Delivery of Cardioactive Therapeutics in a Porcine Myocardial Infarction Model
Summary
The present protocol describes three methods of administering cardioactive therapeutic agents in a porcine model. Female landrace swine received treatment through either: (1) thoracotomy and transepicardial injection, (2) catheter-based transendocardial injection, or (3) intravenous infusion via jugular vein osmotic minipump.
Abstract
Myocardial infarction is one of the leading causes of death and disability worldwide, and there is an urgent need for novel cardioprotective or regenerative strategies. An essential component of drug development is determining how a novel therapeutic is to be administered. Physiologically relevant large animal models are of critical importance in assessing the feasibility and efficacy of various therapeutic delivery strategies. Due to their similarities to humans in cardiovascular physiology, coronary vascular anatomy, and heart weight to body weight ratio, swine is one of the preferred species in the preclinical evaluation of new therapies for myocardial infarction. The present protocol describes three methods of administering cardioactive therapeutic agents in a porcine model. After percutaneously induced myocardial infarction, female landrace swine received treatment with novel agents through either: (1) thoracotomy and transepicardial injection, (2) catheter-based transendocardial injection, or (3) intravenous infusion via jugular vein osmotic minipump. The procedures employed for each technique are reproducible, resulting in reliable cardioactive drug delivery. These models can be easily adapted to suit individual study designs, and each of these delivery techniques can be used to investigate a variety of possible interventions. Therefore, these methods are a useful tool for translational scientists pursuing novel biological approaches in cardiac repair following myocardial infarction.
Introduction
Coronary artery disease (CAD) and associated ST-elevation myocardial infarction (STEMI) are the preeminent causes of death worldwide. In the past two decades, great progress has been made in reducing in-hospital mortality of patients presenting with STEMI, through the advent of percutaneous coronary intervention, fibrinolytic therapies, and standardization of treatment algorithms to ensure that reperfusion is achieved in a timely manner1,2,3. Despite this, the morbidity associated with STEMI remains a significant burden, thus creating a great need for developing novel cardioprotective and regenerative therapies2,3. An essential component of therapeutic development is the determination of how a novel therapy is to be administered4. The safety, efficacy, and feasibility of each method need to be matched with the characteristics of the therapy itself.
Physiologically relevant large animal models are critical in assessing these attributes of various therapeutic delivery strategies5. Due to their similarities to humans in cardiovascular physiology, coronary vascular anatomy, and heart weight to body weight ratio, swine is one of the preferred species in the preclinical evaluation of new therapies for myocardial infarction6. We have previously used a porcine STEMI model to demonstrate the reparative capacity of a recombinant protein therapy7, and continue to investigate novel pharmacologic, cellular, and genetic therapies using this model. Here, three techniques of therapeutic administration used in swine models after infarct creation are described: thoracotomy and transepicardial injection, percutaneous transendocardial injection, and jugular venous osmotic minipump implantation. The first two methods enable local tissue delivery, reducing required dosages, off-target effects, and hepatic first-pass metabolism8,9,10. The osmotic minipump allows continuous delivery of a drug with a short half-life, negating reliance on an infusion pump and patent intravenous cannula, both of which are challenging to institute in large animal models.
By describing these techniques, it is hoped that this article can aid translational scientists in investigating novel cardioprotective or regenerative agents following myocardial infarction in large animal models.
Protocol
All experiments were performed following the 'Australian code for the care and use of animals for scientific purposes' and were approved by the Western Sydney Local Health District Animal Ethics Committee. Pre-pubescent large white x landrace gilts, weighing 18-20 kg, were used for the present study.
1. Animal husbandry
- House the animals according to local animal ethics committee regulations.
NOTE: The animals included in this protocol are housed individually to avoid fighting and injury and ensure there is no competition for food. However, all animals could see, hear, and smell conspecifics. Their pens have concrete flooring for ease of cleaning, and have both an indoor and outdoor sheltered portion. Pigs are provided with straw bedding in the indoor portion and enrichment 'toys' in the outdoor portion.
2. Sedation and general anesthesia
- Sedation
- Ensure the animal has been fasted for at least 12 h prior to commencing any heavy sedation or general anesthesia.
- If the animal doesn't yet have vascular access in the form of a central line, use an intramuscular sedative protocol.
- Intramuscular sedation consists of 8 mg/kg of ketamine, 0.3 mg/kg of midazolam, 0.2 mg/kg of methadone, and 10 µg/kg of medetomidine (see Table of Materials). Administer this mixture into the epaxial musculature of the animal with a 21 G needle. On average, animals take 5-10 min to become heavily sedated.
NOTE: A stressed pig is more resistant to sedation; ensure a calm and confident handler sedates the animal and that the animal is not unduly stressed. If the animal becomes stressed, it is best to leave them alone for 5-10 min and try again. - Perform intravenous sedation if a central line is in place and patent. Intravenous sedation consists of 2 mg/kg of ketamine, 0.2 mg/kg of midazolam, 0.2 mg/kg of methadone, and 2 µg/kg of medetomidine.
- Flush the intravenous line with 0.9% sodium chloride first to ensure patency. Then, slowly push half of the mixed syringe through the line and flush with 0.9% sodium chloride.
- Observe the animal, which must be heavily sedated within 20-30 s. If the animal is not yet sedated, flush the remaining half of the syringe, as described in step 2.1.5.
- Vascular access
- Once appropriately sedated, transport the pig into the operation theatre.
- Pre-oxygenate the pig with a face mask and an oxygen flow rate of 5 L/min. Connect a pulse oximeter to the pig's tail, ears, or tongue to monitor the animal throughout.
- Using a 22 or 24 G cannula, gain access to both the left and right marginal ear veins and secure with tape.
- General anesthesia
- Position the pig in sternal recumbency.
- Administer 1-3 mg/kg of propofol (see Table of Materials) intravenously as a slow push titrated to effect. Have an assistant hold the animal's mouth open (the jaw should be slack at this stage) and extend the neck.
- Use a laryngoscope (see Table of Materials) to depress the base of the tongue and epiglottis to expose the arytenoid cartilages of the larynx.
- Pass a lubricated endotracheal tube (size 5.5 or 6.0; see Table of Materials) with a stylet through the arytenoid cartilages and into the trachea. Occasionally, resistance may be felt while passing through the larynx; this can be countered with gentle rotation of the endotracheal tube. If the animal is resisting intubation, administer more propofol intravenously.
- Remove the stylet from the endotracheal tube. Inflate the endotracheal tube cuff until satisfied that there are no air leaks around the tube. Generally, 4-6 mL of air is required, although the volume can vary depending on the tube size and animal.
- Secure the endotracheal tube behind the ears of the animal.
- Connect the animal to a rebreathing anaesthetic circuit with a volume-control ventilator function. When the animal is connected, have the ventilator turned 'off'.
NOTE: Ensure that, at all times, the adjustable pressure limiting (APL) valve, or 'pop-off valve' is open. Failure to leave the valve open can result in fatal barotrauma to the animal. - Reduce the anaesthetic circuit oxygen flow rate to 2 L/min.
- Set the positive pressure ventilator to 'volume-control' mode. Set the tidal volume as 10 mL/kg and respiratory rate as 20 breaths/min. Ensure the inspiratory:expiratory ratio is set at 1:2.
- Throughout the procedure, manipulate the respiratory rate to ensure that the end-tidal carbon dioxide lies between 35-50 mmHg.
NOTE: Inspiratory pressure must not exceed 20 cmH2O. Increases in inspiratory pressure prompt investigation for tubing kinks or blockages. To alleviate increased pressures that aren't associated with tubing, the inspiratory:expiratory ratio can be reduced to 1:1.5, and the tidal volume reduced. - If the animal is hemodynamically stable, maintain them with inhalant anesthesia. If the animal is not hemodynamically stable, maintain them with total intravenous anesthesia, as described in step 2.3.14.
- If the animal is to be administered inhalant anesthesia, commence with 2.5% isoflurane from the moment the anaesthetic circuit is first connected.
- Throughout the next 30 min, progressively wean the animal to 1.5% inhaled isoflurane. Maintain the animal on 1.5% isoflurane until the end of the procedure.
- Assess anaesthetic depth every 10 min by checking palpebral reflexes and testing jaw tone. Adjust the isoflurane as required to maintain anaesthetic depth.
- If the animal is hemodynamically unstable, maintain them with total intravenous anesthesia, allowing for greater anaesthetic depth control without the cardiovascular complications associated with inhalant anaesthetic agents.
- Prepare either syringe pumps or standard fluid pumps with propofol, fentanyl, and midazolam. Connect these to the animal via a common line.
NOTE: Propofol may be run at 0.1-0.6 mg/kg/min, fentanyl may be run at 1-5 µg/kg/h, and midazolam may be run at 0.05-0.2 mg/kg/h. - As with inhalant anesthesia, titrate these drugs to effect throughout anaesthetia to maintain appropriate anaesthetic depth.
- Prepare either syringe pumps or standard fluid pumps with propofol, fentanyl, and midazolam. Connect these to the animal via a common line.
- Provide intravenous fluid support with 0.9% sodium chloride or Hartmann's solution (see Table of Materials) at a rate of 5 mL/kg/h.
- Monitor the animal with blood pressure measurements, capnography, anaesthetic gas monitoring, temperature, pulse oximetry, and electrocardiography.
- Throughout any procedure, provide additional analgesia in the form of 0.2 mg/kg of methadone intravenously every 4 h after premedication.
NOTE: Analgesia may be administered at shorter intervals if the animal demonstrates signs of pain while under anesthesia (tachycardia, lightened anaesthetic depth without changes to maintenance drugs).
3. Central line placement
- Position the pig in dorsal recumbency with the hindlimbs extended, the left forelimb extended, and the right forelimb flexed and secured with a tie.
- Aseptically prepare the pig's neck, and cover with a drape.
- Use a linear ultrasound probe (see Table of Materials) with a sterile covering to locate the right jugular vein. This is best achieved with the probe oriented perpendicular to the trachea and slowly moving it laterally from the larynx.
- Enable the color doppler mode to help identify the jugular vein. The jugular vein can be distinguished from the carotid artery by demonstrating collapsibility and continuous, non-pulsatile flow.
- Use an 18 G Cook needle (see Table of Materials) to access the vein under ultrasound guidance. Once access is achieved, pass a 0.035 inch J-tip wire (see Table of Materials) through the needle and thread it into the vessel. Remove the needle over the wire.
- Thread a pre-flushed central line over the wire and push through into the vein. Ensure that the wire is visible emerging from the distal end of the line at all times.
NOTE: A 5 Fr, two-lumen central venous catheter (see Table of Materials) was used for subjects requiring intravenous access over a 1-4 week period. - If there is difficulty advancing the line through the skin, use a no. 11 scalpel to create a small, 2-4 mm incision to facilitate the passage of the line over the wire. Apply negative pressure followed by a flush to each external line to ensure patency. Clamp each line off.
- Attach the provided anchors to the external lines and secure them in place with 2-0 or 3-0 non-dissolvable sutures (see Table of Materials). Place additional sutures to secure the external lines dorsally to avoid the animal chewing on them.
- Attach flushed extension lines to the external catheter lines and clamp off.
- Fit the animal with a commercial pig jacket (see Table of Materials) and secure the lines within. If other procedures are yet to be performed during the same anaesthetic, fit the jacket just prior to animal recovery.
4. Myocardial infarction
NOTE: Animals used in this model received a myocardial infarction following a previously published method7.
- Perform myocardial infarction 2 weeks prior to transepicardial and transendocardial injection procedures8,9,10. Osmotic minipump implantation was performed immediately following coronary reperfusion within the same procedure.
5. Drug or cell administration
- Thoracotomy and epicardial cell injection
- Administer intravenous prophylactic antibiotics in the form of 22 mg/kg of cefazolin (see Table of Materials). Continue every 90 min throughout the procedure.
- Position the animal in the right lateral recumbency position.
- Apply a 100 µg/h fentanyl patch (see Table of Materials) to the flat plane behind the pig's ears. Cover with an adhesive dressing or 2-0 suture.
NOTE: This patch is considered 'active' 12 h after placement, and provides analgesia for 72 h. - Set up the electroanatomic mapping system cabling and patches following the product specifications (see Table of Materials).
- Mark a 10 cm horizontal line between ribs 4 and 5 on the animal with a crayon or surgical marker.
- Prepare a mixture of lidocaine (2 mg/kg) and bupivacaine (1 mg/kg) in a single syringe with a 25 G needle.
- Aseptically prepare the surgical site and drape the animal. Perform a cutaneous line block at the marked incision site by inserting the needle into the skin at a shallow angle, withdrawing the plunger slightly to ensure no backflow of blood into the syringe, and slowly injecting the solution as the needle is withdrawn from the skin. Repeat this process while moving down the line.
NOTE: Negative pressure must be applied to the syringe at each new injection site to ensure that bupivacaine is not administered intravascularly. Intravascular bupivacaine can be rapidly fatal. - Using a no. 22 scalpel, make a 10 cm skin incision along the marked line.
- Using monopolar cautery (see Table of Materials), deepen the incision through the underlying muscle layers until the intercostal muscles have been reached.
- Prepare a sterile syringe of 0.5 mg/kg of bupivacaine and 1 mg/kg of lignocaine, and attach a 25 G needle.
- Insert the needle at a shallow angle on the caudal edge of the fourth rib. Apply negative pressure on the plunger to ensure the needle has not passed through the intercostal vein or artery. Slowly inject a quarter of the syringe's volume while keeping the needle stationary.
- Repeat step 5.1.12 in three more locations, 3-5 cm apart along the caudal edge of the fourth rib.
- Use Metzenbaum scissors (see Table of Materials) to incise the intercostal muscles carefully, then incise the pleura after confirmation with the anaesthetist.
- As the pleura is incised, turn off the ventilator to allow the lungs to fall away from the pleura. After the incision has been made, turn the ventilator back on and adjust the positive end-expiratory pressure to 4 cmH2O.
- Place self-retaining rib retractors between the ribs and open slowly to expose the heart. Using tissue forceps, gently grasp the pericardium, and incise with Metzenbaum scissors so that the heart may be exteriorized.
NOTE: During this step and the next few steps, arrhythmias are common. The ECG needs to be monitored closely, and any cardiac manipulation must be halted temporarily if the animal becomes hemodynamically unstable (mean arterial pressure below 55 mmHg). Intravenous boluses of metaraminol (0.25 mg) are to be administered as required to improve blood pressure. - Place temporary 2-0 stay sutures at both ends and sides of the pericardial incision to fix it to the thoracic wall and create a pericardial well. Ensure the sides of the pericardium are supported to render the pericardial well as shallow as possible.
- Use moistened swabs or laparotomy sponges to pack the region surrounding the heart. This will aid in keeping the heart stable and preventing the drying of exposed tissues.
- Deliver the apex of the heart from within the pericardial cavity with an index finger behind the left ventricle. Take care to minimize left ventricular compression.
NOTE: A healthy heart can beat comfortably with the apex pointing vertically, sitting on its base with gentle support but no compression. There is an initial decrease in blood pressure associated with the manipulation, but this must recover to an acceptable level in the absence of external ventricular compression.- If no or very sluggish recovery occurs in 15 s, return the heart immediately to the pericardial cavity and take necessary steps to improve hemodynamic parameters before further dislocation.
NOTE: If the ventricle can be dislocated, the position of the apex outside the pericardium can be maintained by placing appropriately sized gauze packs within the pericardium to support the ventricle. Likewise, an elongated, moistened swab can be positioned under the base of the heart, where it acts as a 'sling' that guides the cardiac apex upward toward the incision (Figure 1A).
- If no or very sluggish recovery occurs in 15 s, return the heart immediately to the pericardial cavity and take necessary steps to improve hemodynamic parameters before further dislocation.
- Create an electroanatomic voltage map of the left ventricular epicardial surface using an electrophysiological mapping catheter (Figure 1B). Identify scar, border, and remote zones by standard voltage cut-offs.
NOTE: Scar and remote zone were defined with bipolar cut-offs of <0.5 mV or >1.5 mV, and unipolar cut-offs of <3 mV or >8.3 mV, respectively11,12. - Bend the needle of the 27 G therapeutic delivery syringe to roughly an 80-90° angle.
- Advance the needle into the target tissue at a shallow angle and apply pressure to the syringe plunger to discharge 1/4 to 1/3 of the total volume. Blanching of the tissue must be observed (Figure 1C). Use the electrophysiological mapping catheter to annotate the injection site location on the generated epicardial voltage map (Figure 1D).
NOTE: Vehicle injections consisting of the cell culture medium (RPMI 1640) were used for demonstration purposes in the video protocol. - Partially withdraw the needle and redirect it within the myocardium. Discharge another 1/4 to 1/3 of the volume of the syringe. Continue until the syringe is empty.
- Repeat steps 5.1.20-5.1.24 until the desired dose has been delivered.
- Remove the swabs packing off the heart, and gently remove the 'sling' under the heart, so it returns to the neutral position. Arrhythmias are common during this step, and caution must be exercised as described in step 5.1.17.
- Remove the 2-0 stay sutures from the pericardium. Loosen the retractor and remove it from the thorax.
- Use size 1 polydioxanone sutures (PDS) with a blunt-tipped, round needle to close the thorax by passing it through the spaces between ribs 3 and 4, and ribs 5 and 6.
NOTE: Narrow figure eight sutures provide a pulley effect for approximating the ribs. Two or three such sutures must be employed, depending on animal size. - Before tightening and tying off the rib-approximating sutures, insert a short length of silicone tubing into the ventral edge of the incision.
- Place the free end of the tubing into a bowl of sterile saline for underwater sealed drainage of the pleural cavity.
- Close the overlying muscle layers in a simple continuous pattern with 2-0 absorbable sutures. Close the skin in a simple continuous or ford interlocking pattern13 with a 2-0 or 3-0 non-absorbable suture.
- As the wound closure is completed, expel the free air from the thorax by the effect of positive pressure ventilation.
- Turn the ventilator onto the 'free breathing' setting. Use the reservoir bag on the rebreathing circuit to provide consistent positive pressure to the airways (maintain at 20-30 cmH2O).
- Continue this pressure until bubbling is no longer observed in the saline bowl, indicating no free air within the thorax.
- Remove the silicone tube.
- Apply an adhesive dressing to the surgical site, and place scattered simple interrupted sutures to assist in keeping the dressing in place.
- At recovery, administer 0.3 mg/kg of methadone subcutaneously and 0.2-0.5 mg/kg of ondansetron (see Table of Materials) intravenously.
- Jugular vein osmotic minipump implantation
- Position the animal as described in step 3.1. Aseptically prepare and drape the right side of the animal's neck.
- Using a no. 22 scalpel blade, make an 8-10 cm incision extending cranially from a point 2-3 cm lateral to the manubrium sterni. This incision must become slightly more lateral as it moves cranially.
- Use Metzenbaum scissors to dissect through the cutaneous colli, sternohyoideus, and sternocephalicus muscles. Use blunt dissection techniques to deepen the incision until the external jugular vein is visible.
- Place self-retaining retractors in the incision and open them to improve visibility.
- Using Adson brown tissue forceps (see Table of Materials) and Metzenbaum scissors, remove the soft tissue surrounding the jugular vein, both above and below (Figure 2A). This is a crucial step, as in later stages, soft tissue overlying the vessel can obstruct the passage of the minipump tubing.
- Use a 5-0 absorbable suture through the caudal exposed end of the vein, approximately 1 cm cranial from the most caudal visible edge of the vessel. Pass the suture in 5 mm 'bites' in the following fashion: cranial-to-caudal on the right-hand side, right to left, and caudal-to-cranial on the left-hand side. The resultant pattern must appear as 'three sides of a square'. Ensure the suture tails from both ends are equal in length.
- Assemble the minipump as described in the product instruction manual (see Table of Materials).
- Tie loop elastic vascular ties around the vessel at both the cranial and caudal ends. Keep these loose initially. Have an assistant place tension on the vascular ties to occlude the vessel.
- Using a 14 G needle, create a puncture in the vein in the centre of the 'three-sided square' created with suture material (step 5.2.7; Figure 2B).
- Thread the minipump tubing into the puncture. It must pass easily into the vessel. If resistance is encountered, do not keep pushing; instead, pull the tubing out and try again.
- Advance the tubing until 1-2 cm remains outside the vessel. Tighten the suture material around the catheter tubing and tie it off in a simple interrupted knot. Have the assistant release and remove the vascular ties.
- Wrap a 2-0 non-absorbable suture around the mini pump body several times and tie off so that the suture is secure on the pump. Then, secure the pump to the nearby soft tissue with a simple interrupted knot (Figure 2C,D).
- Remove the retractors from the incision. Close the incision in a standard three-layer simple continuous closure.
- Administer 0.2 mg/kg of meloxicam subcutaneously during recovery.
- Percutaneous transendocardial injection
- Position the animal in dorsal recumbency with forelimbs and hindlimbs extended, and secure with ties.
- Aseptically prepare the caudal abdomen and medial thighs of the animal. Drape the animal with a fenestrated femoral angiography drape (see Table of Materials).
- Set up the electroanatomic mapping system cabling and patches following the product specifications (see Table of Materials).
- Use a linear ultrasound probe to identify the femoral artery. Under ultrasound guidance, puncture the femoral artery with a cook needle and thread a 0.035 inch guidewire into the vessel via the needle. Remove the needle over the wire.
- Thread an 8 Fr arterial sheath and introducer over the arterial wire and push through until only the hub is exposed to the skin. Ensure the wire is always visible, emerging from the hub of the sheath.
NOTE: As this is a larger sheath, occasionally, a no. 11 scalpel may be required to create a small skin incision to facilitate passage. - Remove the sheath introducer and wire. Administer intravenous heparin (100-200 units/kg).
- Introduce the transendocardial injection catheter through the sheath and advance to the left ventricle via a retrograde aortic approach.
- Create an electroanatomic map of the left ventricle by gently dragging the catheter across the endocardial surface. Perform axial rotation and gentle alterations of the tip flexion to achieve good endocardial contact. Identify scar, border and remote zones by standard voltage cut-offs.
- Direct the catheter with fluoroscopic and electroanatomic guidance to the preferred injection location. Perform gentle axial rotation with deflection of the distal tip to engage and maintain stable endocardial contact.
- Confirm placement of the catheter tip with at least two fluoroscopic views. Advance the core catheter gently, then extend the needle to a controlled intramyocardial depth (3.5 mm for apical injection, 5 mm for septal injection).
- Flush 4-6 mL of iodinated contrast (see Table of Materials) through the injection catheter until visible on fluoroscopy. Observe the contrast for the next 10-30 s to ensure it remains in the tissue (Figure 3A).
- Observe the ECG closely during the extension of the needle and injection, as runs of ventricular ectopy are common.
NOTE: Ventricular ectopy is when the needle tip has come into myocardial contact. - If the contrast injection is successful, follow up with an injection of the agent of interest. Flush this through with contrast until observed fluoroscopically.
NOTE: The agent of interest is specific to the user, depending on what therapy they are testing. - Retract the needle. Remove the injection catheter from the arterial sheath.
- Remove the arterial sheath and apply pressure to the sites for up to 20 min until hemostasis is achieved.
6. General anaesthetic recovery
- Turn off inhalant anaesthetic or intravenous anaesthetic pumps being used. Reduce the respiratory rate on the ventilator to 8-10 breaths/min.
- After 2-5 min, switch the ventilator to 'free breathing' mode for no more than 30 s at a time. Observe the animal and the capnograph for spontaneous breathing.
- If spontaneous breathing has occurred, leave the ventilator switched off. If the animal is apnoeic, turn the ventilator back on for another 1-2 min and try again until spontaneous breathing is achieved.
NOTE: The timeframe for recovery is variable depending on procedural and animal characteristics, but may range from 15 min to 1 h. - Once the animal is breathing comfortably (respiratory rate of 15-30 breaths/min, end-tidal carbon dioxide less than 60, SpO2 above 95%), disconnect the oxygen, leaving other monitoring connected.
- Remove temporary sites of vascular access and apply pressure to prevent haematoma formation. If the animal remains stable and continues to oxygenate well over the following 5 min, it can be transported to its recovery area.
- After three consecutive spontaneous swallows, deflate the cuff of the endotracheal tube and gently remove the tube.
- Monitor the animal for at least another 5 min to ensure they continue to oxygenate appropriately and don't encounter any respiratory distress. Flow-by oxygen is to be used as required if an animal desaturates after extubation.
Representative Results
Thoracotomy and epicardial cell injection
Of the 29 animals that underwent thoracotomy and epicardial injection, 26 survived. Histological analysis confirmed the engraftment of human cells delivered by this method in all surviving animals (Figure 1E). One animal experienced fatal arrhythmias during cell injection and could not be resuscitated. Another experienced pulseless electrical activity during closure and prolonged application of positive pressure to the airways and was unable to be recovered. A third animal both vomited and went into respiratory arrest upon extubation. This animal was unable to be resuscitated.
Two animals experienced major complications but were able to be recovered. One animal developed ventricular fibrillation during intramyocardial injection and was able to be resuscitated with internal defibrillation paddles and cardiac massage. The second animal vomited upon extubation and had a brief respiratory arrest but was able to be rapidly re-intubated and recovered well. All of these events occurred during early experiments, with reduced adverse events as team experience with the protocol increased (Table 1).
Jugular vein osmotic minipump implantation
No reported mortality or major complications were associated with jugular osmotic minipump implantation. Most of the seven animals experienced mild swelling at the surgical site within the first 24 h, which resolved without intervention. ELISA performed on serum on day 3 post-pump implantation demonstrated the efficacy of the pump, achieving a significant blood concentration of platelet-derived growth factor-AB human (PDGF-AB) compared to controls7 (Figure 2E).
Percutaneous transendocardial injection
A total of 22 animals received endocardial injections. Of these injections, 17 were considered 'successful', determined by fluorescence or ink staining observed in the target tissue at post-mortem (Figure 3B). There were no mortalities associated with this procedure. One animal developed a small volume pericardial effusion from right ventricular perforation. This was self-limiting and did not result in cardiovascular compromise. This same animal did die; however, this was from an unrelated additional procedure after the intramyocardial injection.
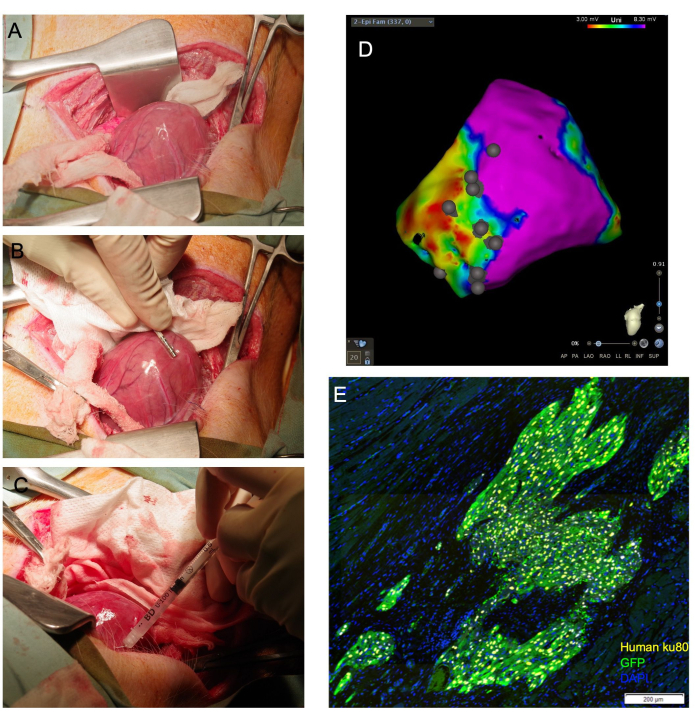
Figure 1: Transepicardial cardiomyocyte injection allows for direct cardiac visualization and achieves a high proportion of viable cells delivered to the myocardium. (A) The cardiac apex is exposed through a moistened gauze sling guided under the base of the heart. (B) An epicardial mapping catheter delineates scar and border zones and annotates injection sites. (C) A 31 G needle is used to transepicardially inject cells into the myocardium. (D) Epicardial voltage map with injection site annotation. Purple: normal voltage, healthy myocardium; Red: abnormal voltage, diseased myocardium; Grey dots: injection sites. Following sacrifice, the heart is collected and formalin-fixed for downstream histological assessment. In (E), engrafted human cells are detected by immunostaining for the human anti-nuclear antibody, Ku80, and an anti-GFP antibody. Scale bar = 200 µm. Please click here to view a larger version of this figure.
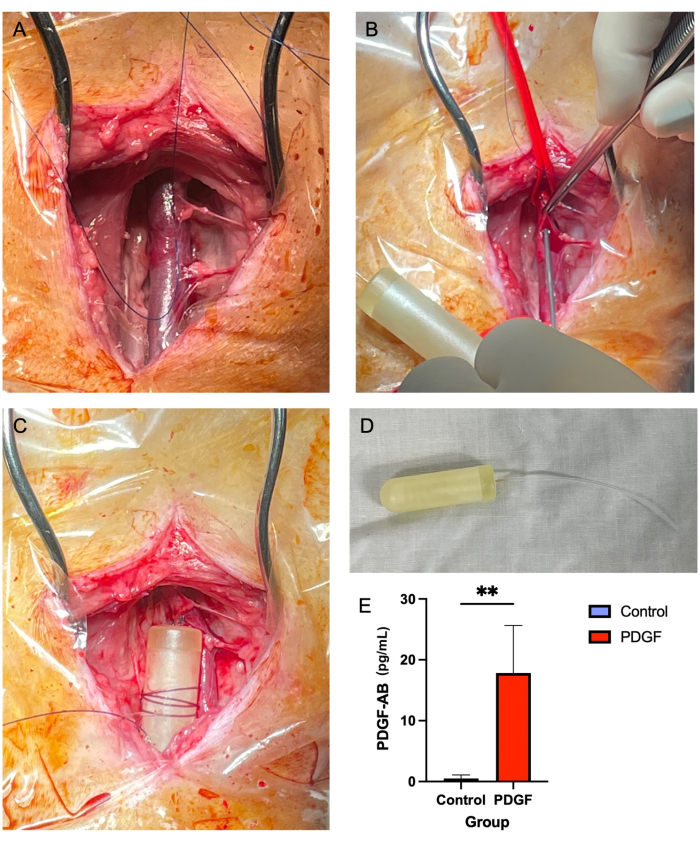
Figure 2: Jugular vein minipump insertion provides a safe and reliable method of PDGF delivery over a 7 day time period. (A) The right jugular vein is exposed, and soft tissue is cleared away from the vessel. (B) Vascular ties occlude the vessel while a 14 G needle is used to make a puncture, through which the minipump tubing is threaded. (C) The minipump tubing is advanced into the vein, and the minipump body is secured to adjacent soft tissue. (D) The minipump body and tubing prior to implantation. (E) The serum concentration of the recombinant protein delivered via the minipump and the PDGF-AB was measured using ELISA from each animal on day 3 post-implantation. Animals receiving PDGF-AB were demonstrated to have a significantly higher blood concentration of PDGF-AB than control animals, confirming the efficacy of the osmotic minipump administration method. **denotes a statistically significant difference between groups (p = 0.005, Mann-Whitney U test) Please click here to view a larger version of this figure.
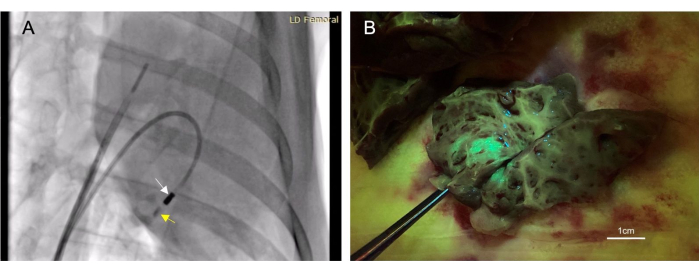
Figure 3: Transendocardial intramyocardial injection allows for a minimally-invasive therapeutics administration method. (A) A right anterior oblique fluoroscopic image demonstrating the injection catheter (white arrow) administering contrast material (yellow arrow) into the myocardium. The contrast material injection both precedes and follows therapeutic injection so that needle placement within the myocardium can be confirmed. (B) The injected vector expressed green fluorescent protein (GFP) so that injected material would fluoresce upon tissue collection, thus confirming the success of the injection. Please click here to view a larger version of this figure.
| Transepicardial Injection (n = 29) | Transendocardial Injection (n = 22) | Osmotic Minipump (n = 7) | |
| Mortality | 3 | 0 | 0 |
| Post-operative vomiting and respiratory arrest | 1 | 0 | 0 |
| Ventricular Fibrillation during injection | 1 | 0 | 0 |
| Pulseless electrical activity during chest closure | 1 | 0 | 0 |
| Morbidity | 0 | 1 | 0 |
| Pneumothorax | 0 | 0 | 0 |
| Pleural effusion | 0 | 0 | 0 |
| Cardiac chamber perforation | 0 | 1 | 0 |
| Hemorrhage | 0 | 0 | 0 |
Table 1: Complications list.
Discussion
Transepicardial intramyocardial injection
This procedure has the benefit of direct cardiac visualization and has been demonstrated to provide greater local retention of therapeutics than systemic administration methods9,10,14. However, thoracotomies are invasive, require considerable technical skill, and present a greater risk of morbidity and mortality than other methods discussed10,15. Knowledge of the critical and precarious stages of the procedure can assist in the mediation of this increased risk.
Great care must be exercised upon manipulating the heart to expose the cardiac apex due to the high risk of arrhythmia and associated hemodynamic compromise. Continuous invasive blood pressure monitoring and electrocardiography allow for rapid identification of hypotension or unstable arrhythmias, facilitating prompt intervention and correction. Transient hypotension can generally be treated with metaraminol boluses. Sustained hypotension may be temporized by reducing inhalant anaesthetic (careful monitoring of anaesthetic depth) and commencing a vasopressor infusion, while concurrently determining the cause of altered hemodynamics. Unstable arrhythmias, such as ventricular tachycardia or ventricular fibrillation, can be treated by electrical cardioversion with or without intravenous antiarrhythmics.
Equally important for animal survival is the successful removal of free gas from the pleural cavity before closing the chest. Failure to do so can culminate in developing a pneumothorax, leaving the animal at great risk of respiratory compromise and death once disconnected from the mechanical ventilator at recovery. Positive airway pressure must be maintained for at least 30 s until bubbling is no longer observed. The silicone tubing is promptly removed upon the cessation of bubbling, and the thorax is then rapidly closed. It is also possible to surgically place a thoracostomy tube at closure, allowing manual air and inflammatory fluid removal over the next 24-72 h. This, however, is difficult to keep clean and intact, especially if animals are housed together. Damage or contamination of the tube can lead to pyothorax, pneumothorax, or sepsis. In our experience, inserting a temporary chest drain is not required if free gas is adequately removed prior to chest closure.
Percutaneous transendocardial intramyocardial injection
This method of therapeutic administration has the benefit of allowing for local tissue delivery with lower risk due to its less invasive nature compared with a surgical approach10,14. This technique is already used in large animal studies, with both fluoroscopy and electromechanical mapping as a guide in the absence of direct visualization10,16,17.
Given the heart isn't under direct vision, it is prudent for the proceduralist to use orthogonal fluoroscopic views when selecting an injection site. Furthermore, the injection of diluted iodine contrast before and delivery of the therapeutic is extremely valuable in confirming myocardial contact. Appropriate contact can be confirmed by observing a characteristic 'myocardial blush', which may be one of the only markers of injection success prior to tissue harvest. Due to the risk of chamber perforation, the myocardial wall thickness at the selected injection site is also recommended to be greater than 9 mm14,16.
Jugular venous osmotic minipump
The osmotic minipump is a popular device commonly employed in small animal studies. There has been increasing interest in using this device in large animal models7,18,19, given its unique advantage of administering a therapeutic agent at a consistent rate over a set time period. A possible limitation of this method is the inability to alter or stop infusion rates of the drug without replacing or removing the pump. This should be considered before trialing therapy in this manner.
This study demonstrated that this method could be performed with a high success rate in swine, with low morbidity and mortality. It must be noted that many vital structures are adjacent to the surgical site, including lymph nodes, the thymus, and the carotid artery. Adherence to the method, and consultation of anatomical texts20, are strongly recommended to prevent inadvertent damage to any of these structures. The most concerning complication of this method is hemorrhagic shock due to inadvertent injury to the jugular vein or a surrounding structure. It is therefore critical that the soft tissue surrounding the jugular vein is carefully removed. Failure to properly complete this step can lead to difficulty in placing the minipump tubing or controlling inadvertent bleeding.
This article has described three methods for the delivery of cardioactive therapeutics. Despite the reported success of each technique, there are inherent limitations to be considered. Invasive procedures (transepicardial injection) allow for increased accuracy of therapeutic delivery; however, they bring a greater risk of potentially fatal complications. Furthermore, invasive delivery has a greater requirement for technical skills to minimize the risk of complications. Similarly, fluoroscopic-guided, transendocardial injection requires a degree of technical skill for catheterization and manipulation of hardware. If this method is performed improperly, injection failure and fatal complications are possible.
The direct injection methods described allow for the one-off administration of a therapeutic into the target tissue. The jugular venous osmotic minipump allows for the systemic administration of a therapeutic over a 7 day period. Comparatively, this method is simpler and associated with less risk, however, it relies on a systemic therapeutic finding its way to the myocardium. Additionally, once the pump is in place, it is impossible to discontinue administration or alter the dose rate without re-anaesthetizing the animal and removing the pump.
All methods described in this article were performed on animals on the day or 2 weeks after myocardial infarction. Therefore, this work cannot report the success of mentioned methods in healthy animals or animals subjected to an alternative cardiac pathology. Finally, the pharmacology and biotechnology of any intended agent are to be carefully considered, as this will be inherently linked to the efficacy of the chosen delivery route. A detailed discussion of this is beyond the scope of this manuscript.
Comprehensive depictions of preclinical methods benefit animal welfare and the wider scientific community. The resultant enhanced reproducibility of procedures and results leads to fewer animal health complications, reduced number of animals required to produce significant results, and greater confidence in experimental outcomes21,22. Three methods of administration of novel therapeutics are described in this article for the treatment of myocardial infarction in a porcine model. By detailing the techniques used and articulating the benefits and risks of each, it is anticipated that researchers will be able to comfortably create consistent and reliable preclinical models that suit their research goals.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by grants from the National Health and Medical Research Council APP1194139/APP1126276 (JC), National Stem Cell Foundation of Australia and New South Wales Government Office of Health and Medical Research (JC). DS was supported by the Royal Australasian College of Physicians, the Institute of Clinical Pathology and Medical Research, and the Australian Government Research Training Program. TD was supported by the Institute of Clinical Pathology and Medical Research, Penfolds Family Scholarship, National Health and Medical Research Council (APP2002783) and the National Heart Foundation of Australia (104615).
Materials
| Central line placement | |||
| 2-0 sutures | Ethicon | JJ9220 | |
| Arrow' Paediatric Two-Lumen Central Venous Catheterisation Set with Blue FlexTip Catheter (contains 18G cook needle and 0.035" J-tip wire) | Teleflex | CS-14502 | Central Line |
| Green Fluorsence Protein (GFP) | Abcam | ab13970 | 1:100 dilution ratio |
| Histology antibodies | |||
| Ku80 | Cell Signalling Technology | C48E7 | 1:500 dilution ratio |
| No. 11 scalpel | Swann-Morton | 203 | |
| Sparq' Ultrasound System | Philips | MP11742 Medpick | |
| Sterile ultrasound probe cover | Atris | 28041947 | |
| Swine Jacket with Pocket, size 'Medium' | Lomir Biomedical | SS J2YJJET | |
| Jugular vein osmotic minipump implantation | |||
| Adson Brown Tissue Forceps | Icon Medical Supplies | KLINI316012 | |
| Bellucci Self-Retaining Retractor | surgicalinstruments.net.au | group-24.26.02 | Self retaining tissue retractor |
| Electrosurgical Pencils with 'Edge' Coated Electrodes | Covidien | E2450H | Cautery Pencil |
| Metzenbaum Scissors | Icon Medical Supplies | ARMO3250 | |
| No. 22 scalpel blade | Swann-Morton | 208 | |
| Nylon Suture (2-0, 3-0) | Ethicon | D9635, 663G | |
| Osmotic Infusion Minipump | Alzet | 2ML1, 2ML2, 2ML4 | |
| Vascular Silicone Ties | Vecmedical | 95001 | |
| Vicryl suture (5-0) | Ethicon | W9982 | |
| Percutaneous transedocardial injection | |||
| Artis Zee' C-Arm Fluoroscopy | Siemens | IR-19-1994 | |
| CARTO' 3 System | Biosense Webster | Electrophysiological Mapping Software & System | |
| Cook Access Needle | Cook Medical | G07174 | Cannulation needle |
| Fast-Cath' Introducer (6 French, 8 French) | Abbott | 406204, 406142 | Vascular sheath with introducer and guidewire |
| Myostar' Injection Catheter | Biosense Webster | 121117S, 121119S, 1211120S | Intramyocardial injection catheter |
| No.11 scalpel | Swann-Morton | 203 | |
| Omnipaque' Iohexol Contrast | GE Healthcare | AUST R 39861 | Iodinated contrast agent |
| Sparq' Ultrasound System | Philips | MP11742 Medpick | |
| Sedation & general anaesthesia | |||
| Compound Sodium Lactate Hartmann's Solution | Free flex | 894451 | |
| Fentanyl 50 mcg/mL | Pfizer | AUST R 107027. | Intravenous anaesthesia and analgesia |
| Forthane' Isoflurane | Abbott | AUST R 29656 | Inhalant anaesthetic |
| GE Aestiva 5 Anaesthesia Machine | Datex Ohmeda | 17002-9, 17002A9 Avante Health Solutions | Anaesthetic Machine |
| Hypnovel' Midazolam 5 mg/mL | Roche | AUST R 13726 | Sedative |
| Intravenous cannula | BD Angiocath | 381137 | 20 gauge cannula |
| Ketamil' Ketamine 10 mg/mL | Ilium | APVMA number: 51188c | Sedative |
| Laryngoscope | Miller | VDI-6205 | |
| Medetomidine 1 mg/mL | Ilium | APVMA number 64251; ACVM number A10488 | Sedative |
| Metaraminol 10 mg/mL | Phebra | AUST R 284784 | Short-acting vasopressor |
| Methadone 10 mg/mL | Ilium | APVMA number: 63712 | Sedative, Restricted drug |
| Onsetron' Ondansetron 2 mg/mL | Accord Healthcare | AUST R 205593 | Anti-emetic |
| Propofol-Lipuro' Propofol 10 mg/mL | Braun | AUST R 142906 | Intravenous anaesthetic |
| Pulse Oximeter | Meditech | GVPMT-M3S | Portable pulse oximeter |
| Shiley' Cuffed Basic Endotracheal Tube (Size 5.5 & 6.0) | Medtronic | 86108-, 86109- | |
| Shiley' Intubating Stylet, 10 Fr | Medtronic | 85864 | |
| Sodium Chloride 0.9% | Free flex | FAH1322 | |
| Thoracotomy and epicardial Cell Injection | |||
| 27 G Insulin needle | Terumo | 51907 | |
| Adson Brown Tissue Forceps | Icon Medical Supplies | KLINI316012 | |
| CARTO' 3 System | Biosense Webster | Electrophysiological Mapping Software & System | |
| Cefazolin 1 g Vial | AFT Pharmaceuticals | 9421900137367 CH2 | Antibiotic Prophylaxis |
| Chest drainage tube | SurgiVet | SKU-336 | |
| Cook Access Needle | Cook Medical | G07174 | Cannulation needle |
| Cooley Sternotomy Retractor Paediatric | Millennium Surgical | 9-61287 | |
| Durogesic' 100 mcg/h Fentanyl Patch | Janssen | AUST R 112371 | Postoperative analgesia |
| Electrosurgical Pencils with 'Edge' Coated Electrodes | Covidien | E2450H | Cautery Pencil |
| Electrosurgical Pencils with 'Edge' Coated Electrodes | Covidien | E2450H | Cautery Pencil |
| Fast-Cath' Introducer (6 French, 8 French) | Abbott | 406204, 406142 | Vascular sheath with introducer and guidewire |
| Lignocaine 20 mg/mL | Pfizer | AUST R 49296, AUST R 49297, AUST R 49293 and AUST R 49295. | Local anaesthesia, anti-arrhythmic |
| Marcaine' Bupivacaine 0.5% | Pfizer | AUST R 48328 | Local anaesthesia. |
| Metzenbaum Scissors | Icon Medical Supplies | ARMO3250 | |
| No. 22 scalpel | Swann-Morton | 208 | |
| Nylon Suture (2-0, 3-0) | Ethicon | D9635, JJ76264 | |
| Size 1 PDS suture | Ethicon | JJ75414 | |
| Sparq' Ultrasound System | Philips | MP11742 Medpick | |
| Sterile gauze | Kerlix | KE5072 | |
| Sterile laparotomy sponges | Propax | 2907950 | |
| Thermocool Smartouch' Catheter | Biosense Webster | D133601, D133602, D133603 | Epicardial Mapping Catheter |
References
- Vogel, B., et al. ST-segment elevation myocardial infarction. Nature Reviews Disease Primers. 5 (1), 39 (2019).
- Niccoli, G., et al. Optimized treatment of ST-elevation myocardial infarction. Circulation Research. 125 (2), 245-258 (2019).
- Ezekowitz, J. A., et al. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. Journal of the American College of Cardiology. 53 (1), 13-20 (2009).
- Hastings, C. L., et al. Drug and cell delivery for cardiac regeneration. Advanced Drug Delivery Reviews. 84, 85-106 (2015).
- Silva, K. A. S., Emter, C. A. Large animal models of heart failure: a translational bridge to clinical success. JACC: Basic to Translational Science. 5 (8), 840-856 (2020).
- Suzuki, Y., Yeung, A. C., Ikeno, F. The representative porcine model for human cardiovascular disease. Journal of Biomedicine and Biotechnology. 2011, 195483 (2011).
- Thavapalachandran, S., et al. Platelet-derived growth factor-AB improves scar mechanics and vascularity after myocardial infarction. Science Translational Medicine. 12 (524), (2020).
- Hou, D., et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 112, 150-156 (2005).
- Tousoulis, D., Briasoulis, A., Antoniades, C., Stefanadi, E., Stefanadis, C. Heart regeneration: what cells to use and how. Current Opinion in Pharmacology. 8 (2), 211-218 (2008).
- Bonnet, G., Ishikawa, K., Hajjar, R. J., Kawase, Y. Direct myocardial injection of vectors. Methods in Molecular Biology. 1521, 237-248 (2017).
- Marchlinski, F. E., Callans, D. J., Gottlieb, C. D., Zado, E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 101 (11), 1288-1296 (2000).
- Polin, G. M., et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 8 (1), 76-83 (2011).
- Tatay, J. . Veterinary Sutures Handbook. , (2018).
- McCall, F. C., et al. Myocardial infarction and intramyocardial injection models in swine. Nature Protocol. 7 (8), 1479-1496 (2012).
- Sun, S., et al. Establishing a swine model of post-myocardial infarction heart failure for stem cell treatment. Journal of Visualized Experiments. (159), e60392 (2020).
- Gwon, H. C., et al. The feasibility and safety of fluoroscopy-guided percutaneous intramyocardial gene injection in porcine heart. International Journal of Cardiology. 79 (1), 77-88 (2001).
- Krause, K., et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart. 95 (14), 1145-1152 (2009).
- Wang, X., Shangguan, W., Li, G. Angiotensin-(1-7) prevents atrial tachycardia induced-heat shock protein 27 expression. Journal of Electrocardiology. 51 (1-7), 117-120 (2018).
- Klatt, N., et al. Development of nonfibrotic left ventricular hypertrophy in an ANG II-induced chronic ovine hypertension model. Physiological Reports. 4 (17), 12897 (2016).
- Singh, B., Dyce, K. M. . Dyce, Sack, and Wensing’s Textbook of Veterinary Anatomy. , (2018).
- Percie du Sert, N., et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLOS Biology. 18 (7), 3000411 (2020).
- Schüttler, D., et al. A practical guide to setting up pig models for cardiovascular catheterization, electrophysiological assessment and heart disease research. Lab Animals. 51 (2), 46-67 (2022).

