Isolation of Group 2 Innate Lymphoid Cells from Mouse Nasal Mucosa to Detect the Expression of CD226
Summary
Group 2 innate lymphoid cells (ILC2s), implicated in type 2 inflammation, mainly participate in response to helminth infection, allergic diseases, metabolic homeostasis, and tissue repair. In this study, a procedure to isolate ILC2s from murine nasal mucosa and detect the expression of CD226 is demonstrated.
Abstract
With abundant research on group 2 innate lymphoid cells (ILC2s) published over the years, ILC2s are widely known to be implicated in regulating various pathological processes, including anti-helminth immunity, tissue repair, thermogenesis, and autoimmune diseases such as asthma and allergic rhinitis (AR). ILC2s permanently reside in peripheral tissues such as the skin, gut, lungs, and nasal cavity; however, there is limited information about their exact functions in nasal mucosal immunity. CD226 is an activating costimulatory molecule, mainly expressed on natural killer (NK) cells, T cells, and inflammatory monocytes. However, whether ILC2s express CD226 or play a role in the pathogenesis of ILC2s-related diseases remains unknown. Here, we established a method to isolate and identify ILC2s from the nasal mucosa and detected CD226 expression on ILC2s obtained from healthy and AR mice. Herein, we describe this protocol for the isolation and identification of ILC2s from mouse nasal mucosa, which will help explore the internal pathological mechanism of immunological disorders in nasal mucosal diseases.
Introduction
Group 2 innate lymphoid cells (ILC2s) were first discovered in the peritoneal cavity tissues of mice and were subsequently demonstrated to be present in the blood and other peripheral tissues such as the lungs, skin, and nasal cavity1,2,3. As tissue-resident cells, ILC2s are mainly maintained and proliferated locally and function as the first guards responding to exogenous harmful stimuli through producing numerous type 2 cytokines and inducing type 2 immunity4,5,6. ILC2s can also exert their effects by trafficking toward the infected tissues7,8.
Similar to T-helper 2 (Th2) cells, the complicated regulatory networks of ILC2s ensure their significant involvement in the progression of various type 2 inflammatory diseases, including airway allergic diseases8,9. In asthma, epithelial cell-derived alarmins can activate ILC2s, which further promote pulmonary inflammation through the secretion of interleukin (IL)-4, IL-5, and IL-1310. Clinical studies have also indicated that ILC2 levels were significantly elevated in the sputum and blood of patients with severe asthma, suggesting an association of ILC2s with asthma severity and their function as a predictor of asthma progression11.
Allergic rhinitis (AR) is a common chronic inflammatory disease that affects millions of people annually, and effective treatments for this disease are limited12,13. ILC2s play crucial roles in the pathophysiology of AR, whether in the sensitization phase or symptom generation and inflammation phase14. In patients with AR, the levels of ILC2 in the peripheral blood have been reported to be elevated both locally and systemically15. However, certain effects and the underlying mechanisms of ILC2s on the pathophysiology and progression of AR still require further exploration.
CD226 – a transmembrane glycoprotein that serves as a costimulatory molecule – is primarily expressed on natural killer (NK) cells, T cells, and other inflammatory monocytes16,17. The interaction of CD226 and its ligands (CD155 and/or CD112) or competitor (TIGIT) allows it to participate in the biological functions of various immune cells18. The binding of the ligands on antigen-presenting cells to CD226 on cytotoxic lymphocyte (CTL) promotes the activation of both cells simultaneously, while the activation of CTL can be further suppressed by TIGIT (T cell immunoreceptor with Ig and ITIM domains), the competitor of CD22619,20. A human ex vivo study revealed that CD226 and CD155 on T cells regulate the balance between Th1/Th17 and Th2 through differentially modulating Th subsets21. CD226 can likewise mediate platelet adhesion and NK tumor-killing activity22,23. Meanwhile, CD226 is well-studied in the pathogenesis of various infectious diseases, autoimmune diseases, and tumors18,24,25. At present, CD226 has become a new bright spot for immunotherapy. Studies have found that extracellular vesicles can reverse CD226 expression on NK cells to reinstate their cytotoxic activity and intervene in the progression of lung cancer26. A recent study has revealed a subcluster of fetal intestinal group 3 ILCs characterized with high CD226 expression by single-cell RNA sequencing27, which indicated that CD226 might exert roles in the innate lymphoid cell-mediated immunity.
Our knowledge about ILC2s in airway inflammation is primarily based on studies on asthma; however, little is known about their functions in nasal mucosal immunity. Thus, a protocol was established to isolate and identify ILC2s from the nasal mucosa. The study focuses on the expression of CD226 on ILC2s in nasal tissues and its variation between healthy and AR mice. This may provide novel insights into the underlying mechanisms of ILC2-mediated regulation in the local immunity and serve as a basis for developing new approaches for AR treatment.
Protocol
All experiments were performed in accordance with the Care and Use of Laboratory Animals Guidelines. All procedures and protocols were approved by the Scientific Research Ethics Committee of the Fourth Military Medical University (No. 20211008).
1. Murine AR model establishment
- House male and female wild-type (WT) C57BL/6 mice aged 8-12 weeks under specific pathogen-free conditions and provide standard laboratory chow and water.
- Emulsify 50 µg of ovalbumin (OVA) in 0.2 mL of sterile PBS containing 2 mg of aluminum hydroxide on a clean bench to maintain sterility. On days 0, 7, and 14, intraperitoneally inject female or male mice with 50 µg each of emulsified OVA.
- On days 21, 22, 23, 24, and 25, intranasally instill mice with 50 µg of OVA dissolved in 30 µL of sterile PBS (15 µL per nostril) under inhalation anesthesia (2-3% isoflurane with an Oxygen flow rate or 0.5 L/min).
- Euthanize the mice 24 h after the last challenge (day 26).
2. Isolation of mononuclear cells (MNCs) from the nasal mucosa
- Euthanize the mice by cervical dislocation under deep anesthesia. Soak the mice head up in 75% ethanol for 5 min and avoid ethanol entering the external nostril. Place the abdomen downward on the operating table.
- Cut off the fore-teeth. At the midline of the head, make an incision, and cut open the skin using scissors.
- Remove the lower jaw, cut off the entire nose along the end of the palate, and place the tissue into a 60 mm Petri dish containing 5 mL of ice-cold PBS. Using scissors and forceps, remove the flesh and muscles adhering to the bones.
- Transfer the mouse nose to a new 60 mm Petri dish containing 5 mL of ice-cold PBS. Wash the bones twice with 5 mL of ice-cold PBS.
- Transfer the nose into a 1.5 mL microcentrifuge tube.
- Sufficiently smash and transfer the nose to a 15 mL tube containing 2 mL of prewarmed digest buffer (RPMI 1640 medium supplemented with 1 mg/mL of collagenase IV and 10 U/mL DNase I).
- Fasten the lid and place the tube vertically in an orbital shaker at 37 °C, with continuous agitation at 120-150 rpm for 40 min.
NOTE: Pre-warm the digest buffer to 37 °C to achieve the highest enzyme activity. - Add 5 mL of ice-cold RPMI 1640 medium containing 10% fetal bovine serum (FBS) to stop the digestion process.
- Filter through a 70 µm cell strainer to remove solid fragments.
- Centrifuge at 500 x g for 5 min, and then gently discard the supernatant.
- Resuspend the pellet in ice-cold RPMI 1640 medium and centrifuge at 500 x g for 5 min. Gently discard the supernatant.
- Resuspend the cell pellet in 4 mL of 40% density gradient media (160 µL of 10x PBS + 1.44 mL of density gradient media stock solution + 2.4 mL of RPMI 1640 medium).
- Gently insert a Pasteur pipette to the bottom of the tube and slowly add 2.5 mL of 80% density gradient media (200 µL of 10x PBS + 1.8 mL of density gradient media stock solution + 0.5 mL of RPMI 1640 medium).
- Set the acceleration and deceleration rate of the centrifuge lower than the third gear and centrifuge at 400 x g for 15 min at room temperature (RT).
- Remove the top layer of impurities before draining the cells at the interface to avoid potential contamination. Carefully drain the mononuclear cells (MNCs) layer at the 40%/80% density gradient media interface into a 15 mL tube containing 2 mL of ice-cold PBS using a pipette. Wash the cells with ice-cold PBS twice.
3. Surface staining for FCM analysis
- Harvest the cells and centrifuge at 500 x g for 5 min at 4 °C. Resuspend the cell pellet in staining buffer (PBS supplemented with 2% FBS and 0.1% NaN3) and centrifuge at 500 x g for 5 min at 4 °C. Discard the supernatant.
CAUTION: Be cautious when preparing the staining buffer. NaN3 is very toxic and may cause damage to organs if swallowed, inhaled, or in contact with skin. Also, avoid release to the environment. - Resuspend the cells in 80 µL blocking solution (anti-CD16/32 antibody (1:100) diluted in staining buffer) per tube. Incubate for 30 min in the dark at 4 °C.
- Without washing, add 20 µL of the appropriate dilution of the surface-staining antibody cocktail (Table 1).
- Add 1 µL (per test) of fixable viability dye 520 (FVD) just before adding the antibody cocktail to the samples. Incubate in the dark at 4 °C for 30 min. Set matching isotype antibodies and fluorescence minus one (FMO) as the negative controls.
- Wash the cells in 500 µL of staining buffer by centrifuging at 500 x g for 5 min at 4 °C.
- Resuspend the cell pellet in 200 µL of staining buffer. Add 50 µL of vortexed absolute counting beads to the stained cells and agitate. Subject them to a flow cytometry (FCM) analysis.
NOTE: FCM data were obtained using a spectral cell analyzer and analyzed with flow cytometry (FCM) analysis software.
Representative Results
An OVA-induced murine model was developed to explore the role of ILC2s in AR. The construction of AR murine model was based on previous studies with slight modifications28,29,30,31. A 10 min video was captured to measure the frequency of sneezing and nasal scratching after the last nasal challenge. Allergic symptoms of the OVA-induced-AR mice were presented in Figure 1. The frequency of nasal rubbing and sneezing in the AR mice was significantly higher than in the control group.
Next, we described a procedure to isolate MNCs from murine nasal mucosa and perform their characterization using FCM analysis. About 2-3 x 106 lymphocytes were obtained from each murine nose. The dead cells were excluded by FVD staining. The gating strategy for ILC2s among the isolated cells was Lin (CD11b, CD11c, CD3, and B220)− CD45+ CD90.2+ KLRG1+ cells. All gates were drawn based on isotype or FMO control. Typically, ~2%-3% of Lin− CD45+ cells were identified in the nasal mucosa of healthy control mice (Figure 2). The absolute number of ILC2s in murine nasal mucosa varies from 2,000-4,000.
We then isolated the ILC2s from the AR mice using the above-described method. There was no difference in the absolute numbers of lymphocytes between HC and AR mice. The FCM results indicated that the fraction of ILC2s in the AR mice was 9%-14% of the total number of Lin− CD45+ lymphocytes, indicating a remarkable increase in the number of ILC2s in AR nasal mucosa compared with that in healthy control mice (Figure 3).
We detected the expression of CD226 on the ILC2s. On average, 51.9% of ILC2s expressed CD226 under unimmunized conditions, whereas the average ratio of ILC2s expressing CD226 was 54.8% in the AR mice, which showed a nonsignificant tendency to increase compared with that in control mice. In addition to cell frequency, the cell-surface expression level of CD226 was also determined using mean fluorescence intensity (MFI) that was auto-calculated by FCM analysis software. Interestingly, the MFI of CD226 was significantly upregulated in the AR mice compared with the control mice (Figure 4), indicating an elevated expression of CD226 on the ILC2s of the allergic nasal mucosa.
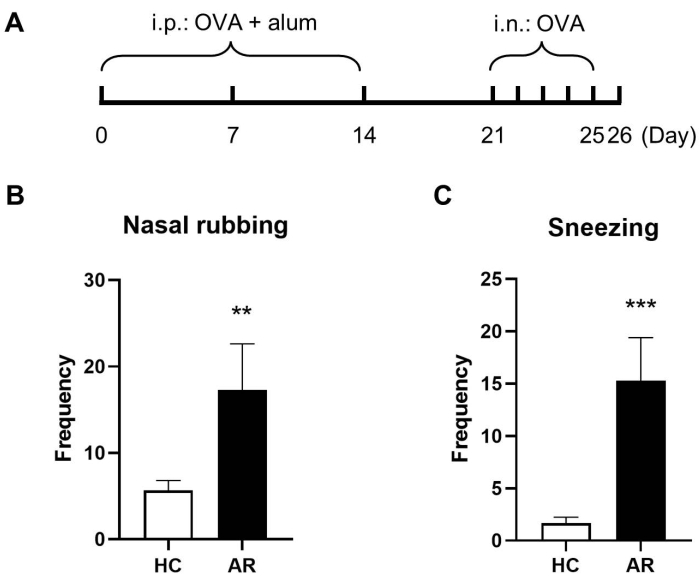
Figure 1: OVA-induced murine AR model. (A) The schematic diagram of the murine AR model establishment. The frequency of nasal rubbing (B) and sneezing (C) in a 10 min period. Values are presented as mean ± standard deviation (SD) (n = 3). For comparing the two groups, Student's t-test was performed; P < 0.05 was considered statistically significant. Please click here to view a larger version of this figure.
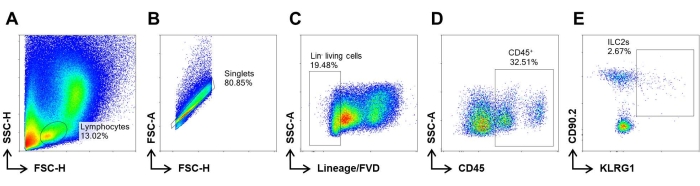
Figure 2: FCM gating strategy for ILC2s. MNCs were isolated as described above. (A) The density graphic demonstrates the distribution of isolated MNCs along the FSC and SSC axes. (B) Singlets were gated based on FSC characteristics. (C) Lin− cells were defined as CD11b, CD11c, CD3, and B220 negative expression to exclude the myeloid cells, B cells, and T cells in MNCs. Dead cells were excluded by means of fixable viability dye (FVD) staining. (D) ILCs are characterized by Lin− CD45+. (E) ILC2s are gated by CD90.2+ KLRG1+ among ILCs. Please click here to view a larger version of this figure.
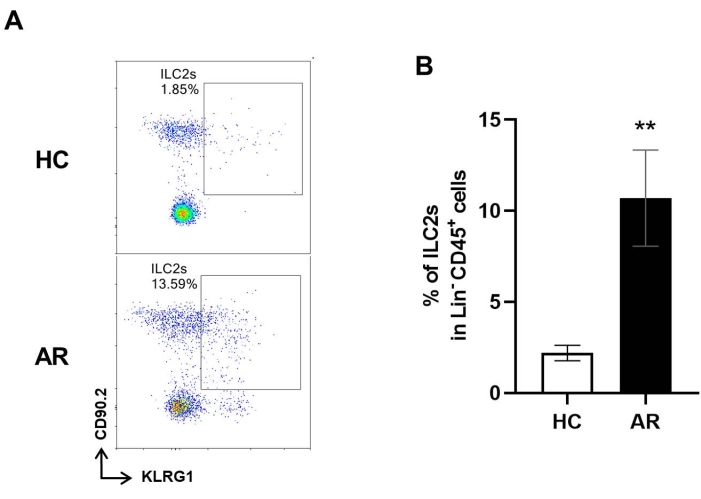
Figure 3: ILC2s in the nasal mucosa of healthy and AR mice. (A) Representative FCM images illustrate the proportions of ILC2s among ILCs from the nasal cavity mucosa of healthy control (HC) and AR groups. (B) The proportions of ILC2s in Lin− CD45+ cells in HC and AR groups. Values are presented as the mean ± SD (n = 3). Student's t-test was performed for a two-group comparison. P < 0.05 was considered statistically significant. Please click here to view a larger version of this figure.
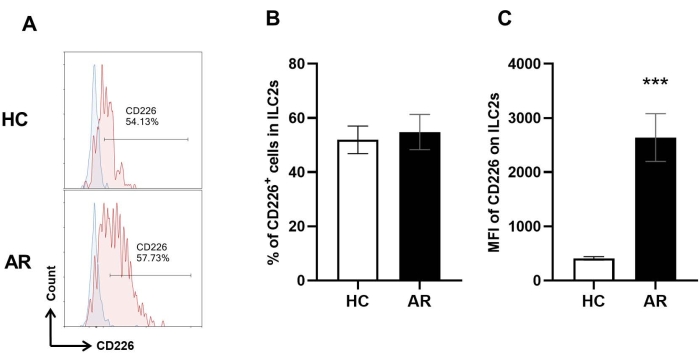
Figure 4: CD226 expression in nasal mucosal ILC2s. ILC2s were characterized using the above-mentioned strategy. The expression of CD226 on ILC2s was evaluated using FCM. (A) Representative histograms of CD226 expression in HC and AR groups; blue area represents the CD226 FMO control. (B,C) The proportions of CD226-positve ILC2s and mean fluorescence intensity (MFI) of CD226 in the HC group and AR group. Values are expressed as the mean ± SD (n = 3). Student's t-test was performed for comparison between the two groups. P < 0.05 was considered significant. Please click here to view a larger version of this figure.
| Antibody | Fluorochrome | Clone | Dilution |
| CD11b | FITC | M1/70 | 1:100 |
| CD11c | FITC | N418 | 1:200 |
| CD226 | PE/Cyanine7 | 10E5 | 1:50 |
| CD3e | FITC | 145-2C11 | 1:100 |
| CD45 | Alexa Fluor 700 | 30-F11 | 1:200 |
| CD45R(B220) | FITC | RA3-6B2 | 1:100 |
| CD90.2 | PE | 30-H12 | 1:100 |
| KLRG1 | APC | 2F1 | 1:160 |
Table 1: Surface staining antibody cocktail.
Discussion
ILC2s are closely associated with type 2 inflammation and inflammatory disorders, as demonstrated by an increasing number of studies. Both mouse models and human observation contribute to a better understanding of its function in the upper airway. In asthma pathophysiology, ILC2s are activated through thymic stromal lymphopoietin, IL-25, and IL-33, which are mostly produced by epithelial cells. Then mirroring Th2 cells, ILC2s produce IL-4, IL-5, and IL-13 to aggravate type 2 inflammation32. Furthermore, different subsets of ILC2s also produce IL-10 and IL-1733,34,35. AR is also a type of airway allergic inflammation driven by type 2 cells. The participation of ILC2s in AR pathophysiology has been demonstrated recently14. However, the potential regulatory mechanisms of ILC2s under AR remain largely unknown.
Evidence indicates that the interaction between different ILC2s may rely on the surface molecules36. For instance, both ICOS and ICOS-ligands expressed on ILC2s are necessary for their survival and functions37. In addition, ILC2s also express ICAM-1 and LFA-1, which exert important effects on cell migration and cell-cell interaction7,38. In addition, ILC2s can crosstalk with other immune cells through these cell-surface molecules. For instance, OX40L is a dominant mediator for the interaction between CD4+ T cells and ILC2s in lung inflammation39. Because CD226, as a transmembrane costimulatory molecule, expresses on various immune cells, we also evaluated its expression on ILC2s in nasal tissues from healthy and AR mice in this study.
General OVA-induced AR modeling includes intraperitoneally systemic sensitization and intranasally local stimulation, which usually require at least 3-4 weeks to achieve satisfactory modeling results31. Another OVA-induced nasal-only delivery AR model takes a similar time as the general model method to achieve an approximate level of type 2 inflammation and AR symptoms40. However, the number and function of nasal ILC2s by these two AR modeling methods still need further research. We assessed sneezing and nasal rubbing to evaluate AR symptoms. Although it would be more convincing to include the assessment for rhinorrhea, we found it is hard to catch sight of the rhinorrhea without interfering with mice behavior during evaluation, so we regrettably excluded these criteria. In addition, the evaluation for sneezing and nasal rubbing might be profound enough to illustrate the severity of murine AR symptoms for many of the relevant articles that employed only these two criteria41,42,43.
Previous studies about mice nasal ILC2s have described the isolation of murine nasal mucosa44,45. Their protocol is as follows: Briefly, remove the soft tissue around the skull, open the nasal bone to expose the nasal cavity, and scrape the nasal mucosal tissue off the surface of the skull with forceps. While most of the isolated mucosa was used for IF, IHC, or qPCR, little is reported about the following isolation of mononuclear cells (MNCs) and FCM analysis. In this study, considering the low amounts of ILC2s located in the nasal mucosa, the operation retained the maximum volume of the nasal mucosa by digesting the entire nose instead of stripping the mucosa from the surface of the nasal bone. To optimize the digestion protocol, we evaluated three digest media: 0.5 mg/mL of collagenase IV, 1 mg/mL of collagenase IV, and 1 mg/mL of collagenase IV and 10 U/mL DNase I, and compared the percentage of living cells and lymphocyte frequency obtained by these three digest media. The proportion of living cells acquired by all three media has no difference (90%-95%); however, the first two types of digestion were inadequate with lower lymphocyte frequency (~5%). Thus, 1 mg/mL of collagenase IV and 10 U/mL of DNase I were chosen as the digest media in this study. However, other enzymes such as liberase and dispase that may replace collagenase have not been studied. Since the type and concentration of enzymes are critical for cell viability and cell-surface molecule expression46,47, the usage and dosage of these alternatives during nose digestion still need careful exploration and optimization. We used FVD eFluor 520 to check cell viability, because it can be detected using a 530/30 band pass filter which is equivalent to FITC, so we could exclude the dead cells while gating Lin– cells. Other cell viability dyes are also optional according to specific antibody panels. Furthermore, instead of intracellular staining of transcription factors GATA3, which is usually performed in other ILC2-related studies, ILC2s in the present study were characterized based on cell-surface markers such as Lin (CD11b, CD11c, CD3, and B220), CD45, CD90.2, and KLRG1 in FCM analysis. Among them, KLRG1 expressed on ILC2s has been identified as a specific marker of mature tissue ILC2s48,49. Although CD90.2 is less specific, it is still accepted as an ILC2-associated surface marker50. In this study, mice were not distinguished by sex. Research has shown that the abundance and cell-surface antigens of pulmonary ILCs in mice are different in gender, but the correlation of nasal ILC2s with gender still needs to be studied50. In addition, the protocol did not focus on the proportion of other hematopoietic cells. Different FCM antibody panels would help to solve this issue.
This protocol described a method to isolate ILC2s from the murine nasal mucosa. The isolated ILC2s can be stained for FCM characterization, sorted for cell culture, or stimulated in vitro for further analyses. Consistent with the findings of the previous studies10,45,51, a substantial accumulation of ILC2s was observed in the nasal mucosa of AR mice. Notably, CD226 was expressed on local ILC2s, and its level significantly increased further in allergic conditions. Therefore, the findings in this study suggest the involvement of CD226 in ILC2-dependent AR. Whether ILC2s in patients with AR express CD226 and whether the CD226 expression on ILC2s increases in allergic conditions should be investigated in future studies.
In conclusion, we established a protocol to isolate and identify ILC2s from the nasal mucosa and detected the expression of adhesion molecules within them. The protocol requires further optimization; however, the lack of studies that systematically and minutely describe the procedure for isolating and characterizing lymphocytes, particularly innate lymphoid cells in the nasal mucosa, makes this protocol a preliminary reference for researchers exploring the local nasal immunological environment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
R.Z. was supported by the National Natural Science Foundation of China (No. 81871258) and funds provided by Fourth Military Medical University (No.2020rcfczr). Y.Z. was supported by the Natural Science Basic Research Program of Shaanxi (No. 2021JM-081).
Materials
| Aluminum hydroxide | Meilun biological Technology | 21645-51-2 | |
| CD11b | eBioscience | 11-0112-82 | Used in antibody coctail |
| CD11c | BioLegend | 117306 | Used in antibody coctail |
| CD16/32 | BioLegend | 101302 | Clone: 93; Dilution 1:100 |
| CD226 | BioLegend | 128812 | Used in antibody coctail |
| CD3e | BioLegend | 100306 | Used in antibody coctail |
| CD45 | BioLegend | 103128 | Used in antibody coctail |
| CD45R | eBioscience | 11-0452-82 | Used in antibody coctail |
| CD90.2 | BD Pharmingen | 553014 | Used in antibody coctail |
| Collagenase IV | DIYIBio | DY40128 | |
| CountBright absolute counting beads | Invitrogen | C36950 | absolute counting beads |
Dnase  |
Beyotime | D7076 | |
| Fetal Bovine Serum | gibco | 10270-106 | |
| Fixable Viability Dye eFluor 520 (FITC) | eBioscience | 65-0867-14 | FVD |
| HBSS, calcium, magnesium | Servicebio | G4204-500 | |
| KLRG1 | eBioscience | 17-5893-81 | Used in antibody coctail |
| NaN3 | SIGMA | S2002 | |
| NovoExpress software | AgilentTechnologies | Version 1.5.0 | flow cytometry (FCM) analysis software |
| OVA | SIGMA | 9006-59-1 | |
| PBS, 1x | Servicebio | G4202-500 | |
| PBS, 10x | Servicebio | G4207-500 | |
| Percoll | Yeasen | 40501ES60 | density gradient media |
| RPMI 1640 culture media | Corning | 10-040-CVRV | |
| Spectral cell analyzer | SONY | SA3800 |
References
- Huang, Y., et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 359 (6371), 114-119 (2018).
- Price, A. E., et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 107 (25), 11489-11494 (2010).
- Ebihara, T., et al. Trained innate lymphoid cells in allergic diseases. Allergology International. 70 (2), 174-180 (2021).
- Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y., Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 350 (6263), 981-985 (2015).
- Moro, K., et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nature Immunology. 17 (1), 76-86 (2016).
- Helou, D. G., et al. LAIR-1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. The Journal of Allergy and Clinical Immunology. 149 (1), 223-236 (2022).
- Karta, M. R., et al. beta2 integrins rather than beta1 integrins mediate Alternaria-induced group 2 innate lymphoid cell trafficking to the lung. The Journal of Allergy and Clinical Immunology. 141 (1), 329-338 (2018).
- Helou, D. G., et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nature Communications. 11 (1), 3998 (2020).
- Kabata, H., Moro, K., Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunological Reviews. 286 (1), 37-52 (2018).
- Zheng, H., et al. The role of Type 2 innate lymphoid cells in allergic diseases. Frontiers in Immunology. 12, 586078 (2021).
- Maggi, L., et al. The dual function of ILC2: From host protection to pathogenic players in type 2 asthma. Molecular Aspects of Medicine. 80, 100981 (2021).
- Meltzer, E. O., et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. The Journal of Allergy and Clinical Immunology. 124, 43-70 (2009).
- Wheatley, L. M., Togias, A. Clinical practice. Allergic rhinitis. The New England Journal of Medicine. 372 (5), 456-463 (2015).
- Bousquet, J., et al. Allergic rhinitis. Nature Reviews. Disease Primers. 6 (1), 95 (2020).
- Kato, A. Group 2 innate lymphoid cells in airway diseases. Chest. 156 (1), 141-149 (2019).
- Nakamura-Shinya, Y., et al. DNAM-1 promotes inflammation-driven tumor development via enhancing IFN-gamma production. International Immunology. 34 (3), 149-157 (2022).
- Braun, M., et al. CD155 on Tumor cells drives resistance to immunotherapy by inducing the degradation of the activating receptor CD226 in CD8(+) T cells. Immunity. 53 (4), 805-823 (2020).
- Huang, Z., Qi, G., Miller, J. S., Zheng, S. G. CD226: An emerging role in immunologic diseases. Frontiers in Cell and Developmental Biology. 8, 564 (2020).
- Gilfillan, S., et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. Journal of Experimental Medicine. 205 (13), 2965-2973 (2008).
- Zhang, D., et al. TIGIT-Fc alleviates acute graft-versus-host disease by suppressing CTL activation via promoting the generation of immunoregulatory dendritic cells. Biochimica et Biophysica Acta: Molecular Basis of Disease. 1864, 3085-3098 (2018).
- Lozano, E., Joller, N., Cao, Y., Kuchroo, V. K., Hafler, D. A. The CD226/CD155 interaction regulates the proinflammatory (Th1/Th17)/anti-inflammatory (Th2) balance in humans. Journal of Immunology. 191 (7), 3673-3680 (2013).
- Kojima, H., et al. CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. Journal of Biological Chemistry. 278 (38), 36748-36753 (2003).
- Martinet, L., Smyth, M. J. Balancing natural killer cell activation through paired receptors. Nature Reviews. Immunology. 15 (4), 243-254 (2015).
- Yeo, J., Ko, M., Lee, D. H., Park, Y., Jin, H. S. TIGIT/CD226 axis regulates anti-tumor immunity. Pharmaceuticals. 14 (3), 200 (2021).
- Nakano, M., et al. Association of elevated serum soluble CD226 levels with the disease activity and flares of systemic lupus erythematosus. Scientific Reports. 11 (1), 16162 (2021).
- Chang, W. A., et al. miR-150-5p-containing extracellular vesicles are a new immunoregulator that favor the progression of lung cancer in hypoxic microenvironments by altering the phenotype of NK cells. Cancers. 13 (24), 6552 (2021).
- Stehle, C., et al. T-bet and RORalpha control lymph node formation by regulating embryonic innate lymphoid cell differentiation. Nature Immunology. 22 (10), 1231-1244 (2021).
- Piao, C. H., Fan, Y. J., Nguyen, T. V., Song, C. H., Chai, O. H. Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-kappaB signaling pathways. International Journal of Molecular Sciences. 21 (10), 3415 (2020).
- Zhao, Y., Tao, Q., Wu, J., Liu, H. DMBT1 has a protective effect on allergic rhinitis. Biomedicine and Pharmacotherapy. 121, 109675 (2020).
- Piao, C. H., et al. Ethanol extract of Dryopteris crassirhizoma alleviates allergic inflammation via inhibition of Th2 response and mast cell activation in a murine model of allergic rhinitis. Journal of Ethnopharmacology. 232, 21-29 (2019).
- Liang, M. J., et al. Immune responses to different patterns of exposure to ovalbumin in a mouse model of allergic rhinitis. European Archives of Oto-Rhino-Laryngology. 273 (11), 3783-3788 (2016).
- Ebbo, M., Crinier, A., Vely, F., Vivier, E. Innate lymphoid cells: major players in inflammatory diseases. Nature Reviews. Immunology. 17 (11), 665-678 (2017).
- Seehus, C. R., et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nature Communications. 8 (1), 1900 (2017).
- Cai, T., et al. IL-17-producing ST2(+) group 2 innate lymphoid cells play a pathogenic role in lung inflammation. The Journal of Allergy and Clinical Immunology. 143 (1), 229-244 (2019).
- Golebski, K., et al. IL-1beta, IL-23, and TGF-beta drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nature Communications. 10 (1), 2162 (2019).
- Lei, A., Zhou, J. Cell-surface molecule-mediated cell-cell interactions in the regulation of ILC2-driven allergic inflammation. Cellular and Molecular Life Sciences. 76 (22), 4503-4510 (2019).
- Maazi, H., et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 42 (3), 538-551 (2015).
- Lei, A. H., et al. ICAM-1 controls development and function of ILC2. The Journal of Experimental Medicine. 215 (8), 2157-2174 (2018).
- Drake, L. Y., Iijima, K., Kita, H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 69 (10), 1300-1307 (2014).
- Wang, Y., et al. The comparation of intraperitoneal injection and nasal-only delivery allergic rhinitis model challenged with different allergen concentration. American Journal of Rhinology & Allergy. 33 (2), 145-152 (2019).
- Niu, Y., et al. HIF1alpha deficiency in dendritic cells attenuates symptoms and inflammatory indicators of allergic rhinitis in a SIRT1-dependent manner. International Archives of Allergy and Immunology. 181 (8), 585-593 (2020).
- Van Nguyen, T., et al. Anti-allergic rhinitis activity of alpha-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling. Scientific Reports. 10 (1), 12528 (2020).
- Pyun, B. J., et al. Gardenia jasminoides attenuates allergic rhinitis-induced inflammation by inhibiting periostin production. Pharmaceuticals (Basel). 14 (10), 986 (2021).
- Liu, Z., et al. Analysis of expression of ILC2 cells in nasal mucosa based on animal model of allergic bacterial infection rhinitis. Journal of Infection and Public Health. 14 (1), 77-83 (2021).
- Hu, B., Wang, Y., Zheng, G., Zhang, H., Ni, L. Effect of parasympathetic inhibition on expression of ILC2 cells in a mouse model of allergic rhinitis. The World Allergy Organization journal. 14 (9), 100582 (2021).
- Autengruber, A., Gereke, M., Hansen, G., Hennig, C., Bruder, D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. European Journal of Microbiology & Immunology. 2 (2), 112-120 (2012).
- Krisna, S. S., et al. Optimized protocol for immunophenotyping of melanoma and tumor-bearing skin from mouse. STAR Protocols. 2 (3), 100627 (2021).
- Hoyler, T., et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 37 (4), 634-648 (2012).
- Huang, Y., et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nature Immunology. 16 (2), 161-169 (2015).
- Loering, S., et al. Differences in pulmonary group 2 innate lymphoid cells are dependent on mouse age, sex and strain. Immunology and Cell Biology. 99 (5), 542-551 (2021).
- Lin, L., et al. Allergic inflammation is exacerbated by allergen-induced type 2 innate lymphoid cells in a murine model of allergic rhinitis. Rhinology Journal. 55 (4), 339-347 (2017).

