Analysis of the Mitochondrial Density and Longitudinal Distribution in Rat Live-Skeletal Muscle Fibers by Confocal Microscopy
Summary
Here, we present a protocol to analyze changes in mitochondrial density and longitudinal distribution by live-skeletal muscle imaging using confocal microscopy for mitochondrial network scanning.
Abstract
The mitochondrion is an organelle that can be elongated, fragmented, and renovated according to the metabolic requirements of the cells. The remodeling of the mitochondrial network allows healthy mitochondria to meet cellular demands; however, the loss of this capacity has been related to the development or progression of different pathologies. In skeletal muscle, mitochondrial density and distribution changes are observed in physiological and pathological conditions such as exercise, aging, and obesity, among others. Therefore, the study of the mitochondrial network may provide a better understanding of mechanisms related to those conditions.
Here, a protocol for mitochondria imaging of live-skeletal muscle fibers from rats is described. Fibers are manually dissected in a relaxing solution and incubated with a fluorescent live-cell imaging indicator of mitochondria (tetramethylrhodamine ethyl ester, TMRE). The mitochondria signal is recorded by confocal microscopy using the XYZ scan mode to obtain confocal images of the intermyofibrillar mitochondrial (IMF) network. After that, the confocal images are processed by thresholding and binarization. The binarized confocal image accounts for the positive pixels for mitochondria, which are then counted to obtain the mitochondrial density. The mitochondrial network in skeletal muscle is characterized by a high density of IMF population, which has a periodic longitudinal distribution similar to that of T-tubules (TT). The Fast Fourier Transform (FFT) is a standard analysis technique performed to evaluate the distribution of TT that allows finding the distribution frequency and the level of their organization. In this protocol, the implementation of the FFT algorithm is described for the analysis of the longitudinal mitochondrial distribution in skeletal muscle.
Introduction
Mitochondria form highly dynamic networks that are mainly regulated by the balance between its elongation (fusion) and fragmentation (fission)1,2, which are modulated by the expression and activity of proteins such as Mitofusin 1 and 2 (Mfn1 and Mfn2), and Optic protein atrophy 1 (Opa1), which regulate the fusion of the outer mitochondrial membrane and inner membrane, respectively1,2. Dynamin-related protein (Drp1) predominantly regulates mitochondrial fission when it is phosphorylated in the Ser6163.
In skeletal muscle, it has been well established that the mitochondrial network is arranged in structurally well-defined subpopulations based on their proximity to different cell regions (myofibrils, sarcolemma, and nuclei)4,5. Those mitochondria located right beneath the sarcolemma are called subsarcolemmal mitochondria (SSM), those located between the contractile filaments are called intermyofibrillar mitochondria (IMF), and the mitochondrial subpopulation around the nuclei are called perinuclear mitochondria network (PMN). Moreover, it has been suggested that these mitochondrial subpopulations have region-specific functions and are metabolically specialized4,5.
The maintenance of cellular energy homeostasis, which allows metabolic and contractile function, depends to a large extent on the interaction and communication on specific sites through the mitochondrial network (e.g., IMF and SSM interaction)4,6. In addition to mitochondria network interactions, the mitochondrion can also interact with other organelles, forming structural and functional complexes. In this regard, it has been shown that IMF can be located adjacent to the sarcoplasmic reticulum (SR) and in proximity to the Ca2+ release units (CRU), formed by the transverse tubules (TT)7. This fact is relevant due to the role of mitochondrial Ca2+ uptake in regulating ATP synthesis and apoptosis. Recently, a potential role in regulating cytosolic Ca2+ transients has also been suggested8.
TT are invaginations of sarcolemma that have a periodic distribution along the longitudinal axis of cardiomyocytes and skeletal muscle fibers9,10, similar to the IMF distribution5. Changes in the distribution of TT have important physiological implications, given their role in contractile function. However, these changes have been mainly evaluated in cardiomyocytes. Using Fast Fourier Transform (FFT) analysis allows the conversion of periodic signals from the distance domain to the frequency domain, resulting in an FFT spectrum that indicates the frequency and the regularity of the signal11,12,13. Although there is evidence that the organization of the mitochondrial network in skeletal muscle fibers is essential for adaptation to different metabolic conditions, as during regeneration after muscle injury14,15, most analyses are performed qualitatively.
Additionally, given that mitochondrial dysfunction has been associated with several skeletal muscle-related (e.g., disuse atrophy)2 and non-muscle diseases, particularly metabolic diseases, and the associated loss of muscle mass (i.e., atrophy)16, the quantitative evaluation of the mitochondrial network and distribution in skeletal muscle takes relevance. Recently, a significant difference in the longitudinal distribution of mitochondria of gastrocnemius muscle fibers between an obese group (Ob; Zucker fa/fa rats), and a lean group (Lean; Zucker +/+ rats) was identified through FFT17. This study demonstrated the usefulness of the FFT in analyzing the mitochondrial distribution. Therefore, this protocol presents a methodology to study mitochondria in live-skeletal muscle fibers from images obtained by fluorescence confocal microscopy. Mitochondrial density is quantified by background thresholding, and the analysis of longitudinal mitochondrial distribution by FFT analysis is also described. A workflow scheme is presented in Figure 1.
Protocol
All animal experimentation procedures were evaluated and approved by the Animal Use and Care Committee (CICUAL) of Tecnologico de Monterrey (Protocol 2019-007). Male Zucker (+/+ and fa/fa) rats aged 12 to 13 weeks were used for this study. Animals were kept in standard husbandry conditions (12 h/12 h light/dark cycle, 40-60% humidity) and had access to food (standard rat chow) and water ad libitum.
1. Solution composition
- Prepare fresh Relax solution by mixing the components shown in Table 1. Adjust pH to 7.3 using sodium hydroxide (NaOH).
- Calculate the free calcium concentration in the Relax solution using a simulation program for determining the free metal concentration.
- Prepare a 5 mM tetramethylrhodamine methyl ester (TMRE) stock in dimethyl sulfoxide (DMSO) and a subsequent dilution of 0.1 mM in DMSO.
2. Dissection of the gastrocnemius muscle fiber bundles
- Place the animal inside the induction chamber and close the lid. Turn the oxygen source on, set the gas flow rate at 0.5 L/min, and set the vaporizer at 4% sevoflurane to induce anesthesia.
- Once the rat is asleep, place it outside the chamber in a supine position while maintaining anesthesia. Pinch the toe or tail to verify the lack of reflexes.
- With scissors, cut through the abdomen's skin and muscles. Then, cut the thoracic cage to gain access to the heart.
- To perform a cardiectomy, grasp the heart from the topmost veins and arteries with forceps and cut the blood vessels with scissors. Remove the heart.
- Immediately after euthanasia, disinfect the hindlimb with ethanol and shave it using a shaving machine.
- Hold the hindfoot and make an incision with scissors through the skin at the level of the Achilles tendon.
- Cut the hindlimb with scissors at the level of the proximal tibia. Transfer it to a 60 mm Petri dish containing 3 mL of ice-cold Relax solution in a dorsal position. Cover it with sufficient solution to prevent the muscles from drying out.
- Identify the Achilles tendon, carefully raise it with forceps, and dissect the muscles away from the bone with iris scissors. Use a stereomicroscope from this step onward of the dissection.
- Identify and separate the whole gastrocnemius muscle, the main bulk at the back of the hindlimb.
- Dissect and discard the connective and fat tissue surrounding the muscle with fine-tip forceps. Transfer the lateral head of the muscle to a new Petri dish with ice-cold Relax solution (see Figure 2A).
- Gently hold the muscle with forceps from one end and carefully separate it into bundles with microscissors. Always manipulate bundles by gently holding them at one end with forceps.
NOTE: Bundles are approximately 10 mm long and 2 mm wide. - Transfer the fiber bundles to a new Petri dish with 2 mL of ice-cold Relax solution. Only select the ones that have a smooth appearance, are complete from one end to the other, and are not shortened (Figure 2B).
3. Live-cell imaging acquisition of mitochondria in skeletal muscle by confocal microscopy
- Incubate the fibers in 2.5 × 10-4 mM TMRE in Relax solution for 20 min at room temperature.
NOTE: With the recommended working concentrations of TMRE, it is expected to be in a non-quenching mode. Protect from light exposure from this point onwards. - During the incubation time:
- Open the confocal microscope standard software, select the configuration framework, and in the hardware configuration dialog box select laser and check HeNe 543 option.
- In the acquire framework, select the acquisition dialog box, and in the acquisition mode, select XYZ panel.
- In the XY dialog box, select the 512 x 512 format, 400 Hz speed, and check pinhole panel. In the displayed pinhole dialog box, select AU for unit and add a value of 3 Airy for the pinhole.
NOTE: Consider reducing the pinhole size closest to the 1 Airy optimal criterion, if an adequate fluorescence signal is conserved. - In the Beam Path Settings dialog box, select a water immersion objective of 20x and numerical aperture (NA) of 0.7 (20x/0.7 IMM), and select the emission wavelength window of 576-700 nm. Select DD488/543 and 15% of laser power for the 543 laser.
NOTE: A water immersion objective lens for live imaging scanning is strongly recommended (if available). Since a similar refractive index of the immersion medium and the incubation medium is achieved.
- After 20 min of incubation, exchange the incubation medium twice. Ensure that the confocal images are acquired within 20-30 min after incubation.
- Prepare the recording chamber with a 0.15 mm coverslip of borosilicate glass.
NOTE: Select the thickness of the coverslip according to the available recording chamber, considering that thickness usually ranges from 0.15 to 0.22 mm for optimal performance. - Add 200 µL of Relax solution to the recording chamber and transfer the fiber bundles.
- In the confocal microscope, use the brightfield mode to identify viable fibers for the fluorescent recording of mitochondria. Viable fibers are complete, not contracted, and have an intact striation pattern.
- Separate the fiber bundles from each other and align them with forceps. Select the ones that are closest to the coverslip.
- Scan the fluorescence using the live button to adjust gain and offset in the Control Panel console considering the following:
- Select low fluorescent intensity values for a background of nearly 0 Arbitrary Units (A.U.).
- Select a gain level between 100 and 200 A.U. to avoid saturation of the recording system. Hence, do not record the highest fluorescence levels.
- Select a pixel size of 150-190 nm to acquire the full width of the fiber, adjusting the zoom factor in the XY dialog box.
NOTE: Consider using the pixel size closer to the Nyquist criteria (90 nm), which allows scanning of the full width of the fiber.
- In the Z stack dialog box, adjust the Z distance to acquire the fluorescence signal starting at a fiber depth of 15 µm (begin button) up to 22 µm (end button). Select 3 µm as the Z-step size.
- Click the Start button to acquire the confocal images. Obtain a Z-stack composed of three confocal images acquired at 15, 18, and 21 µm of depth.
4. Analysis of mitochondrial density
- In the open-source platform for biological image analysis18 open the Z-stack file and rotate the images to place the fiber horizontally, as shown in Figure 3B.
- Randomly select a rectangular region of interest (ROI) that includes the area occupied by the mitochondria (ROImito). Select ROImito sizes ranging from 65 to 90 µm for X. For Y, the size will depend on the fiber width, avoiding its periphery.
- Duplicate the Z-stack with the selected ROImito and save it as a new Z-stack (Mito-stack) in TIFF format. Save the ROImito position selected on the original stack with the ROI Manager tool.
- Calculate the threshold for background subtraction as follows:
- Use the shortcut command+shift+t to open the Threshold dialog box.
- Select the threshold algorithm Otsu |B&W | Dark background option. Observe that the image is now binarized.
- In the Threshold dialog box, observe the histogram of the fluorescence intensity distribution and the threshold value that is displayed.
NOTE: Mitochondria-positive pixels have fluorescence intensity values greater than the threshold and appear as white pixels in the binary image. - Apply the threshold to the binary image stack by selecting Apply. In the displayed Convert Stack to Binary dialog box, select the options Calculate threshold for each image, Black background, Create new stack, and click OK.
- Observe that a stack with the three binary images is generated (BinDMito-stack). Save it in TIFF format.
- Calculate mitochondrial density in the BinDMito-stack as follows:
- Select the Analyze menu. Next, click Histogram. In the displayed histogram dialog box, click Yes to include all the images of the stack for analysis.
- In the Histogram of Stack dialog box, click List to obtain the histogram data. Transfer the histogram data to a spreadsheet.
- In a spreadsheet, identify Mito-pixels that are those with the 255 value. Calculate Mitochondrial density using equation (1):
Mitochondrial density =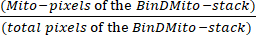 × 100 (1)
× 100 (1)
Total pixels are identified as N in the Histogram dialog box or calculated by summing in the spreadsheet the pixels count of the histogram. - To calculate the area occupied by mitochondria (µm2), multiply the Mito-pixels of the BinDMito-stack by the pixel size.
5. Analysis of mitochondrial distribution by Fast Fourier Transform
- In the open-source platform for biological image analysis, open the BinDMito-stack and draw a rectangular ROI of 256 pixels width and 5 µm height. Locate an ROI in a central position and another in a lateral position, as shown in Figure 4A,B.
- Use the ROI Manager tool to configure the ROIs for FFT analysis and save it.
NOTE: According to the necessities of the analysis, other ROIs can be selected in different positions, and their sizes can be modified. Nevertheless, the width must equal 2n pixels to perform FFT (e.g., 128, 256, 512 pixels). - Obtain the plot profile of the ROIs using the shortcut command + k and transfer the data to a spreadsheet.
- In the spreadsheet, fill B and C columns with the data obtained from the plot profile. The B column contains the distance in µm, and the C column the corresponding gray value of the fluorescence intensity (A.U.).
- The D column corresponds to the FFT frequency (event/µm), which will be filled as follows:
- Calculate the sampling frequency (Fs): Fs = 1/Δd, where Δd is the distance step (the second value of B).
- Calculate the ΔFs: ΔFs = Fs/N, where N is the number of data points of B (256).
- Calculate the stop value of FFT frequency (S): S = (N/2) ×ΔFs.
- Fill D column as follows:
- The first value of D column equals 0.
- Select the second cell in D column, go to the home menu, select fill, and click series. Select columns. For the step value, use the calculated Δ Fs. For the stop value, use the calculated S.
- Next, fill the E column with the FFT complex values as follows:
- Insert 0 in the first cell of the C column, because the distance 0 must coincide with a signal 0 for the calculation of the FFT.
- Then, go to the Data menu, click Data Analysis, and select Fourier Analysis.
- In the new window, select the range of data points of the fluorescent signal described in column C for the input range.
NOTE: The number of data points must be 2n (e.g., 256 or 128 data points of fluorescent signal). - Select the corresponding range of the E for the output range.
- Select Ok and let the FFT complex values be filled automatically.
- Fill F column with the FFT magnitude as follows:
- Use the IMABS function to return the absolute value in F from the complex number in E and multiply by 2/N to normalize (equation (2)):
FFT magnitude = (IM.ABS (E1…En) × (2/N) (2)
- Use the IMABS function to return the absolute value in F from the complex number in E and multiply by 2/N to normalize (equation (2)):
- Plot the FFT spectrum using the FFT magnitude in F, as a function of FFT frequency in D, until S. Find the point of the maximum peak and its corresponding FFT frequency.
- Convert the FFT frequency of the maximum peak to distance with equation (3). This calculated distance represents the longitudinal distance of the mitochondrial distribution.
Distance (µm) = 1/FFT frequency (3)
NOTE: Due to FFT symmetry, do not plot the FFT frequency beyond the S value, which corresponds to half of the data points, avoiding duplication of the FFT spectrum.
6. Optional preprocessing steps to reduce the image noise before image analysis
- Apply a median filter or a 2D deconvolution as follows:
- For median filter:
- Select Process | Filters and click on the Median option.
- In the displayed Median dialog box, select 2.0 for Radius and click OK. Choose Yes to apply the process to all images in the Mito-stack.
- Observe the reduction of noise in the images.
- For 2D deconvolution:
- Generate a theoretical Point Spread Function (PSF).
- Download the plugin PSF_Generator.jar and place the file into the "Plugins" folder.
- In the PSF generator dialog box, click the option Born & Wolf 3D optical model, enter the Refractive index immersion value of 1.33 used during confocal scanning, and select Best for the Accuracy computation option.
- Capture 576 nm for emission wavelength, the NA of the objective lens used of 0.7, and the Z-step of 3,000 nm.
- Capture the pixel size XY of the confocal image to be deconvoluted, as well as the size XYZ indicating the number of pixels of X and Y and the number of Z steps (3 for this protocol).
- In the Display menu of the PSF generator, click the option linear, select 8 bits resolution, and then select the grays option for the lookup table (LUT).
- Run the PSF generator and save the theoretical PSF created in TIFF format.
- Make a sum of slices of the PSF created by opening the Z Project tool of the option Stacks that is located in the Image menu.
- In the displayed ZProjection dialog box, select Sum slices for the Projection type, and click OK. Save the new PSF in TIFF format.
NOTE: A PSF obtained experimentally by confocal scanning fluorescent microbeads with a known diameter can be used instead of the theoretical PSF. - Download the plugin "DeconvolutionLab_2.jar"19 and place it into the plugin folder.
- Click the menu Plugins. Next, click DeconvolutionLab2.
- Separate the confocal images from the Mito-stack using the tool Images to stack from the Stacks option located in the Image menu and save it in TIFF format.
- In the DeconvolutionLab2 dialog box, select one of the images obtained from the Mito-stack. Select the theoretical PSF created, and select the algorithm Richardson-Lucy with 15 iterations.
- Run DeconvolutionLab2, verify signal improvement and noise reduction in the image, and save it in TIFF format.
- For median filter:
- Continue with step 4.4 for image analysis.
- For the thresholding of the deconvoluted images, convert them to an 8-bit mask during the thresholding process. Create a stack with the binary and deconvoluted images by selecting Images to Stack in the Stacks tool of the Image menu.
- For the FFT analysis of deconvoluted images, the plot profile contains distance in pixel units. Transform the pixel units to µm as follows:
- In the spreadsheet, after completing step 5.4 transfer the data from B to A. Next, fill B with the distance in µm by multiplying each pixel number from A by the pixel size.
Representative Results
Following the present protocol, the analysis of the density and distribution of mitochondria can be achieved in live-skeletal muscle. The protocol is divided into three main stages: Skeletal muscle bundle dissection, confocal microscopy scanning, and image analysis. The workflow overview is presented in Figure 1. Figure 2A shows a whole rat gastrocnemius muscle in a Petri dish, marking the lateral head from which the fibers are obtained, while Figure 2B shows the fiber bundles in Relax solution. By confocal microscopy, the mitochondria can be recorded along the depth of live-skeletal muscle fiber using the fluorescent indicator TMRE. TMRE is a lipophilic cationic fluorophore that is selectively accumulated within mitochondria according to the mitochondrial membrane potential20.
An optimal confocal image of IMF can be obtained by selecting a Z distance above 15 µm of depth within the fiber (Figure 3A). Figure 3B shows representative XY confocal images of mitochondria loaded with TMRE acquired along gastrocnemius muscle fibers' Z distance (15 to 21 µm). The confocal images were processed by thresholding to transform them into binary images to allow mitochondrial analysis. Figure 3B (left panel) shows a fiber from an exercised Lean rat. It represents an expected confocal record of skeletal muscle fiber mitochondria since it has a consistent pattern along the fiber. In contrast, we selected a fiber from an Ob rat (Figure 3B, right panel) that shows substantial alterations in mitochondrial content and distribution. Figure 3C shows the quantification of the fiber area occupied by the mitochondria expressed as mitochondrial density, obtained from each binarized image (shown in panel B). As expected, the Ob fiber presented a lower mitochondrial density. It was quantified consistently along the Z distance analyzed, which is also observed in Figure 3D when the mitochondrial density is calculated per stack conformed by the three confocal images acquired at 15, 18, and 21 µm.
Similar to the mitochondrial density analysis, the confocal scanning of a fluorescent indicator for live-cell imaging, such as TMRE, allows for studying the longitudinal organization of mitochondria in live-skeletal muscle. IMF presents a periodic organization in the I-band close to TT7, which can be analyzed by FFT to quantify the frequency of the mitochondrial signal and the level of organization17. Figure 4 shows the differences in the organization of IMF found in gastrocnemius derived from Lean and Ob rats and how FFT allows finding the changes in the distribution of the mitochondrial signal. Figure 4A,B exhibit longitudinal ROIs selected in a central and lateral position in the fiber for FFT analysis. Before performing FFT, the thresholding for background subtraction is calculated. Then, the image is binarized; these procedures eliminate the variations in fluorescence intensity levels of the mitochondrial signal. The binary image provides the plot profile of fluorescence distribution necessary to perform the FFT.
Figure 4C,D show the selected ROIs in panels A and B with their plot profiles (upper panels). From the plot profiles, differences in the fluorescence distribution between the fibers derived from Lean and Ob rats can be observed, as well as the variations between ROIs within the same fiber. For every plot profile, their respective FFT spectrum is presented (lower panels). The peak of the maximum FFT spectrum indicates the FFT frequency (X-axis) of the mitochondrial signal distribution along the longitudinal axis. It can be transformed into a distance value close to 2 µm in the lateral and central ROIs from the Lean rat. Notably, the FFT magnitude of the peak is an index of the regularity of the mitochondrial signal, and changes in this amplitude reveal alterations in the mitochondrial distribution.
Figure 4E,F show the differences in the FFT spectrum between Lean and Ob-derived fibers where lateral and central ROIs were analyzed, respectively. In lateral ROIs (Figure 4E) the frequency of mitochondrial longitudinal distribution was similar in the lean and Ob-derived fibers; nonetheless, the amplitude of the maximum FFT peak in the Ob-derived fibers was higher, which is in agreement with the higher regularity of the signal observed in the image in Figure 4B. Nevertheless, the central ROI (Figure 4F) of the Ob is observed as an example of a critical reduction of the FFT peak compared to Lean when an important alteration of the mitochondrial distribution is present.
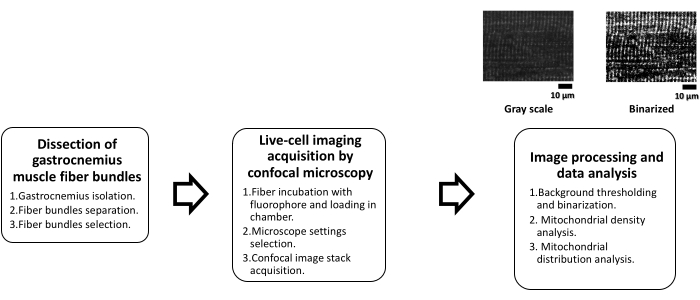
Figure 1: Scheme for mitochondrial analysis in skeletal muscle by confocal microscopy. This scheme summarizes the protocol's main steps in three separate phases. The first phase, dissection of gastrocnemius muscle fiber bundles, is subdivided into three subsequent steps, gastrocnemius muscle isolation, followed by muscle mechanical dissection into bundles to finally make a visual selection of the viable ones. The second phase consists of live-cell imaging acquisition by confocal microscopy, which consists of incubation with the fluorophore (TMRE) for 20 min at room temperature to place the fibers in the chamber. Afterward, the appropriate settings are made in the microscope to conduct the acquisition of the confocal images. During the third phase, confocal image processing and data analysis are conducted. Starting with image processing, where a threshold is required to generate binary images from which mitochondrial density calculations and mitochondrial distribution by FFT are done. Abbreviations: TMRE = tetramethylrhodamine ethyl ester; FFT = Fast Fourier Transform. Please click here to view a larger version of this figure.
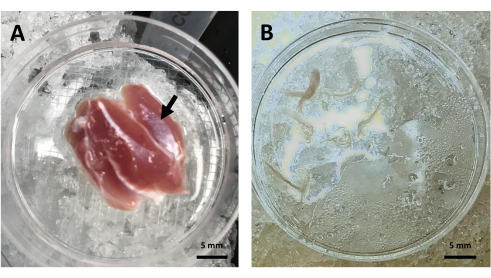
Figure 2: Skeletal muscle bundle dissection. (A) Dissected rat lateral gastrocnemius head (black arrow) in a Petri dish with Relax solution. (B) Representative image of gastrocnemius muscle fiber bundles in Relax solution before TMRE loading. Abbreviation: TMRE = tetramethylrhodamine ethyl ester. Please click here to view a larger version of this figure.
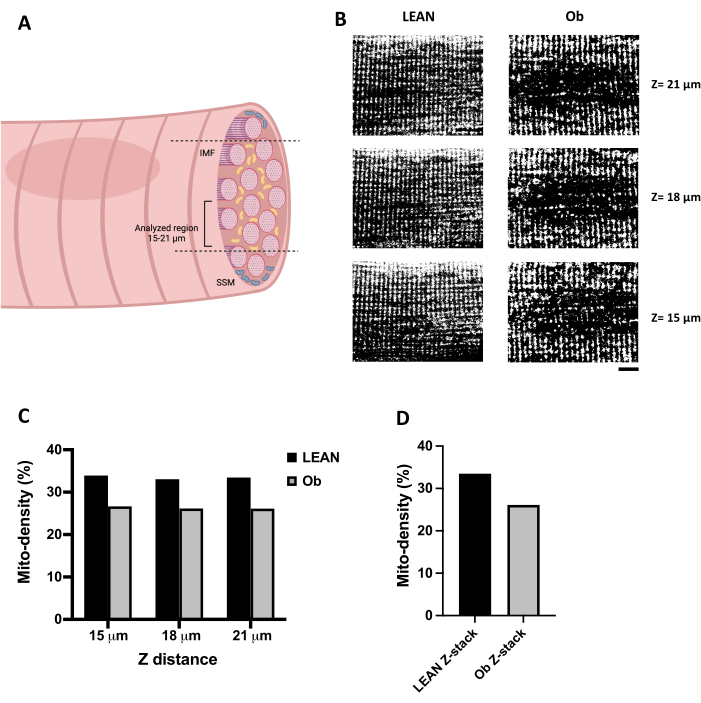
Figure 3: Mitochondrial density analysis. (A) Illustration that represents the Z distance recommended for confocal scanning of IMF mitochondria in skeletal muscle fibers. (B) Series of confocal images of mitochondria loaded with TMRE in skeletal muscle fibers, obtained from an exercised Zucker +/+ rat (Lean) and from an obese Zucker fa/fa rat (Ob) recorded in the Z distance (from 15 to 21 µm of depth). The images were processed by thresholding and transformed into binary images. (C) Calculated mito-density of the confocal slices obtained at different Z distances observed in panel B. (D) The mito-density obtained from the stack composed by the images observed in panel B. Images in panel B are 65 x 50 µm. Scale bar = 10 µm. Abbreviations: Mito-density = Mitochondrial density; IMF = intermyofibrillar mitochondria; Ob = Obese; SSM = subsarcolemmal mitochondria. Please click here to view a larger version of this figure.
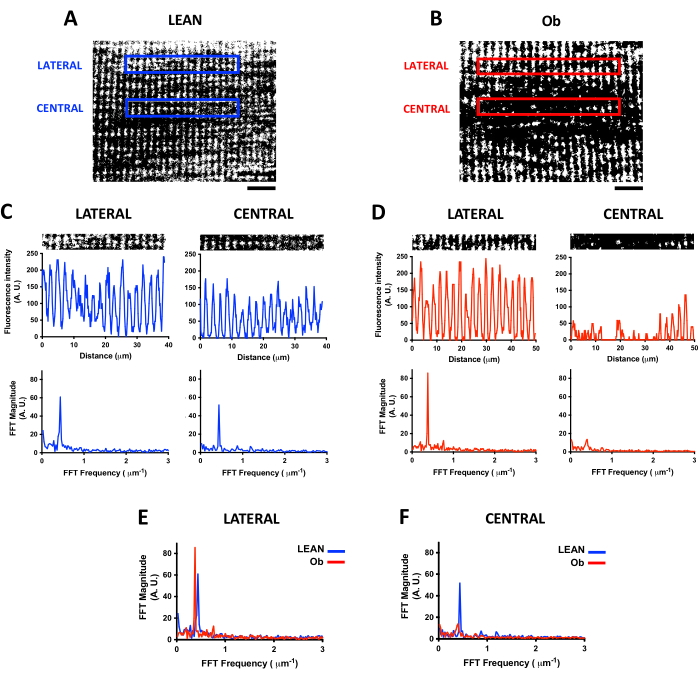
Figure 4: Mitochondrial distribution analysis by FFT. Representative confocal images of mitochondria loaded with the fluorescent indicator TMRE acquired at 21 µm of the depth of skeletal muscle fibers obtained from an exercised Zucker +/+ rat (Lean, panel A) and from an obese Zucker fa/fa rat (Ob, panel B). The images were processed by thresholding and transformed into binary images. Lateral and central ROIs from panel A (panel C) and panel B (panel D) with their respective plot profiles of fluorescence intensity (upper plot) and FFT spectrum (lower plot). FFT was calculated from ROIs with 256 pixels of width, which corresponds to an ROI size of 39 x 5 µm in panel A and an ROI size of 50 x 5 µm in panel B. Panel E shows the differences of the FFT spectrum found between mitochondria derived from Lean and Ob rats in the lateral ROI, while panel F shows the differences of the FFT spectrum of central ROI. Scale bar = 10 µm. Abbreviations: A.U. = Arbitrary Units; FFT = Fast Fourier Transform; ROIs = regions of interest; Ob = Obese. Please click here to view a larger version of this figure.
| Reagent | Final Concentration in 100 mL | Stock |
| K-aspartate | 100 mM | |
| KCl | 20 mM | |
| HEPES | 20 mM | |
| L-glutamic acid | 3 mM | |
| Malic acid | 3 mM | |
| EGTA | 0.1 mM | 10 mM |
| MgCl2 | 1 mM free Mg2+ | |
| CaCl2 | 0.00002 mM free Ca2+ | |
| Phosphocreatine di-Na | 5 mM | 500 mM |
| Creatine phosphokinase | 5 U/mL | 200 U/mL |
| MgATP | 5 mM | |
| pH 7.3 (with NaOH) |
Table 1: Relax solution reagents and concentration.
Supplementary Figure S1: Effect of image preprocessing methods. (A) Representative confocal images of mitochondria loaded with the fluorescent indicator TMRE acquired at a depth of 18 µm in skeletal muscle fibers obtained from an exercised Zucker +/+ rat (Lean, upper panel) and from an obese Zucker fa/fa rat (lower panel), along with their respective images obtained after preprocessing using Otsu´ thresholding, median filter and Otsu´ thresholding, and 2D deconvolution and Otsu´ thresholding. (B) Plot profile of mito-density obtained from the confocal images (panel A) after preprocessing with different methods. Images in panel A are 65 x 50 µm. Scale bar = 10 µm. Abbreviations: 2D-Decon = 2D deconvolution; Mito-density = Mitochondrial density. Please click here to download this File.
Discussion
Mitochondria are organelles with a high remodeling capacity. Their content, density, and distribution can be rapidly modified through the activation of the mitochondrial fusion and fission mechanisms, known as mitochondrial dynamics1, and the balance between the mitochondrial turnover mechanisms: the mitochondrial biogenesis and the specialized mitochondria degradation pathway, mitophagy21,22. Mitochondrial content and morphology can vary according to cell type and stage of development and can be remodeled under different physiological and pathological stimuli17,22,23,24. Therefore, the study of mitochondrial morphology has been relevant for over half a century25. Notably, the analysis of mitochondria through electron microscopy has been the standard technique applied in multiple studies26.
Fluorescent studies by confocal microscopy have gained relevance in the last few years due to their capacity for live-cell imaging of mitochondria at different fiber depths, which could help better understand the role of mitochondria in skeletal muscle in different adaptive and maladaptive conditions27. In this study, a methodology for the analysis of mitochondria density and distribution in live-skeletal muscle fibers by confocal microscopy is described. One of the main challenges of working with live-skeletal muscle fibers is to avoid contraction from the isolation processes up to the mitochondrial confocal recording. To achieve this goal, a high Mg and ATP Relax solution17 is used to keep the fibers relaxed for at least 2 h, which gives enough time to carry out the fiber isolation process, the fluorophore load, and the acquisition of mitochondrial signal by confocal microscopy. A critical point of the protocol is obtaining the fibers mechanically since it requires high precision and fresh tissue; however, it is possible to obtain viable fiber bundles from rat muscle with this previously used and reported technique28. Obtaining intact fibers allows for preserving the sarcolemma and the intracellular environment, keeping the metabolic and functional crosstalk between cell structures28,29.
Unlike working with tissues or fixed cells, the acquisition of live-cell imaging fluorescent images by confocal microscopy allows real-time monitoring of the effect of diverse experimental conditions. The present protocol can be used to explore changes in mitochondrial density and distribution in real-time and explore differences between experimental groups, such as the examples presented here between Lean and Ob-derived fibers (Figure 3 and Figure 4). It should always be considered that live-cell imaging implies standardizing optimal working conditions with minor cell damage. The working time, the quality of the solutions used, the acquisition parameters, and the exposure of lasers must be finely controlled. Hence, essential considerations are mentioned below.
Mitochondria of muscle fibers cannot be recorded entirely longitudinally by confocal microscopy due to the size of the fiber and the damage of the fiber that can be caused by long laser exposure. Nevertheless, a representative sample of the fiber is recorded under this technique. Although it is possible to record the entire thickness of the skeletal muscle fiber of a rat by confocal microscopy, this implies a longer recording time and exposure to the laser beam. In the case of control rats, issues with these recordings have not been encountered. However, fibers from pathological conditions may be more susceptible to damage as observed in the fibers from Ob rats. Consequently, acquiring a stack of representative confocal images obtained at different Z distances is preferred. When only a section of fiber thickness is recorded, it is recommended to take the stack at the same depth on all fibers tested since mitochondrial distribution and density can vary according to its position within the fiber. Acquisition of the signal at a depth above 15 µm is recommended to obtain representative confocal images of IMF, avoiding SSM populations that are situated close to the periphery.
During the confocal acquisition, some important considerations must be taken into account. First, the selection of the immersion objective lens considering the magnification, high NA, and immersion medium. Since cells are maintained in a hydrophilic incubation medium, the refractive index of the incubation and immersion medium must be similar to obtain a good signal and scan deep into the tissue. Usually achieved using a water immersion objective lens. Confocal images of Figure 3 and Figure 4 were acquired with a 20x, 0.7 NA, water immersion objective. This objective allows the record of the fiber in all its depth, but scanning at 15, 18, and 21 µm was decided since representative confocal images of IMF can be obtained with high fluorescence intensity signal and minor fiber damage. Other magnification, such as 40x and oil as an immersion medium, can be considered but needs to be evaluated.
Second, the pixel size for imaging acquisition is calculated according to the Nyquist theorem, which allows the selection of an appropriate pixel size that avoids over-sample (higher laser exposure) and under-sample (leads to less resolution)30. The calculation depends on the characteristics of the objective lens selected and the wavelength (~90 nm). It can be adjusted with the zoom; therefore, only one zoom setting provides an optimal pixel size30. Nevertheless, in practice, the zoom also depends on the area of the specimen to be analyzed. Thus, finding balance allows working with a pixel size that is closest to the Nyquist criterion and that also fits the area to be analyzed. Figure 3 and Figure 4 were acquired with a pixel size of 150 and 190 nm, which allowed for analysis of the full width of the fiber which is ~50-80 µm.
Third, an appropriate pinhole diameter that prevents out-of-focus light from reaching the detector should be used. Typically, 1 Airy is considered the optimal pinhole size since it allows the detection of ~80% of photons originating from the plane of focus30. Nevertheless, some stained biological samples that show low fluorescence levels require a pinhole increase30. Confocal images of Figure 3 and Figure 4 were acquired with a pinhole size of 3 Airy due to a low signal captured with a lower Airy. It is important to consider that the rise in the signal intensity resulting from increasing the pinhole size leads to the reduction of the resolution due to increased out-of-focus captured light. For this reason, we recommended using a pinhole size as close to 1 airy as possible.
When adequately acquired, confocal images can be processed to obtain quantitative information on mitochondrial density and distribution. Regardless, the critical processing image step of thresholding needs to be performed before analysis to improve the quantification of the signal. During this crucial step, the fluorescence intensity value that separates the positive pixels for mitochondria from those of the background is defined. The threshold can be defined by a Gaussian fit of the peak representing mitochondria when the histogram of the image displays two peaks, one corresponding to the background and the other to the mitochondria. However, a bimodal distribution is not always achieved in each of the images, so other thresholding methods have to be applied.
In this protocol, the implementation of Otsu's thresholding is described, which is a non-parametric and unsupervised method designed to find the threshold value when the two peaks are not separated, or other peaks are present31. Otsu can easily be applied using an open-source platform for biological-image analysis; however, other thresholding methods can be tested. The same thresholding method must be applied to all the confocal images and has to be calculated independently for each confocal image. Applying the threshold to a whole stack leads to incorrect results. Once the binary images are obtained after the thresholding process, the analysis of mitochondrial density and FFT can be easily carried out by following the instructions described in this protocol. However, care should be taken when performing both analyses to avoid including nuclei and capillaries, as it would lead to quantification errors. Regarding density, it is enough to subtract the pixels, or the area occupied by the nuclei or capillaries, from the pixels or total area to be analyzed. In addition, when performing the FFT analysis, it must be verified that the mitochondria signal is straight. Conversely, when the mitochondria signal is tilted, it can produce profiles that do not represent the mitochondrial longitudinal distribution, yielding incorrect FFT spectrum data. In addition, a preprocessing step can be applied to reduce the noise in the images. This protocol describes two optional preprocessing steps using a median filter and 2D deconvolution. The effects of these preprocessing methods on the image and mitochondrial density content are presented in Supplementary Figure S1. It is important to consider that while these preprocesses can improve the image quality, they can also result in the loss of certain image details. Therefore, they should be used with caution and consistently applied to all the images being analyzed.
Despite its advantages, confocal microscopy is limited by a lateral resolution (XY) of 180-250 nm when the optimal conditions for acquisition are implemented32. Mitochondrial diameter is ~200-700 nm, close to the diffraction limit of confocal microscopy; thus, sub-mitochondrial structures cannot be adequately detected33 and cannot be evaluated by density and FFT analyses shown in this protocol. Other super-resolution techniques of microscopy, such as stochastic optical reconstruction microscopy (STORM), stimulated emission depletion (STED) nanoscopy, or structured illumination microscopy (SIM), can be explored to resolve sub-mitochondrial structures32. In this protocol, the confocal images of mitochondria are obtained using the fluorophore TMRE, which depends on the mitochondrial membrane potential. Therefore, mitochondrial fluorescent intensity can vary according to their membrane potential. A thresholding process is performed before the data analysis to overcome this issue. All the pixels above a defined threshold are considered positive for mitochondrial signal independent of their fluorescence value. Nevertheless, it must be noted that mitochondria with a very low membrane potential cannot be resolved with this technique. Thus, complementary studies of mitochondrial protein content quantification are recommended. An advantage of using TMRE is that confocal images can also be used for mitochondrial membrane potential analysis, but adequate controls with uncoupling agents need to be performed such as carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP). In addition, the instructions for mitochondrial density and distribution analysis can be achieved using green fluorescent indicators for mitochondria, which load mitochondria regardless of their membrane potential, but incubation strategy and confocal acquisition settings need to be standardized.
Given that mitochondria structure is related to essential mitochondrial and cellular functions, the protocol described here can provide valuable information about their remodeling during disease or by a particular stress insult. It could contribute to a better understanding of key functions of skeletal muscle governed by mitochondria, such as energy production, or in which mitochondria play an important role in interacting with other organelles, such as contraction-metabolism coupling. Following the protocol instructions allows the estimation of mitochondrial density and distribution in live-skeletal muscle. The protocol steps are divided into three main stages focused on skeletal muscle bundle dissection, confocal microscopy scanning, and image analysis, where detailed instructions and important considerations are included. Notably, the protocol can be further optimized to explore additional Z steps for full mitochondrial reconstruction within the fiber according to the user's necessities. For instance, the confocal image and analysis steps can be tested to study cellular structures with similar distribution, such as TT in live and fixed samples.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the School of Medicine and The Institute for Obesity Research of Tecnologico de Monterrey. Figure 3A was created with Scientific Image and Illustration software.
Materials
| Adenosine 5’-triphosphate disodium salt hydrate | Sigma-Aldrich | A6419 | |
| Borosilicate glass coverslip | Warner Instruments | 64-0709 | |
| Calcium chloride | Sigma-Aldrich | C5670 | |
| Confocal microscope | Leica | TCS SP5 | |
| Confocal microscope software Leica Application Suite | Leica | 2.7.3.9723 | |
| Creatine Phosphokinase | Sigma-Aldrich | C3755 | |
| DeconvolutionLab2 (DeconvolutionLab_2.jar) | Biomedical Imaging Group, EPFL | http://bigwww.epfl.ch/deconvolution/deconvolutionlab2/ | |
| Dimethyl Sulfoxide | Sigma-Aldrich | D2650 | |
| DL-Aspartic acid potassium sat hemihydrate | Sigma-Aldrich | 11240 | |
| Ethylene glycol-bis(2-aminoethylether)-N,N,N´N´-tetraacetic acid | Sigma-Aldrich | E4378 | |
| Forceps | Miltex | MH-18 | |
| HC PL APO 20x/ 0.7 IMM objective | Leica | 506517 | |
| HEPES | Sigma-Aldrich | H3375 | |
| Iris scissors | Miltex | 5-304 | |
| L-(-)-Malic acid | Sigma-Aldrich | M7397 | |
| L-glutamic acid monosodium salt hydrate | Sigma-Aldrich | G1626 | |
| Magnesium chloride hexahydrate | Sigma-Aldrich | M2393 | |
| Maxchelator | UC Davis Health | https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/downloads.htm | |
| Micro scissors | Miltex | 18-1633 | |
| Open-source platform for biological-image analysis Fiji | Public, maintained by Eliceiri/LOCI group, Jug group, and Tomancak lab.Fiji | https://fiji.sc/ | |
| Phosphocreatine disodium salt hydrate | Sigma-Aldrich | P7936 | |
| Potassium chloride | Sigma-Aldrich | P9333 | |
| PSF Generator (PSF_Generator.jar) | Biomedical Imaging Group, EPFL | http://bigwww.epfl.ch/algorithms/psfgenerator/ | |
| Recording chamber | Warner Instruments | RC-27N | |
| Sodium hydroxide | Sigma-Aldrich | S5881 | |
| Spreadsheet Microsoft Excel | Microsoft | ||
| Stereo microscope | Zeiss | Stemi 508 | |
| Tetramethylrhodamine, ethyl ester | Invitrogen | T669 |
References
- Lackner, L. L. Shaping the dynamic mitochondrial network. BMC Biology. 12, 35 (2014).
- De Mario, A., Gherardi, G., Rizzuto, R., Mammucari, C. Skeletal muscle mitochondria in health and disease. Cell Calcium. 94, 102357 (2021).
- Chang, C. R., Blackstone, C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Annals of the New York Academy of Sciences. 1201, 34-39 (2010).
- Willingham, T. B., Ajayi, P. T., Glancy, B. Subcellular specialization of mitochondrial form and function in skeletal muscle cells. Frontiers in Cell and Developmental Biology. 9, 757305 (2021).
- Kuznetsov, A. V., et al. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role. Biochimica et Biophysica Acta. 1757 (5-6), 686-691 (2006).
- Díaz-Vegas, A. R., et al. Mitochondrial calcium increase induced by RyR1 and IP3R channel activation after membrane depolarization regulates skeletal muscle metabolism. Frontiers in Physiology. 9, 791 (2018).
- Boncompagni, S., et al. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Molecular Biology of the Cell. 20 (3), 1058-1067 (2009).
- Reggiani, C., Marcucci, L. A controversial issue: Can mitochondria modulate cytosolic calcium and contraction of skeletal muscle fibers. The Journal of General Physiology. 154 (9), e202213167 (2022).
- Heinzel, F. R., et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circulation Research. 102 (3), 338-346 (2008).
- Al-Qusairi, L., Laporte, J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skeletal Muscle. 1 (1), 26 (2011).
- Pérez-Treviño, P., Pérez-Treviño, J., Borja-Villa, C., García, N., Altamirano, J. Changes in T-tubules and sarcoplasmic reticulum in ventricular myocytes in early cardiac hypertrophy in a pressure overload rat model. Cellular Physiology and Biochemistry. 37 (4), 1329-1344 (2015).
- Celestino-Montes, A., Pérez-Treviño, P., Sandoval-Herrera, M. D., Gómez-Víquez, N. L., Altamirano, J. Relative role of T-tubules disruption and decreased SERCA2 on contractile dynamics of isolated rat ventricular myocytes. Life Sciences. 264, 118700 (2021).
- Song, L. S., et al. Orphaned ryanodine receptors in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 103 (11), 4305-4310 (2006).
- Houzelle, A., et al. Human skeletal muscle mitochondrial dynamics in relation to oxidative capacity and insulin sensitivity. Diabetologia. 64 (2), 424-436 (2021).
- Call, J. A., et al. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. American Journal of Physiology. Cell Physiology. 312 (6), C724-C732 (2017).
- Fealy, C. E., Grevendonk, L., Hoeks, J., Hesselink, M. K. C. Skeletal muscle mitochondrial network dynamics in metabolic disorders and aging. Trends in Molecular Medicine. 27 (11), 1033-1044 (2021).
- Rivera-Alvarez, I., et al. A single session of physical activity restores the mitochondrial organization disrupted by obesity in skeletal muscle fibers. Life Sciences. 256, 117965 (2020).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2022).
- Sage, D., et al. DeconvolutionLab2: An open-source software for deconvolution microscopy. Methods. 115, 28-41 (2017).
- Perry, S. W., Norman, J. P., Barbieri, J., Brown, E. B., Gelbard, H. A. Mitochondrial membrane potential probes, and the proton gradient: a practical usage guide. Biotechniques. 50 (2), 98-115 (2011).
- Ma, K., et al. Mitophagy, mitochondrial homeostasis, and cell fate. Frontiers in Cell and Developmental Biology. 8, 467 (2020).
- Meinild, L. A. -. K., et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiologica. 222 (1), (2018).
- Memme, J. M., Erlich, A. T., Phukan, G., Hood, D. A. Exercise and mitochondrial health. Journal of Physiology. 599 (3), 803-817 (2021).
- Gan, Z., Fu, T., Kelly, D. P., Vega, R. B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Research. 28 (10), 969-980 (2018).
- Schiaffino, S., Hanzlíková, V., Pierobon, S. Relations between structure and function in rat skeletal muscle fibers. Journal of Cell Biology. 47 (1), 107-119 (1970).
- Vincent, A. E., et al. The spectrum of mitochondrial ultrastructural defects in mitochondria myopathy. Scientific Reports. 6, 30610 (2016).
- Mishra, P., Varuzhanyan, G., Pham, A. H., Chan, D. C. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metabolism. 22 (6), 1033-1044 (2015).
- Cheng, A. J., Westerblad, H. Mechanical isolation, and measurement of force and myoplasmic free [Ca2+] in fully intact single skeletal muscle fibers. Nature Protocols. 12 (9), 1763-1776 (2017).
- Kuznetsov, A. V., Javadov, S., Margreiter, R., Hagenbuchner, J., Ausserlechner, M. J. Analysis of mitochondrial function, structure, and intracellular organization in situ in cardiomyocytes and skeletal Muscles. International Journal of Molecular Sciences. 23 (4), 2252 (2022).
- Pawley, J. B. Fundamental limits in confocal microscopy. In: Pawley, J. (eds) Handbook of Biological Confocal Microscopy. , (2006).
- Otsu, N. A. Threshold selection method from gray-level histogram. IEEE Transactions on System Man Cybernetics. 9, 62-66 (1979).
- Schermelleh, L., Heintzmann, R., Leonhardt, H. A guide to super-resolution fluorescence microscopy. The Journal of Cell Biology. 190 (2), 165-175 (2010).
- Jakobs, S., Stephan, T., Ilgen, P., Brüser, C. Light microscopy of mitochondria at the nanoscale. Annual Review of Biophysics. 49, 289-308 (2020).

