The Establishment of a Murine Mandibular Molar Extraction Socket Healing Model
Summary
This protocol demonstrates step-by-step details of how to extract the mandibular first molar in the mouse. It provides an alternative method for researchers focusing on jawbone healing and regeneration.
Abstract
This study introduces the development of a molar extraction model in the murine mandible to provide a practicable model for studying alveolar bone regeneration and intramembranous ossification. C57/J6 mice were used to extract the mandibular first molar to establish this model. They were euthanized, and the bilateral mandibles harvested, at 1 week and 4 weeks post-surgery, respectively. Subsequent serial stereoscopic harvest, histological assessment, and immunofluorescence staining were performed to demonstrate successful surgery. Immediately after surgery, the stereoscopic images displayed an empty extraction socket. The hematoxylin and eosin (H&E) at 1 week and Masson staining at 4 weeks post-surgery showed that the area of the original root was partially and fully filled with bone trabeculae, respectively. The immunofluorescence staining showed that, compared with the homeostasis side, the Sp7 expression increased at 1 week post-surgery, suggesting vigorous osteogenesis in the alveolar fossa. All these results demonstrated a practicable murine tooth extraction socket healing model. Upcoming studies revealing the mechanisms of jawbone defect healing or socket healing could adopt this method.
Introduction
Socket healing after tooth extraction is a common clinical scenario, which can result in unbecoming complications such as socket hemorrhage, dry socket, or even jaw osteomyelitis under undesirable healing1,2,3. These comorbidities may impair the quality of life of patients and, even worse, vitally challenge prosthetic rehabilitation due to massive bone loss4. Though the socket healing stages have been elucidated, they are inadequate to direct clinical care post-tooth extraction surgery when encountering various prognosis challenges4.
Multiple studies based on animal models have been conducted to gain a better understanding of the underlying mechanisms in the socket healing process and avert the above situations. Sp7 is a master regulator in osteoblast differentiation, playing a vital role in skeleton development, bone hemostasis, and bone regeneration5,6. Rational socket healing models could display the redundancy of Sp7 post-traumatic in bone regeneration. In addition, distinct from long bone fracture healing, only a single osteogenic process, intramembranous ossification, involves the healing process of the extraction socket7. This makes the animal tooth extraction model optimal for studying implant-based therapies, as implant osseointegration obeys the same osteogenic rule8.
For decades, the tooth extraction model has been performed in rats, rabbits, and dogs, since these species have big teeth that are convenient to operate on9,10,11. However, given the flourishing demands for genetic modification and as a more adaptive genetic background to humans, mice are increasingly being used to establish a tooth extraction model. Thenceforward, researchers could unravel a specific cell population's role in the socket healing process using genome-modified mice instead of observing phenotypes only12. Among murine tooth extraction socket models, preceding studies have demonstrated the establishment and healing process of murine maxillary tooth and incisor extraction sockets13,14,15,16. However, the healing pattern of the prognosis, and the detective and observative time points, may differ across protocols. This appeals to a universal criterion for scholars to establish a murine socket healing model.
This study aimed to constitute a practicable murine socket healing model for the above issues. Mandible molars in mice have distinctive morphological traits compared to maxillary molars and incisors, bringing unique advantages and disadvantages. As the models focusing on the murine mandible are currently vacuum-based, this protocol tried to provide an accomplished method to extract the mandibular first molar in mice. We hope this protocol will enlighten basic researchers with new ideas to uncover underlying mechanisms of socket healing and indicate clinical care.
Protocol
All animal procedures in this study were reviewed and approved by the Ethical Committee of the West China School of Stomatology, Sichuan University (WCHSIRB-D-2017-041). Adult C57BL/6 mice, obtained from a commercial source (see Table of Materials), were used for the present study.
1. Presurgical preparation
- Instrument preparation
- Prepare various needles of disposable syringes (26G, 25G, 23G; see Table of Materials) to be used as elevators. Inflect the needle head approximately 20°-40°, as shown in Figure 1.
- Prepare toothed ophthalmic tweezers as forceps. Ensure that it fits the size of the mice's molars and can grab the molars firmly. The ideal tweezer size is shown in Figure 1A, B3.
- Obtain a rubber band (which can be torn from a medical latex glove) to be used as a mouth opener, a foam board or corkboard as the surgical platform, a headlamp to light up the surgical area, and a heating pad for postoperative revival. Tear a dry cotton ball into small pieces and apply it to the surgical site if bleeding occurs during the procedure.
- Anesthesia preparation
- The mouse can be anesthetized by any suitable anesthetic protocol that achieves general anesthesia confirmed through the 'toe pinch' method. Following anesthesia, apply veterinary ointment on the eyes of the mouse.
NOTE: Preferred general anesthetic protocol could be: induction and maintenance with xylazine (10 mg/kg) and ketamine (100 mg/kg) intraperitoneally (IP);
For this study, isoflurane and 1% pentobarbital sodium (50 mg/kg, IP) were used for anesthesia induction and maintenance with additional doses as needed.
- The mouse can be anesthetized by any suitable anesthetic protocol that achieves general anesthesia confirmed through the 'toe pinch' method. Following anesthesia, apply veterinary ointment on the eyes of the mouse.
- Disinfection and Sterilization preparation
- Disinfect the operation platform and the open-air overhead with a 75% ethanol sprayer. Prior to each mouse procedure, the use of a new set of disposable needles is recommended.
NOTE: The toothed tweezers are reusable and can be sterilized using any preferred method such as steam sterilization.
- Disinfect the operation platform and the open-air overhead with a 75% ethanol sprayer. Prior to each mouse procedure, the use of a new set of disposable needles is recommended.
2. Surgical process
- Fixation of the mouse and its mandible
- Tie the mouse to a surgical platform in a supine position using adhesive tape. Pin two 26 G needles in line with the orbital ear plane and another two 26 G needles at ease below the mandible.
- Apply the rubber band around the needles and cross the incisors to hold the mouth open. Slightly pull the tongue out and fix it under the rubber band opposite the surgical side, to prevent it from obstructing the field of view (Figure 1C).
- Eliminating the distal resistance
- Hold the molar with tweezers mesially, force a 23 G needle into the buccal alveolar bone of the distal root, and render an interval.
NOTE: This step must be taken with great caution, as a needle that is embedded too deep will likely snap the root. - Next, change to a 25 G needle to continue to expand the interval, and progress delicately toward the periapical area while slowly rotating the needle forward and lingually (with the mouse anatomical position) to press the root out of the alveolar fossa.
- Hold the molar with tweezers mesially, force a 23 G needle into the buccal alveolar bone of the distal root, and render an interval.
- Eliminating the mesial resistance
- When there is enough space, use a 23 G needle to insert into the root fork and lift the molar occlusally. After holding the molar tightly, take another 23 G needle and force it into the lingual mesial periodontal membrane to create an interval.
- Then, substitute with a 25 G needle and slowly rotate forward and buccally. If some underlying hindrances impede the luxation of the molar, use a 26 G needle to penetrate the root apex, and repeat the operations.
- Final extraction
- Extract the tooth and, while extracting, ensure that the crown rises high above the occlusal plane and that two intact roots can be clearly seen.
NOTE: The luxation (dislocation) of the tooth is considered the most painful and distressing moment of the procedure. Prior to dislocating the tooth, the anesthetic depth should be reassessed using the toe pinch test, and accordingly, a moderate dose of an anesthetic agent should be administered if necessary.
- Extract the tooth and, while extracting, ensure that the crown rises high above the occlusal plane and that two intact roots can be clearly seen.
- Post-operative care
- After extracting the tooth, apply dry cotton to stop bleeding, reposition the tongue, administer carprofen (5 mg/kg) subcutaneously, and put the mouse on a constant temperature heating pad until recovery from anesthesia.
3. Imaging of the mouse mandible and the extraction socket
- Preparation of the samples
- Euthanize the mouse by cervical dislocation. Use ophthalmic scissors to cut the skeleton muscles attached to the mandible and the zygomatic arch. Cut from the throat along the lower edge of the mandible to the ascending branch and then to the backside of the condyle, and then pull the mandible down and cut along the lower incisor midline. This way, the two separate mandibles are harvested.
- Perform fixation, demineralization, and dehydration following the standard procedure17. When embedding17, ensure that the occlusal plane is parallel with the edge of the cassette (see Table of Materials). Fix the mandible on the bottom of the cassette, with its lower edge cocked (Figure 2).
NOTE: This protocol offers the sagittal plane of the mandible area.
- Fixing the sample on the microtome
- Regulate the specimen clamp in the microtome to protrude the condyle and the crown side 5°-20° more, to attain an integrated image of crown pulp concomitant with root pulp (Figure 2).
- Preparing the sections
NOTE: The condyle is always the first anatomical structure to be cut. When it fades away, the molar area can then be cut into several slices.- Shift the microtome range to 5 µm, collect every eight slices, and scout for dentin in the last slice. If the crown dentin first appears without any root apical dentin, adjust the specimen clamp to make the root area protrude, and vice versa.
- Collecting the sections
NOTE: The cutting angle is appropriate until the dentin is equally attained in both the crown and apical area.- Cut along this direction and watch the slices under the microscope until the dental pulp appears in both the crown and the roots. Collect the sections when an obscure contour of molars appears on the surface of the paraffin sample17.
Representative Results
To elucidate the practical use of this method, the right mandibular first molar of two healthy C57BL/6 mice (3 months old, both female) were extracted and followed for 1 week and 4 weeks, respectively. The undamaged left mandibles were used as healthy controls. Figure 1A shows the specific features of the surgical appliance, including 26-23 G needles and a toothed ophthalmic tweezer. The 26 G needle is pinpoint-removed and bent. The 25 G needle is bent at approximately 25° at the pinpoint. The 23 G needles are the linchpin for the surgery and are bent at approximately 25° and 35°, respectively, at the pinpoint (Figure 1B1,B2). The tweezers have a 1 mm long tooth that fits the shape of the murine molars (Figure 1B3). Figure 1C shows the state of the mouse before surgery. The key points are the location of the four pins around the head and fixing the tongue under the rubber band on the left.
Figure 3A shows the position and morphology of the right mandibular first molar. Figure 3B indicates the tooth socket immediately filled with clot post-extraction. At 1 week and 2 weeks, the mice were euthanized, and the mandibles were demineralized, embedded in paraffin17, and sectioned into slices. Figure 4A displays the healing process of the socket at 1 week. Some sponge-like trabecular bones were formed, but the clot remained. In the healing process at 2 weeks (Figure 4B), the socket was fully filled with sponge bones, meaning the regeneration was roughly completed. The immunofluorescence (IF) staining also corroborated the results of the histopathology staining. In Figure 5, the Sp7 was widely expressed in the bone marrow cells, especially in the margin, which is the bone-forming front. Under the homeostasis state, the trabecular bones were consistent and confluent, with bone marrow cell blocks sprinkled like islands. However, at 1 week post-surgery, numerous Sp7-expressing cells filled the extraction socket, with newly formed trabecular bone sprinkled around. At 4 weeks post-surgery, the condition reversed and turned to largely merged trabecular bone again, and the activity of Sp7-expressing cells declined to a level approaching the homeostasis state.
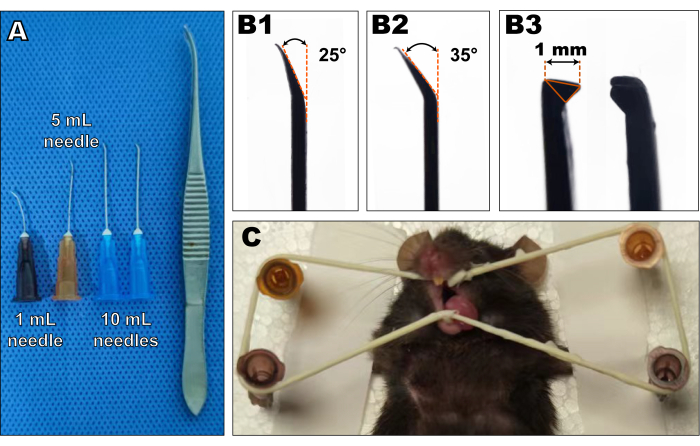
Figure 1: The object images of surgical appliances and surgery-prepared mice. (A) The surgical appliances mainly consisted of 26, 25, and 23 G needles and a toothed tweezer. (B) Needles were bent at the pinpoint at 20°-40° (B1,2). The tweezers' tooth must be approximately 1 mm in size (B3). Please click here to view a larger version of this figure.
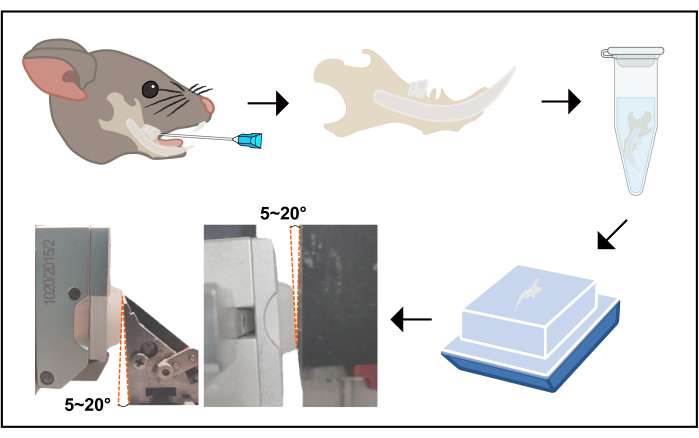
Figure 2: The flowchart of the procedure from tooth extraction to sectioning. This image displays a schematic flow from tooth extraction to sectioning. The right mandibular first molar was extracted according to the protocol, then the mouse was euthanized, and the mandible was harvested. Upon harvesting, the mandible was soaked in 4% POM for 24 h, then re-soaked in 10% EDTA; the fluid was renewed every day for 14 days. Next, the samples were dehydrated and embedded in paraffin following a universal protocol. The buccal or lingual side must be face down, as sagittal plane slices are supposed to be sectioned. Finally, when fixing the sample on the specimen clamp, the condyle and crown sides must be 5°-20° protruded. Abbreviations: POM = paraformaldehyde; EDTA = ethylenediamine tetra-acetic acid. Please click here to view a larger version of this figure.
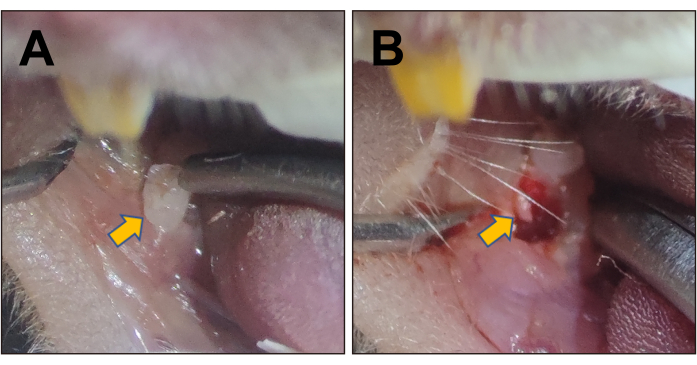
Figure 3: Stereoscopic images of the mouse mandibular first molar and the extraction socket. (A) Stereoscopic image of the right mandibular first molar, indicated by the yellow arrow. (B) The extraction socket of the first right mandibular molar post-surgery, indicated by the yellow arrow. Please click here to view a larger version of this figure.
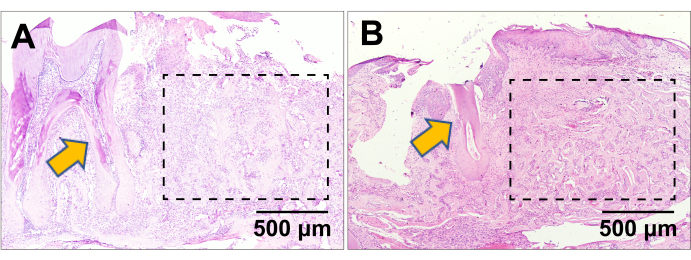
Figure 4: H&E staining. H&E staining of the extraction socket at (A) 1 week and (B) 4 weeks post-surgery. The yellow arrows indicate the mandibular second molar in the proximity of the first molar extraction socket healing. The dashed rectangle line indicate the healing first molar extraction socket area. Scale bars: 500 µm. Please click here to view a larger version of this figure.
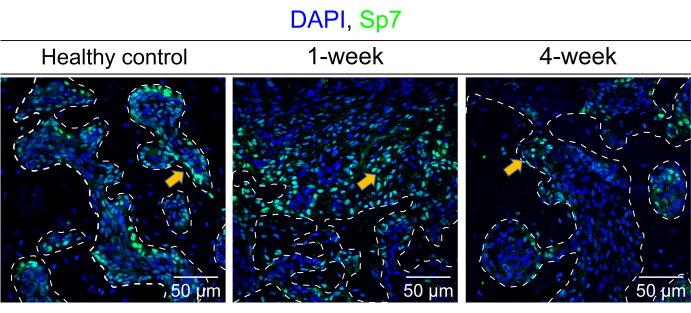
Figure 5: Detection of Sp7 via IF staining. This image shows the distribution of Sp7 expression in the mouse mandible bone marrow cells and the surrounding trabecular bone under a homeostasis state (healthy control), 1 week and 4 weeks post-surgery, respectively. The white dashed line contoured the estimated scale of the trabecular bone. The yellow arrows indicated the typical Sp7-expressing cells. Scale bars: 50 µm. Please click here to view a larger version of this figure.
Discussion
The murine socket healing model is an important method for unraveling the underlying mechanisms in bone healing and regeneration, ultimately solving clinical challenges. Existing studies have demonstrated the possibility of the incisor extraction model and the maxillary molar extraction model, whereas studies have not used the mandibular first molar model13,17,18. However, incisors are crucial for rodent living, and their impairment may be fatal. Moreover, as the bone in the maxilla is more cancellous than the mandible, there may be some dissimilarity in the underlying mechanisms during the healing process. Hence, it is necessary to establish a feasible mandibular first molar extraction model.
The most important consideration in tooth extraction is to avoid root breaks19. Murine molars are small and vulnerable, and the residue roots in the mandible cannot be extracted. In this protocol, however, the root-breaking risk exists throughout the whole surgery. Thus, it is critical to hold the molar tightly and rigorously control the force. To be specific, when eliminating the distal resistance, one needs to be careful not to scrunch the crown; when eliminating the mesial resistance, one needs to be cautious so that the needle does not surge from the root fork; when using needles to render intervals, it is important to remember that the intervals are supposed to between the root and the alveolar process, therefore one should not apply much rotational force, as this can lead to a high risk of the root breaking.
Extracting the murine mandibular first molar is arduous and needs sufficient training to master. During the operation, emergencies including but not limited to the following may occur: (1) tooth extraction instruments tend to slip and stab soft tissues around due to the mobility of the mandible and tongue, which may cause mild to severe bleeding. In this case, one can deposit a clean dry cotton ball into the mouth, release the rubber band, and let it close spontaneously for a while. Compression to stop bleeding is not suggested, as this may cause wider lacerations and bleeding. (2) Operators are often confronted with a binary choice on alveolar bone plate reservation or molar root reservation, as the first molars are tightly joined with the mandible. To alleviate this bond force, the operator could break the lingual or buccal bone plate; otherwise, a ferocious rotation force in the socket leads to the root(s) being broken. Whether one chooses to preserve the bone plate depends on the research objective. (3) Extracting the mandibular first molar may be more traumatic than the maxillary first molar or the incisor. We suggest watching with scrutiny until the mouse awakes after surgery. (4) The mesial root of the mandibular second molar is vulnerable because of the limited operation room in the buccal area; sometimes, even the crown of the second molar can be destroyed. Because the absence of the second molar does not affect the observation of the first molar extraction socket healing, this accident can be ignored. (5) Monitor the anesthesia depth of the mouse closely during the tooth extraction, as this is a painful procedure for both humans and rodents. Administer anesthetic agents as needed to maintain a surgical plane of anesthesia until extraction is complete. In addition, prolonged pressure from the rubber band may cause oxygen deprivation in the tongue, characterized by pale membranes. Operations must be halted immediately in such cases, with instruments removed, allowing the mouse time to recover.
Taken together, unskilled operators may inadvertently inflict unintended injuries to the mice, as listed in point 1. In order to ensure the safety and well-being of all laboratory animals, it's strongly advocated that ample practice be conducted on murine cadavers before mastering this skill and performing live procedures on actual mice.
Although this protocol provides an alternative method for researchers focusing on jawbone healing and regeneration, it has several drawbacks. (1) The mandible cannot be fixed and has broad flexibility. As a result, elevators are difficult to hold a steady-acting point on the alveolar bone, causing some emergencies. (2) Adult male mice have intensified mandible bones, so eliminating the mesial resistance may be tough. (3) This protocol does not apply to left mandibular first molar extraction.
In conclusion, though this protocol has demonstrated the details of extracting the murine mandibular first molar, operators still need much practice and caution to perform a successful surgery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by National Natural Science Foundation of China 81825005 (L.Y.), 82201045 (F.Y.), and 82100982 (F.L.), and by Sichuan Province Science and Technology Program 2021JDRC0144 (F.L.), 2022JDRC0130 (F.Y.).
Materials
| 23/25/26 G needle | Chengdu Xinjin Shifeng Medical Apparatus & Instruments Co. LTD. | SB1-074(IV) | |
| C57/B6J | Gempharmatech Experimental Animals Company, Chengdu, China | C57/B6J | |
| DAPI Staining Solution | Beyotime | Cat#C1005 | |
| Embedding Cassettes | CITOTEST Scientific | 80106-1100-16 | |
| Hematoxylin and Eosin Stain Kit | Biosharp | BL700B | |
| Isoflurane | RWD Life Science Co.,Ltd | R510-22-10 | |
| Masson’s Trichrome Stain Kit | Solarbio | G1340 | |
| Microtome | Leica | RM2235 | |
| Pentobarbital Sodium | Huaxia Chemical Reagent Co., Ltd | 2018042001 | |
| Rabbit polyclonal | anti-Sp7 | Abcam Cat# ab22552 | |
| Tweezers | Chengdu Xinjin Shifeng Medical Apparatus & Instruments Co. LTD. | SB2-115 |
References
- Mamoun, J. Dry socket etiology, diagnosis, and clinical treatment techniques. Journal of the Korean Association of Oral and Maxillofacial Surgeons. 44 (2), 52-58 (2018).
- Laraki, M., Chbicheb, S., El Wady, W. Alveolitis: review of the literature. Odonto-Stomatologie Tropicale = Tropical Dental Journal. 35 (139), 19-25 (2012).
- Soundia, A., et al. Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone. 90, 133-141 (2016).
- Araújo, M. G., Silva, C. O., Misawa, M., Sukekava, F. Alveolar socket healing: what can we learn. Periodontology 2000. 68 (1), 122-134 (2015).
- Hojo, H., Ohba, S. Sp7 Action in the skeleton: its mode of action, functions, and relevance to skeletal diseases. International Journal of Molecular Sciences. 23 (10), 5647 (2022).
- Zhou, X., et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proceedings of the National Academy of Sciences. 107 (29), 12919-12924 (2010).
- Ito, S., et al. Pathological differences in the bone healing processes between tooth extraction socket and femoral fracture. Bone Reports. 16, 101522 (2022).
- Vasak, C., et al. Early bone apposition to hydrophilic and hydrophobic titanium implant surfaces: a histologic and histomorphometric study in minipigs. Clinical Oral Implants Research. 25 (12), 1378-1385 (2014).
- Araújo, M. G., Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of Clinical Periodontology. 32 (2), 212-218 (2005).
- Kim, I. -. S., Ki, H. -. C., Lee, W., Kim, H., Park, J. -. B. The effect of systemically administered bisphosphonates on bony healing after tooth extraction and osseointegration of dental implants in the rabbit maxilla. The International Journal of Oral & Maxillofacial Implants. 28 (5), 1194-1200 (2013).
- Kanyama, M., et al. Connective tissue growth factor expressed in rat alveolar bone regeneration sites after tooth extraction. Archives of Oral Biology. 48 (10), 723-730 (2003).
- Zhou, S., et al. The role of IFT140 in early bone healing of tooth extraction sockets. Oral Diseases. 28 (4), 1188-1197 (2022).
- Apaza Alccayhuaman, K. A., et al. FasL is required for osseous healing in extraction sockets in mice. Frontiers in Immunology. 12, 678873 (2021).
- Avivi-Arber, L., Avivi, D., Perez, M., Arber, N., Shapira, S. Impaired bone healing at tooth extraction sites in CD24-deficient mice: A pilot study. PLoS One. 13 (2), 0191665 (2018).
- Vieira, A. E., et al. Intramembranous bone healing process subsequent to tooth extraction in mice: micro-computed tomography, histomorphometric and molecular characterization. PLoS One. 10 (5), 0128021 (2015).
- Min, K. -. K., et al. Effects of resveratrol on bone-healing capacity in the mouse tooth extraction socket. Journal of Periodontal Research. 55 (2), 247-257 (2020).
- Yu, F., Li, F., Zheng, L., Ye, L. Epigenetic controls of Sonic hedgehog guarantee fidelity of epithelial adult stem cells trajectory in regeneration. Science Advances. 8 (29), (2022).
- Kuroshima, S., et al. Transplantation of noncultured stromal vascular fraction cells of adipose tissue ameliorates osteonecrosis of the jaw-like lesions in mice. Journal of bone and Mineral Research. 33 (1), 154-166 (2018).
- Ahel, V., et al. Forces that fracture teeth during extraction with mandibular premolar and maxillary incisor forceps. The British Journal of Oral & Maxillofacial Surgery. 53 (10), 982-987 (2015).

