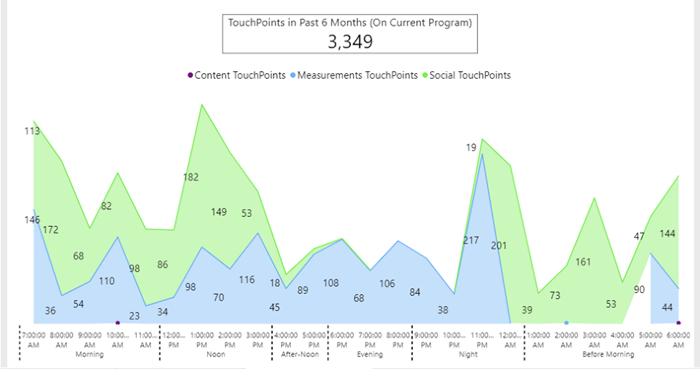
Figure 7: Daily behavior of one patient as aggregate activity during 6 months. Patient's engagement and frequency points of contact, through the app, between him and his coordinator. The green color shows the extent and time of communication between the coordinator and the patient displayed as touchpoints. The blue color indicates the number of measurements of the various indices as sent by the patient throughout one day, displayed as touchpoints.
Discussion
Home-based digital monitoring allows health care providers to acquire the patient's physiological data and have a follow-up of the patient's physical activity, as well as the patient's engagement and behavioral adaptation to a new, healthier regime. The software makes it possible to receive data in a continuous manner, allowing the interpretation of the data by the clinical team while collaborating with the patient, thereby increasing the patient's engagement regarding their rehabilitation process and their awareness regarding their physical condition. The methodology can detect the contacts and cooperation between coordinator, team, and patient and can postulate the degree of compliance (Figure 7). It can also summarize the patient's activity during the rehabilitation period (in months) and their time of preference to perform the assignments (Figure 7).
Regularly performing physical activity is a well-established method for preventing and treating cardiovascular diseases1,6. CR is an important tool to promote healthfulness and enhance therapeutic efficacy7,8.
Consensus statements among all agencies American Heart Association (AHA), American Association of Cardiovascular Pulmonary Rehabilitation (AACVPR), Agency for Health Care Policy and Research (AHCPR), American College of Cardiology (ACC), state that cardiac rehabilitation programs should offer a multidisciplinary approach to risk reduction and that programs that consist of exercise training alone are not enough25,26. Currently, CR programs address not only exercise but education (lifestyle) counseling as well27,28. Despite interventions such as utilizing electronic medical records, only a minority of eligible patients are willing to participate in the existing programs13. Moreover, patients' adherence to CR programs is not satisfactory. Home-based models have been developed to overcome obstacles such as distance as well as time constraints29,30,31,32,33,34,35.
Tele-CR has been shown to be as effective as conventional facility-based CR31,36.A meta-analysis comparing home-based to facility-based CR, examining exercise capacity, modifiable risk factors (blood pressure, blood lipid concentrations, and smoking), QoL, and cardiac events showed no differences in outcomes for those receiving home-based as opposed to facility-based rehabilitation in either the short term (<12 months) or long term (>12 months)37. Moreover, remote monitoring has an added value by allowing more patients to participate in rehabilitation programs and, specifically designed for those patients who cannot enroll in the facility-based CR, it can be used as an alternative for low-to-moderate cardiac risk patients36. A cost-benefit analysis comparing the two regimes found a benefit for either combined facility-home monitoring CR or telerehabilitation alone36,38.
Currently, home-based physical activity programs utilize diaries/questionnaires and mobile phone-based interventions29,30,31,32,35,39. However, all current remote home-based monitoring devices lack the technology to share data with the clinical team in real-time. Moreover, the clinical team's evaluation depends on the patient's subjective reports, which may not accurately reflect their condition. Another disadvantage is the lack of a daily team and group interaction and support. Furthermore, physical activity diaries should be interpreted cautiously unless the participants have an adequate understanding of physical activity intensity40.
Unlike with other telemedicine programs, which depend on the coordination of telephone calls and subjective reporting, clinical data using the current system can also be obtained retrospectively. The coordinator and team can communicate with patients via daily chat or calls, according to the mutual time convenience of patients and staff. Therefore, it is expected to find an improved collaboration between the patient and the multidisciplinary team. The AI system allows for the monitoring and identification of abnormal results that require the attention of the staff, such as an increase in blood pressure indices, changes in heart rate, failure to reach daily goals, etc. The assemblage of physiological information, including the parameters actively transmitted by the patient, such as blood pressure and ECG transmission, enables the rehabilitative team to treat the patient's complaints, to relieve anxieties, and to address symptoms such as a sense of palpitations, weakness, and fatigue. The patient learns to adopt new habits with the help of behavioral tools, daily feedback, and motivational elements, increasing the level of satisfaction and motivation to cooperate.
The methodology represented here, via the graph analysis, reflects the patient's new adapted lifestyle and provides scientists with information about the patient's engagement and interaction with the program, which cannot be done with standard questionnaires. However, this method has its limitations. First, the ECG platform is not connected or synchronized with the digital platform. For now, there is no interface between the two systems, requiring patients and staff to switch between apps. Second, technology usability barriers, difficulties using smartphone apps, and the fact that there is data that patients need to enter on their own make the methodology not suitable for all patients. It all depends on the capabilities and skills of the patients in using digital appliances and also on their ability to deal with the content and digital control.
Currently, the study is underway. The main goal is to encourage long-term health promotion and adherence with the aim to be economically cost efficient as well. In terms of technological capabilities, the goal is to make the digital system accessible to the entire population so that every patient could use the system without restrictions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to express our gratitude to Vera and Joseph Eden for their generous contribution to our research, in particular support the research work of Dr. Sara K.
Materials
| FitBit Charge 2 | FitBit | Model Name: FB407 | |
| Master Caution Garment | HealthWatch | MCG-M-XL | |
| Master Caution Device | HealthWatch | MCD | |
| Spectra 360 electrode gel | Parker labs | 12-08 |
References
- Gupta, S., et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 123, 1377-1383 (2011).
- Ekblom, O., Ek, A., Cider, A., Hambraeus, K., Borjesson, M. Increased Physical Activity Post-Myocardial Infarction Is Related to Reduced Mortality: Results From the SWEDEHEART Registry. Journal of American Heart Association. 7 (24), e010108 (2018).
- Sui, X., Sarzynski, M. A., Lee, D. C., Kokkinos, P. F. Impact of Changes in Cardiorespiratory Fitness on Hypertension, Dyslipidemia and Survival: An Overview of the Epidemiological Evidence. Progress in Cardiovascular Diseases. 60, 56-66 (2017).
- Spencer, R. M., Heidecker, B., Ganz, P. Behavioral Cardiovascular Risk Factors- Effect of Physical Activity and Cardiorespiratory Fitness on Cardiovascular Outcomes. Circulation Journal: Official Journal of the Japanese Circulation Society. 80, 34-43 (2016).
- Dor-Haim, H., et al. Improvement in cardiac dysfunction with a novel circuit training method combining simultaneous aerobic-resistance exercises. A randomized trial. PLoS ONE. 13 (1), e0188551 (2018).
- Piercy, K. L., et al. The physical activity guidelines for americans. Journal of the American Medical Association. 320, 2020-2028 (2018).
- Shaya, G. E., et al. High Exercise Capacity Attenuates the Risk of Early Mortality After a First Myocardial Infarction: The Henry Ford Exercise Testing (FIT) Project. Mayo Clinic Proceedings. 91, 129-139 (2016).
- Martin, B. J., et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clinic Proceedings. 88, 455-463 (2013).
- Hung, R. K., et al. Cardiorespiratory fitness attenuates risk for major adverse cardiac events in hyperlipidemic men and women independent of statin therapy: The Henry Ford ExercIse Testing Project. American Heart Jurnal. 170, 390-399 (2015).
- Schreuder, T. H., Duncker, D. J., Hopman, M. T., Thijssen, D. H. Randomized controlled trial using bosentan to enhance the impact of exercise training in subjects with type 2 diabetes mellitus. Experimental Physiology. 99, 1538-1547 (2014).
- Leggett, L. E., et al. Optimizing Value From Cardiac Rehabilitation: A Cost-Utility Analysis Comparing Age, Sex, and Clinical Subgroups. Mayo Clinic Proceedings. 90, 1011-1020 (2015).
- Forman, D. E., et al. Utility and efficacy of a smartphone application to enhance the learning and behavior goals of traditional cardiac rehabilitation: a feasibility study. Journal of Cardiopulmonary Rehabilitation and Prevention. 34, 327-334 (2014).
- Ades, P. A., et al. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clinic Proceedings. 92, 234-242 (2017).
- Cardinale, M., Varley, M. C. Wearable Training Monitoring Technology: Applications, Challenges and Opportunities. International Journal of Sports Physiology and Performance. 12 (Suppl 2), S255-S262 (2016).
- Wilde, L. J., Ward, G., Sewell, L., Muller, A. M., Wark, P. A. Apps and wearables for monitoring physical activity and sedentary behaviour: A qualitative systematic review protocol on barriers and facilitators. Digital Health. 4, 1-2 (2018).
- McCallum, C., Rooksby, J. Evaluating the Impact of Physical Activity Apps and Wearables: Interdisciplinary Review. Journal of Medical Internet Research Mhealth Uhealth. 6 (3), e58 (2018).
- McConnell, M. V., Turakhia, M. P., Harrington, R. A., King, A. C., Ashley, E. A. Mobile Health Advances in Physical Activity, Fitness, and Atrial Fibrillation: Moving Hearts. Journal of the American College of Cardiology. 71, 2691-2701 (2018).
- de Arriba-Perez, F., Caeiro-Rodriguez, M., Santos-Gago, J. M. Collection and Processing of Data from Wrist Wearable Devices in Heterogeneous and Multiple-User Scenarios. Sensors (Basel, Switzerland). 16 (9), e1538 (2016).
- Kamisalic, A., Fister, I. Sensors and Functionalities of Non-Invasive Wrist-Wearable Devices: A Review. Sensors (Basel, Switzerland). 18 (6), e1714 (2018).
- Arigo, D. Promoting physical activity among women using wearable technology and online social connectivity: a feasibility study. Health Psychology and Behavioral. 3, 391-409 (2015).
- Gordon, R., Bloxham, S. Influence of the Fitbit Charge HR on physical activity, aerobic fitness and disability in non-specific back pain participants. The Journal of Sports Medicine and Physical Fitness. 57 (12), 1669-1675 (2017).
- Diaz, K. M., et al. Fitbit(R): An accurate and reliable device for wireless physical activity tracking. International Journal of Cardiology. 185, 138-140 (2015).
- Leth, S., Hansen, J., Nielsen, O. W., Dinesen, B. Evaluation of Commercial Self-Monitoring Devices for Clinical Purposes: Results from the Future Patient Trial, Phase I. Sensors (Basel, Switzerland). 17 (1), e211 (2017).
- Thorup, C. B., Andreasen, J. J. Accuracy of a step counter during treadmill and daily life walking by healthy adults and patients with cardiac disease. Journal of Medical Internet Research Mhealth Uhealth. 7 (3), e011742 (2017).
- Yeboah, J. Road to the American Heart Association 2020 Impact Goals: The Metric for Monitoring Progress. Circulation Cardiovascular Imaging. 11, e007385 (2018).
- Treat-Jacobson, D., et al. Optimal Exercise Programs for Patients With Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 13, CIR0000000000000623 (2018).
- Balady, G. J., et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 124, 2951-2960 (2018).
- Balady, G. J., et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 115, 2675-2682 (2007).
- Rawstorn, J. C., et al. End Users Want Alternative Intervention Delivery Models: Usability and Acceptability of the REMOTE-CR Exercise-Based Cardiac Telerehabilitation Program. Archives of Physical Medicine and Rehabilitation. 99, 2373-2377 (2018).
- Frederix, I., Solmi, F., Piepoli, M. F., Dendale, P. Cardiac telerehabilitation: A novel cost-efficient care delivery strategy that can induce long-term health benefits. European Journal of Preventive Cardiology. 24, 1708-1717 (2017).
- Hwang, R., Bruning, J., Morris, N. R., Mandrusiak, A., Russell, T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. Journal of Physiotherapy. 63, 101-107 (2017).
- Maddison, R., et al. HEART: heart exercise and remote technologies: a randomized controlled trial study protocol. BioMed Central Cardiovascular Disorders. 11, 26 (2011).
- Piotrowicz, E., et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. European Journal of Heart Failure. 12, 164-171 (2010).
- Zwisler, A. D., et al. Home-based cardiac rehabilitation for people with heart failure: A systematic review and meta-analysis. International Journal of Cardiology. 221, 963-969 (2016).
- Frederix, I., Sankaran, S., Coninx, K., Dendale, P. MobileHeart, a mobile smartphone-based application that supports and monitors coronary artery disease patients during rehabilitation. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. , 513-516 (2016).
- Kraal, J. J., et al. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. European Journal of Preventive Cardiology. 24, 1260-1273 (2017).
- Buckingham, S. A., et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 3, e000463 (2016).
- Frederix, I., Vandijck, D., Hens, N. Economic and social impact of increased cardiac rehabilitation uptake and cardiac telerehabilitation in Belgium – a cost-benefit analysis. Acta Cardiologica. 73, 222-229 (2018).
- Rohrbach, G., et al. The design and implementation of a home-based cardiac rehabilitation program. Federal Practitioner. 34 (5), 34-39 (2017).
- Freene, N., Waddington, G., Chesworth, W., Davey, R., Cochrane, T. Validating two self-report physical activity measures in middle-aged adults completing a group exercise or home-based physical activity program. Journal of Science and Medicine in Sport/Sports Medicine Australia. 17, 611-616 (2014).

