Dissection of Pelvic Autonomic Ganglia and Associated Nerves in Male and Female Rats
Summary
The major pelvic ganglia contain parasympathetic and sympathetic neurons that innervate pelvic organs. Here we describe a dissection method and provide schematics for identification of these ganglia and their associated nerves. These methods can be applied to experimental manipulation of these ganglia in vivo or removal post-mortem for further study.
Abstract
The bilateral major pelvic ganglia (MPG; synonym, pelvic ganglia) are the primary source of postganglionic sympathetic and parasympathetic neurons innervating pelvic organs of rodents; the functionally equivalent structure in humans is the inferior hypogastric plexus. The major pelvic ganglia also provide the route by which lumbar and sacral sensory axons reach the pelvic organs. These complex, mixed ganglia can prove challenging to identify and dissect for further experimental study of normal autonomic mechanisms or to establish preclinical models of disease, injury or visceral pain. Here we describe a protocol to access and visualize these ganglia and their associated nerve tracts. We provide this protocol with schematics for both male and female rats, as the ganglion size and landmarks for identification differ between sexes. The protocol describes removal of the ganglion for in vitro studies, but this method can be integrated into a surgical recovery protocol for experimental interventions (e.g., nerve crush, nerve resection) or for mapping neuronal circuits (e.g., by microinjection of neural tracers). We also demonstrate the primary structures of the ganglion and its associated nerves immediately following dissection and following immunohistochemical staining.
Introduction
The rat is the one of the best characterized species used in the study of the pelvic organ physiology and anatomy. While excellent resources exist for descriptions of these organs1,2, they generally do not provide information on the related neural structures or do so at insufficient resolution to guide an experimental study. As detailed further below, the organization of the autonomic ganglia that regulate pelvic organ function is quite different to the rest of the autonomic nervous system, making it difficult to accurately infer pelvic innervation features from neuroanatomical information available for other autonomic ganglia. This deficiency in resources to guide researchers entering this area may have slowed research on neural regulation of pelvic organs. Here we describe protocols for accessing this region of the nervous system for further in vitro studies or experimental intervention.
The bilateral major pelvic ganglia (MPG; synonyms: pelvic ganglia; paracervical ganglia [female]; Frankenhäuser's ganglion [female]) are the primary source of postganglionic sympathetic and parasympathetic neurons innervating pelvic organs of rodents; the inferior hypogastric plexus comprises the equivalent neuronal structure in humans3,4,5,6. Sensory projections from lumbar and sacral dorsal root ganglia also travel via the MPG to reach the pelvic organs. Therefore, understanding the neural circuitry and biology of the MPG is critical for preclinical studies on a myriad of clinical conditions relating to the development and adult function of pelvic organs. Several excellent descriptions of rodent MPG have been published7,8, but our experience is that in general these descriptions do not always provide sufficient guidance to practically inform an experimental dissection or manipulation of these structures when recovery of the animal is required. Moreover, the majority of MPG studies focus on male rats. In female rats, the MPG are smaller9 and have distinct anatomical landmarks, and therefore require a distinctly tailored guide to visualization and dissection.
Sympathetic and parasympathetic pathways are distinguished by their anatomy, specifically the location of their preganglionic neurons, with sympathetic pathways having preganglionic neurons in the thoraco-lumbar spinal cord and the parasympathetic preganglionic neurons located in the brainstem (cranial nerve projections) and sacral spinal cord. In most other regions of the autonomic system, their target ganglion neurons are located in distinct sympathetic or parasympathetic ganglia. However, the MPG are unusual in being mixed sympathetic-parasympathetic ganglia, and therefore at a macroscopic scale are sites of convergence from preganglionic axons of both thoraco-lumbar and sacral spinal regions. We have therefore included in our protocols the location and description of these primary nerve tracts that connect each spinal region with the MPG, facilitating experimental analysis or separate manipulation of these neural components. We also note for readers specifically comparing these ganglia across species, that in rodents the spinal preganglionic neurons that are 'functionally sacral', e.g., are active and required during micturition, defecation and penile erection, are located at spinal levels L6-S1 rather than exclusively in sacral segments10; likewise L6 and S1 dorsal root ganglia provide the major 'sacral' sensory input to pelvic organs. In rodents, sensory and preganglionic input from more rostral neural circuits is concentrated in spinal levels L1 and L210.
Here we describe a protocol to access the MPG and their associated nerve tracts in male and female rats, and support this with schematics to illustrate specific landmarks. This protocol guides surgical access to these structures in an experimental context of removing the tissue for in vitro studies, e.g., isolating MPG neurons for molecular characterization or primary culture. It can also be adapted to MPG removal after intracardiac perfusion with fixative, although this is a more difficult dissection because the neural tissue becomes more difficult to visualize when the adjacent tissues are devoid of blood. This protocol can also be integrated into a surgical setting for experimental intervention of these nerve pathways (e.g., nerve resection, microinjection of neural tracers). These types of dissections are increasingly important for the growing field of bioelectronic medicine, where new targets and approaches for neuromodulation to treat clinical conditions of the pelvic viscera are being developed11. We present the complete protocol first for male rats, then a replicate of the protocol tailored specifically for female rats.
Protocol
All procedures are to be conducted according the institutional and funding body requirements for animal experimentation. The use of animals for this dissection and the protocol for euthanasia have been approved by the Animal Ethics Committee at the University of Melbourne (Protocol number 1814639).
NOTE: The dissections illustrated here were performed on adult (~10 weeks) male and female Sprague-Dawley rats (Biomedical Sciences Animal Facility, University of Melbourne), weighing 280 g (female) and 350 g (male). Prior to these dissections, the rats were euthanized in a CO2 chamber for 4−5 min. Immediately following death, MPG were dissected. If dissecting tissue from an animal that has undergone transcardial perfusion with fixative, take precautions to protect the operator from exposure to fixative, i.e., perform dissection in fume cupboard or downdraft cabinet and wear suitable personal protective equipment. A protocol for transcardial perfusion has been published in detail12.
1. Major pelvic ganglion and adjacent nerves: access and resection in a male rat
NOTE: Figure 1 shows anatomical landmarks for MPG visualization in a male rat.
- Access to the abdominal cavity and pelvis
- Place the rat in a supine position and access the abdomen and pelvis through a ventral midline incision, taking care to avoid contamination of the surgical field with fur.
- Gently move the abdominal organs to one side using forceps or cotton-tipped applicators. Note the location of the ventral lobes of the prostate gland and the urinary bladder.
- Move the seminal vesicle to the contralateral side.
- Cut the vas deferens to provide better access to the area overlying the ganglion.
NOTE: From this point of the dissection, the tissue must not dry out; keep the tissue moist with physiological saline (for fresh tissue dissection) or fixative (for perfusion-fixed animal). Keeping the tissue moist with saline not only benefits tissue structure but also makes the dissection easier as dry nerves are more fragile and tear more easily during handling. - Identify the dorsolateral lobe of the prostate gland, on the dorsal surface of which is the location of the ganglion; this will not yet be visible.
- To visualize the ganglion, carefully clear away tissues near and overlying the ganglion. If necessary, use a retractor to keep the dissection field clear.
- Remove a nearby aggregate of adipose tissue and open the lateral fascia of the pelvis.
- Dissection of the MPG and its associated nerves
- Identify the following locations that provide landmarks for the next steps of the dissection: the dorsolateral lobe of the prostate gland (the ganglion is located on the surface of this lobe, slightly more caudal than the junction between seminal vesicle and prostate) and the seminal vesicles (where they converge at the midline indicates the ganglion location on the animal's rostrocaudal axis).
- As required from this point, carefully remove any tissue that impedes complete view of the neural structures, avoiding damage to the thin capsule of the prostate gland or major vessels.
- Identify the pelvic nerve by visualizing the following landmarks and features.
- Find the internal iliac vein and its fine branch projecting towards the MPG and the bladder. This vascular branch runs parallel to and is sometimes embedded within the pelvic nerve, then traverses the ganglion.
- Gently place fine-tipped angled forceps under the pelvic nerve and slide the forceps along to free it from surrounding tissue.
NOTE: It may also be possible to isolate the pelvic nerve from the small vessel running parallel to it, but for most types of experiments this is not essential. Confirm that the structure is the pelvic nerve by viewing under high magnification to determine that the nerve contains several loosely aggregated fascicles, which are easily distinguished under the dissecting microscope and are characteristic of the pelvic nerve, as none of the other major nerves associated with the ganglion show this clear fasciculation.
- Identify the cavernous nerve by visualizing the following landmarks and features.
- After following the pelvic nerve to its junction with the ganglion, follow the cavernous nerve as it travels across the prostate and then caudally towards the cavernous bodies of the penis.
- If microscope magnification permits, note that there is a small group of delicate nerves emerging from the ganglion between the pelvic and cavernous nerves; these are the rectal nerves that travel to the lower bowel.
- Identify the hypogastric nerve by visualizing the following landmarks and features.
- Identify where the hypogastric nerve joins the ganglion at its cranial edge, after travelling alongside the ureter.
- Confirm that the hypogastric nerve is much thinner than either the pelvic or cavernous nerves and is not accompanied by large vessels.
- Identify the MPG by visualizing the following features.
- Visualize the ventral, dorsal and cranial edges of the ganglion, forming a triangular shape.
- Confirm the location of each major nerve: the pelvic nerves emerging from the ganglion's dorsal edge, the cavernous nerve at the most caudal corner of the ganglion, the hypogastric nerve from its cranial edge, and the accessory nerves emerging from the ganglion's ventral edge.
- Identify the accessory nerves by visualizing the following landmarks and features.
- After clearing tissue to enable visualization of the ventral edge of the ganglion, identify a cluster of nerves that project towards the urinary and reproductive tracts.
- If microscope magnification permits, identify one caudal group of nerves that enter between the prostate lobes and one rostral group between the seminal vesicle and the bladder.
- Removal of the MPG with its associated nerves
- Gently slide forceps between the ganglion and the underlying prostate gland, being careful not to puncture the thin capsule of the prostate. Disrupt any connections between the ganglion and the prostate.
- Clear any final connections with surrounding tissues for the lengths of nerves required for the experiment, then cut each nerve.
- Using fine forceps, move the ganglion with its nerves to the appropriate solution for the experiment and confirm that each of the main nerves are intact.
2. Major pelvic ganglion and adjacent nerves: access and resection in a female rat
NOTE: Figure 2 shows anatomical landmarks for MPG visualization in a female rat.
- Access to the abdominal cavity and pelvis
- Place the rat in a supine position and access the abdomen and pelvis through a ventral midline incision, taking care to avoid contamination of the surgical field with fur.
NOTE: From this point of the dissection, the tissue must not dry out; keep the tissue moist with physiological saline (for fresh tissue dissection) or fixative (for perfusion-fixed animal). - Gently move the abdominal organs to one side using forceps or cotton-tipped applicators. Note the location of the uterine horn, urinary bladder and rectum.
- Cut the ovarian and uterine vessels and retract the uterine horn.
- Enter the peritoneal space and gently clear away an aggregate of adipose tissue located near the uterine cervix.
- Place the rat in a supine position and access the abdomen and pelvis through a ventral midline incision, taking care to avoid contamination of the surgical field with fur.
- Dissection of the MPG and its associated nerves
- Identify the lateral wall of the uterine cervix, just caudal to its junction with the uterine horns; this region is the primary landmark for defining the MPG location on the animal's rostrocaudal axis.
- As required from this point, carefully remove any tissue that impedes complete view of the neural structures, avoiding damage to major vessels.
- Identify the pelvic nerve by visualizing the following landmarks and features.
- Find the internal iliac vein and its fine branch projecting towards the MPG and the bladder. This branch runs parallel to and is sometimes embedded within the pelvic nerve, then traverses the ganglion.
- Confirm that the structure is the pelvic nerve by viewing under high magnification to determine that the nerve contains several loosely aggregated fascicles, which are easily distinguished under the dissecting microscope and are characteristic of the pelvic nerve, as none of the other major nerves associated with the ganglion show this clear fasciculation.
- Identify the hypogastric nerve by visualizing the following landmarks and features.
- Identify where the hypogastric nerve joins the ganglion at its cranial edge, after travelling alongside the ureter.
- Confirm that the hypogastric nerve is much thinner than either the pelvic or cavernous nerves and is not accompanied by large vessels.
- Identify the cavernous nerve by visualizing the following landmarks and features.
- After following the pelvic nerve to its junction with the ganglion, follow the cavernous nerve as it travels caudally along the lateral wall of the cervix towards the vagina.
- If microscope magnification permits, note that there is a small group of delicate nerves emerging from the ganglion between the pelvic and cavernous nerves; these are the rectal nerves that travel to the lower bowel.
- Identify the accessory nerves by visualizing the following landmarks and features.
NOTE: The accessory nerves are difficult to see but project from the medial aspect of the MPG. After clearing tissue to enable visualization of the ventral edge of the ganglion, identify a cluster of very delicate nerves that project towards the urinary and reproductive tracts. - Identify the MPG by visualizing the following features.
- Visualize the ventral, dorsal, and cranial edges of the ganglion, which form a triangular shape.
- Confirm the location of each major nerve: the pelvic nerves emerging from the ganglion's dorsal edge, the cavernous nerve at the most caudal corner of the ganglion, the hypogastric nerve from its cranial edge, and the accessory nerves emerging from the ganglion's ventral edge.
- Removal of the MPG with its associated nerves
- Gently place fine-tipped angled forceps under the pelvic nerve and slide the forceps along to free it from the underlying uterine cervix and surrounding tissue.
NOTE: It may also be possible to isolate the pelvic nerve from the small vessel running parallel to it, but for most types of experiments this is not essential. If demonstrating the dissection, place a suture under the pelvic nerve, to facilitate its visualization. - Repeat the process for the cavernous nerve, then the hypogastric nerve, and finally the accessory nerves.
- Gently slide forceps between the ganglion and the underlying uterine cervix. Disrupt any connections between the ganglion and the cervix.
- Clear any final connections with surrounding tissues for the lengths of nerves required for the experiment, then cut each nerve.
- Using fine forceps, move the ganglion with its nerves to the appropriate solution for the experiment and confirm that each of the main nerves are intact.
- Gently place fine-tipped angled forceps under the pelvic nerve and slide the forceps along to free it from the underlying uterine cervix and surrounding tissue.
3. Confirmation of ganglion components (optional)
- After removal of the ganglion, immerse ganglion in a conventional histological fixative (e.g., 4% buffered formalin) for a minimum of 1 h, wash out fixative with 0.1 M phosphate buffer and process tissue for cryosectioning and fluorescence immunohistochemistry, as previously described13.
NOTE: Many high-quality antibodies that specifically recognize these three neural markers are commercially available. See Table of Materials for the reagents used for the labeling shown in Figure 3. - Alternatively, process ganglia intact (wholemounts) for immunohistochemistry using a similar method as above but increasing the incubation times for the antibodies to 4 days (primary antibody) and 2 days (secondary antibody).
- To demonstrate a major population of sensory axons, use antibodies against calcitonin gene-related peptide (CGRP).
NOTE: The recommended dilution of the antibody used in this study is 1:5,000. - To demonstrate noradrenergic sympathetic neurons, use antibodies against tyrosine hydroxylase (TH).
NOTE: The recommended dilution of the antibody used in this study is 1:5,000. - To demonstrate a major population of cholinergic neurons, use antibodies against neuronal nitric oxide synthase (NOS).
NOTE: The recommended dilution of the antibody used in this study is 1:500.
Representative Results
A successful dissection will not only remove the complete body of the MPG intact, but also retain the first segment of each of the major nerves still attached. These nerves are valuable indicators of ganglion orientation in vivo and therefore provide essential information for many types of anatomical studies (e.g., mapping expression patterns or cellular changes after an experimental perturbation). Although preserving the associated nerves can be of less importance for some experiment types (e.g., ganglion dissociation for culture of isolated neurons), the presence of nerves also provides a way of handling the ganglion without touching (and potentially damaging) the neuronal cell bodies.
An unsuccessful dissection will have an incomplete or damaged ganglion, or where the primary nerves are no longer attached. It is also possible that ganglia or nerves are unknowingly damaged during dissection, either because the physical damage is too subtle to detect under the dissecting microscope or because the damage only becomes apparent during certain types of assays. For example, if the ganglion tissue becomes dry during dissection, the tissue may appear normal during later handling, but will show high levels of non-specific fluorescence on the surface.
Examples of dissected MPG are shown in Figure 3, which provides examples of the entire MPG visualized as a whole thickness complete ganglion (Figure 3A) and an MPG that has been cryosectioned for performing immunofluorescence to demonstrate noradrenergic and cholinergic neurons (Figure 3B,C).
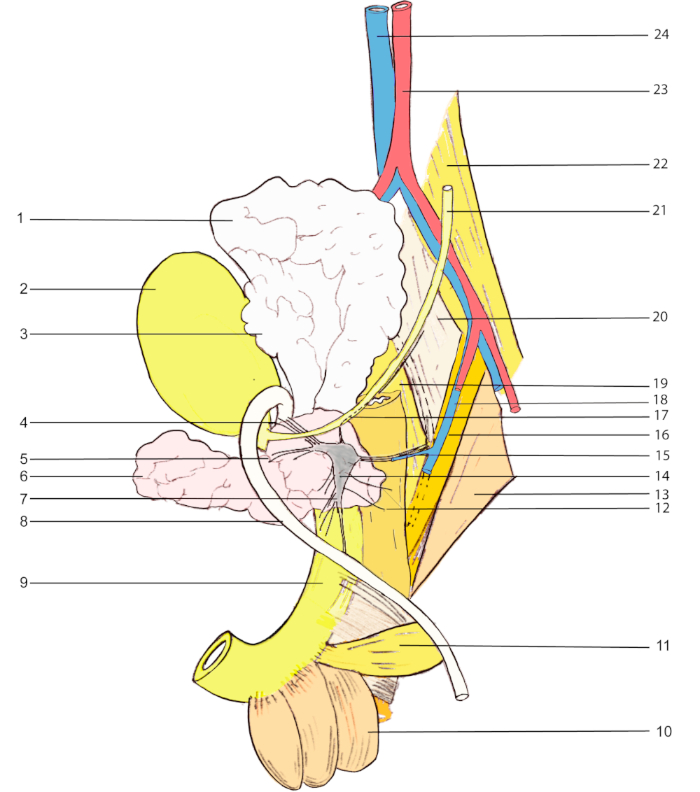
Figure 1: Anatomical landmarks for MPG visualization in a male rat. 1, seminal vesicle; 2, urinary bladder; 3, coagulating gland; 4 & 5, accessory nerves; 6, prostate (ventral lobe); 7, cavernous nerve; 8, vas deferens; 9, urethra; 10, bulbocavernosus muscle; 11, ischiocavernosus muscle; 12, rectal nerves; 13, abductor caudae externus; 14, major pelvic ganglion; 15, pelvic nerve; 16, abductor caudae internus; 17, hypogastric nerve; 18, internal iliac vein; 19, flexor caudae brevis; 20, flexor caudae longus; 21, ureter; 22, psoas major; 23, abdominal aorta; 24, inferior vena cava. Please click here to view a larger version of this figure.
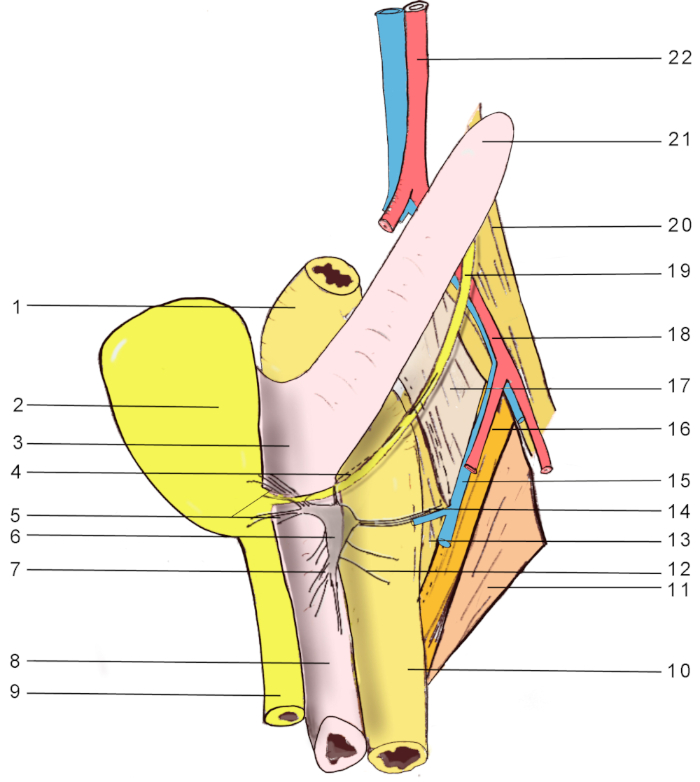
Figure 2: Anatomical landmarks for MPG visualization in a female rat. 1, distal colon; 2, urinary bladder; 3, uterine body; 4, hypogastric nerve; 5, accessory nerves; 6, major pelvic ganglion; 7, cavernous nerve; 8, vagina; 9, urethra; 10, rectum; 11, abductor caudae externus; 12, rectal nerves; 13, flexor caudae brevis; 14, pelvic nerve; 15, internal iliac vein; 16, abductor caudae internus; 17, flexor caudae longus; 18, external iliac artery; 19, ureter; 20, psoas major; 21, uterine horn; 22, abdominal aorta. Please click here to view a larger version of this figure.
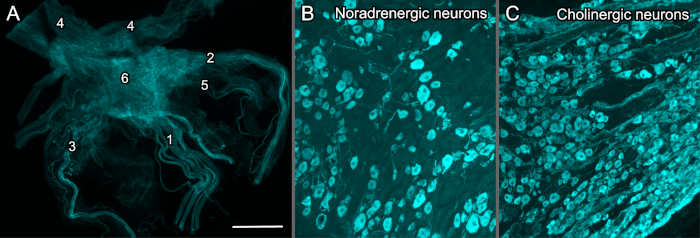
Figure 3: Immunohistochemically labelled MPG from adult male rats. All preparations have been visualized with a conventional widefield fluorescence microscope equipped with a monochrome camera, then digitally colorized. (A) Wholemount (complete thickness), fixed MPG with associated nerves, immunohistochemically labelled for the sensory nerves that express calcitonin gene-related peptide (CGRP); 1, pelvic nerve (showing the multiple fascicles); 2, cavernous nerve; 3, hypogastric nerve; 4, accessory nerves; 5, rectal nerves; 6, major pelvic ganglion (MPG). (B,C) Cryosections (14 µm) of fixed MPG, immunohistochemically labelled to demonstrate the mixed noradrenergic-cholinergic nature of the ganglion; (B) noradrenergic neurons demonstrated by antibody for tyrosine hydroxylase and (C) a major population of cholinergic neurons by antibody for neuronal nitric oxide synthase. Calibration bar represents (A) 1,000 µm, (B,C) 200 µm. Please click here to view a larger version of this figure.
Discussion
Neural control of the pelvic organs is mediated by complex pathways including somatic, parasympathetic, sympathetic and visceral sensory components14,15,16,17. Most of these pathways originate in or pass through the MPG. The dissection protocols outlined here provide an introduction to MPG anatomy, the related associated nerves and nearby macroscopic anatomical landmarks; the latter are illustrated by anatomical schematics. Other approaches to MPG dissection may also be successful, but we find the one described here to be robust and suitable for a researcher new to this area of the nervous system.
The most critical aspects of the protocol are correct identification of each major nerve and the complete removal of MPG tissue. With careful viewing and handling of the tissues, MPG tissues can be removed for anatomical, molecular and electrophysiological in vitro studies18,19,20,21,22. The protocol can also be adapted for in vivo experimental manipulation23,24,25, noting that in this case, great care must be taken to minimise contact with the primary nerves associated with the ganglion or to damage nearby vasculature. If the experiment requires selective denervation by interruption of one or more nerves, it is recommended to ligate the severed nerve to prevent reinnervation and confounding of analyses. This dissection protocol could also be utilized for the mouse, where there is also an MPG with comparable function26,27,28.
For neuroanatomical studies, the best preservation of antigens and tissue structure is obtained by dissecting the MPG from an anesthetized animal that has been perfused transcardially with histological fixative appropriate to the experiment29; however, identification of the ganglion and nerve structures are more difficult after this process, as the tissue coloration is lost. It is recommended to become proficient in identifying and dissecting the ganglion from nonperfused animals before attempting this dissection after perfusion. Likewise, it is recommended to first become proficient in the dissection in males because for animals of equivalent age and body size, the MPG and its associated nerves are much smaller in females.
To validate that the tissue removed is indeed the MPG, the researcher is first advised to check the location and features of each primary nerve. Many dissectors find the pelvic nerve and cavernous nerve the easiest to identify in situ; the hypogastric and accessory nerves are more delicate and more difficult to distinguish from the surrounding tissue. If these nerves are no longer available because of problems during dissection, or if there is uncertainty regarding their structure, it is recommended that initial MPG dissections are characterized with conventional histology (to confirm presence of neuronal cell bodies8) and secondly with immunohistochemistry (to identify that both cholinergic and noradrenergic neurons are present30,31) (Figure 3). To validate correct identification of the major nerves, the cavernous nerves are readily identified by their high density of neuronal cell bodies in their initial portion close to the MPG; most of these neurons express markers of cholinergic, nitrergic neurons32,33. The pelvic, hypogastric and accessory nerves have very few neuronal cell bodies34.
There are several common pitfalls in performing this dissection. If novice dissectors have problems finding any of the major nerves or the MPG, they are encouraged to return to the steps that describe the key landmarks. It is very common to become so focused on finding the microstructures that one loses track of the macroscopic context. Most commonly, novice dissectors either move too far rostral in their dissection site or remain too 'superficial'-i.e., too close to the ventral opening of the abdomen, rather than examining deeper (i.e., more dorsal) structures. A common problem during dissection is damage to the vasculature during dissection. If bleeding starts, gently hold a cotton-tipped applicator over the source until bleeding stops, then flush the area liberally with saline before recommencing dissection. It is possible the MPG will not be usable for experiments if contaminated with too much blood or if the dissection is delayed too long while waiting for bleeding to stop. Another common dissection error is damage to the capsule of the prostate gland which significantly impairs the MPG visualization and removal. This capsule is a very delicate structure that is easily punctured while removing the fat from the lateral wall of the prostate, even if using only a cotton-tipped applicator. Finally, the main nerves associated with the MPG are easily damaged during the process of identifying each one and then during removal of the MPG. Dissectors are encouraged to develop a routine whereby each nerve is isolated in turn, in a particular order, so that there is less opportunity for confusion during the final steps of ganglion removal.
This dissection did not seek to trace each of the components of the accessory nerves to specific organs, or to identify each of the many microganglia that lie at various points between the pelvic ganglia and the pelvic organs8. These are quite difficult to visualize in vivo without using specific stains; however, they can be removed by following each of the nerve tracts towards the organs, and utilizing specific neural stains post hoc to determine ganglion location. These microganglia, even though comprising only small fraction of the neuronal population compared to the MPG, may provide specific types of input to the organs to which they are most closely located. We note here a limitation in the field that neither these microganglia nor many of the small nerve tracts exiting the MPG to travel to pelvic organs yet have broadly accepted names. Moreover, a similarly detailed study of microganglia has not yet been conducted in female rats.
In summary, the protocol and schematics provided here provide researchers with tools to study the primary structures providing the autonomic nerve supply to the pelvic organs, as well as the major peripheral conduits of sensory nerves from lumbosacral dorsal root ganglia that travel via the MPG to pelvic organs.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by the Office of the Director, National Institutes of Health, Stimulating Peripheral Activity to Relieve Conditions (SPARC) Program, Award Number OT2OD023872. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Bertrand's fellowship in Dr. Keast's laboratory was financed by: The University Hospital of Nîmes, the faculty of medicine of Montpellier-Nîmes, The Association Française de Chirurgie (AFC), The Société Interdisciplinaire Francophone d'UroDynamique et de Pelvipérinéologie (SIFUD-PP) and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement No PCOFUND-GA-2013-609102, through the PRESTIGE programme coordinated by Campus France.
Materials
| Anti-calcitonin gene-related peptide; RRID AB_259091 | Merck | C8198 | |
| Anti-nitric oxide synthase, RRID AB_2533937 | Invitrogen | 61-7000 | |
| Anti-rabbit IgG, Cy3 tag, RRID AB_2307443 | Jackson | 711-165-152 | |
| Anti-tyrosine hydroxylase, RRID AB_390204 | Millipore | AB152 | |
| Dissecting microscope | Olympus | SZ40, SC | |
| Dumont AA epoxy coated forceps | Fine Science Tools | 11210-10 | |
| Dumont #5 forceps | Fine Science Tools | 11255-20 | |
| Dumont #5/45 curved forceps | Fine Science Tools | 11251-35 | |
| LED light source | Schott | KL 1600 | |
| Micro-Adson forceps | Fine Science Tools | 11019-12 | |
| Student Vannas spring scissors | Fine Science Tools | 91500-09 | |
| Surgical scissors | Fine Science Tools | 14054-13 |
References
- Greene, E. C. . Anatomy of the Rat. , (1935).
- Krinke, G. J. . The Laboratory Rat. , (2000).
- Keast, J. R., McLachlan, E. M. Pelvic ganglia. Autonomic Ganglia. , 445-480 (1995).
- Keast, J. R. Unusual autonomic ganglia: connections, chemistry, and plasticity of pelvic ganglia. International Review of Cytology. 193, 1-69 (1999).
- Alsaid, B., et al. Coexistence of adrenergic and cholinergic nerves in the inferior hypogastric plexus: anatomical and immunohistochemical study with 3D reconstruction in human male fetus. Journal of Anatomy. 214 (5), 645-654 (2009).
- Keast, J. R., Smith-Anttila, C. J., Osborne, P. B. Developing a functional urinary bladder: a neuronal context. Frontiers in Cell and Developmental Biology. 3, 53 (2015).
- Purinton, P. T., Fletcher, T. F., Bradley, W. E. Gross and light microscopic features of the pelvic plexus in the rat. Anatomical Record. 175 (4), 697-705 (1973).
- Arellano, J., Xelhuantzi, N., Mirto, N., Hernández, M. E., Cruz, Y. Neural interrelationships of autonomic ganglia from the pelvic region of male rats. Autonomic Neuroscience. 217, 26-34 (2019).
- Greenwood, D., Coggeshall, R. E., Hulsebosch, C. E. Sexual dimorphism in the numbers of neurons in the pelvic ganglia of adult rats. Brain Research. 340 (1), 160-162 (1985).
- Nadelhaft, I., Booth, A. M. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. Journal of Comparative Neurology. 226 (2), 238-245 (1984).
- Kessler, T. M., Birder, L. A., Gomery, P. Neuromodulation of urinary tract function. New England Journal of Medicine. 380 (21), 2067-2069 (2019).
- . Intracardiac perfusion with fixative for anatomical studies [keast-001-stage02] Available from: https://www.protocols.io/view/intracardiac-perfusion-with-fixative-for-anatomica-w3ffgjn (2019)
- . Immunohistochemical analysis of ganglion neurons innervating the lower urinary tract [keast-001-stage03] Available from: https://www.protocols.io/view/immunohistochemical-analysis-of-ganglion-neurons-i-w3efgje (2019)
- Fowler, C. J., Griffiths, D., de Groat, W. C. The neural control of micturition. Nature Reviews Neuroscience. 9 (6), 453-466 (2008).
- Keast, J. R., Booth, A., de Groat, W. C. Distribution of neurons in the major pelvic ganglion of the rat which supply the bladder, colon or penis. Cell and Tissue Research. 256 (1), 105-112 (1989).
- Dail, W. G., Minorsky, N. Composition of the pelvic nerve. Experimental Neurology. 92 (1), 278-283 (1986).
- Dail, W. G. The pelvic plexus: innervation of pelvic and extrapelvic visceral tissues. Microscopy Research and Technique. 35 (2), 95-106 (1996).
- Purves-Tyson, T. D., Arshi, M. S., Handelsman, D. J., Cheng, Y., Keast, J. R. Androgen and estrogen receptor-mediated mechanisms of testosterone action in male rat pelvic autonomic ganglia. Neuroscience. 148 (1), 92-104 (2007).
- Nangle, M. R., Keast, J. R. Semaphorin 3A inhibits growth of adult sympathetic and parasympathetic neurones via distinct cyclic nucleotide signalling pathways. British Journal of Pharmacology. 162 (5), 1083-1095 (2011).
- Tan, H., Mawe, G. M., Vizzard, M. A. Electrical properties of neurons in the intact rat major pelvic ganglion. Autonomic Neuroscience. 134 (1-2), 26-37 (2007).
- Park, K. S., et al. An alpha3beta4 subunit combination acts as a major functional nicotinic acetylcholine receptor in male rat pelvic ganglion neurons. Pflügers Archiv – European Journal of Physiology. 452 (6), 775-783 (2006).
- Park, K. S., et al. Modulation of N-type Ca2+ currents by A1-adenosine receptor activation in male rat pelvic ganglion neurons. Journal of Pharmacology and Experimental Therapeutics. 299 (2), 501-508 (2001).
- Payne, S. C., Belleville, P. J., Keast, J. R. Regeneration of sensory but not motor axons following visceral nerve injury. Experimental Neurology. 266, 127-142 (2015).
- Nangle, M. R., Proietto, J., Keast, J. R. Impaired cavernous reinnervation after penile nerve injury in rats with features of the metabolic syndrome. Journal of Sexual Medicine. 6 (11), 3032-3044 (2009).
- Kepper, M. E., Keast, J. R. Specific targeting of ganglion cell sprouts provides an additional mechanism for restoring peripheral motor circuits in pelvic ganglia after spinal nerve damage. Journal of Neuroscience. 18 (19), 7987-7995 (1998).
- Yan, H., Keast, J. R. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. Journal of Comparative Neurology. 507 (2), 1169-1183 (2008).
- Ritter, K. E., Wang, Z., Vezina, C. M., Bjorling, D. E., Southard-Smith, E. M. Serotonin receptor 5-HT3A affects development of bladder innervation and urinary bladder function. Frontiers in Neuroscience. 11, 690 (2017).
- Tompkins, J. D., Girard, B. M., Vizzard, M. A., Parsons, R. L. VIP and PACAP effects on mouse major pelvic ganglia neurons. Journal of Molecular Neuroscience. 42 (3), 390-396 (2010).
- Forrest, S. L., Payne, S. C., Keast, J. R., Osborne, P. B. Peripheral injury of pelvic visceral sensory nerves alters GFRα (GDNF family receptor alpha) localization in sensory and autonomic pathways of the sacral spinal cord. Frontiers in Neuroanatomy. 9, 43 (2015).
- Keast, J. R., Luckensmeyer, G. B., Schemann, M. All pelvic neurons in male rats contain immunoreactivity for the synthetic enzymes of either noradrenaline or acetylcholine. Neuroscience Letters. 196 (3), 209-212 (1995).
- Keast, J. R., de Groat, W. C. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. Journal of Comparative Neurology. 288 (3), 387-400 (1989).
- Dail, W. G., Moll, M. A., Weber, K. Localization of vasoactive intestinal polypeptide in penile erectile tissue and in the major pelvic ganglion of the rat. Neuroscience. 10 (4), 1379-1386 (1983).
- Keast, J. R. A possible neural source of nitric oxide in the rat penis. Neuroscience Letters. 143 (1-2), 69-73 (1992).
- Kepper, M. E., Keast, J. R. Transmitter profile and spinal inputs of pelvic ganglion cells projecting with preganglionic axons along the hypogastric and pelvic nerves of the male rat. Neuroscience Letters. 280 (2), 123-126 (2000).

