Base Recording: A Technique for Analyzing Responses of Taste Neurons in Drosophila
Summary
A rarely used method of electrophysiological recording, base recording, allows analysis of features of taste coding that cannot be examined by conventional recording methods. Base recording also allows the analysis of taste responses to hydrophobic stimuli that cannot be studied using traditional electrophysiological methods.
Abstract
Insects taste the external world through taste hairs, or sensilla, that have pores at their tips. When a sensillum comes into contact with a potential food source, compounds from the food source enter through the pore and activate neurons within. For over 50 years, these responses have been recorded using a technique called tip recording. However, this method has major limitations, including the inability to measure neural activity before or after stimulus contact and the requirement for tastants to be soluble in aqueous solutions. We describe here a technique that we call base recording, which overcomes these limitations. Base recording allows the measurement of taste neuron activity before, during, and after the stimulus. Thus, it allows extensive analysis of OFF responses that occur after a taste stimulus. It can be used to study hydrophobic compounds such as long-chain pheromones that have very low solubility in water. In summary, base recording offers the advantages of single-sensillum electrophysiology as a means of measuring neuronal activity – high spatial and temporal resolution, without the need for genetic tools – and overcomes key limitations of the traditional tip recording technique.
Introduction
Insects, including drosophilid flies, are endowed with a sophisticated taste system that enables them to extract complex chemical information from their surroundings. This system allows them to discern the chemical composition of various substances, distinguishing between those that are nutritious and those that are harmful1,2.
At the core of this system are specialized structures known as taste hairs or sensilla, strategically located on various body parts. In drosophilid flies, these sensilla are located on the labellum, which is the major taste organ of the fly head1,2,3,4, as well as on the legs and wings1,2,5,6. The labellum is located at the tip of the proboscis and contains two lobes4,7,8. Each lobe is covered with 31 taste sensilla categorized as short, long, and intermediate4,7,8. These sensilla each house 2-4 taste neurons1,2,9,10. These taste neurons express members of at least four different gene families, namely the Gustatory receptor (Gr), Ionotropic receptor (Ir), Pickpocket (Ppk), and Transient receptor potential (Trp) genes1,2,11,12,13. This diversity of receptors and channels equips insects with the ability to recognize a wide array of chemical compounds, including both nonvolatile and volatile cues1,2,14.
For over 50 years, scientists have quantified the response of taste neurons and their receptors using a technique called tip recording3,4,6,8,13,15,16,17,18,19,20,21,22,23,24,25,26,27,28,
29,30,31,32,33,34,35. However, this method has major limitations. First, neural activity can be measured only during contact with the stimulus, and not before or after contact. This limitation precludes the measurement of spontaneous spiking activity and prevents the measurement of OFF responses. Second, only tastants that are soluble in aqueous solutions can be tested.
These limitations can be overcome by a rarely used alternative electrophysiological technique called "base recording." Here we describe this technique, which we have adapted from a method used by Marion-Poll and colleagues24, and show the crucial taste coding features that it can now conveniently measure14.
Protocol
The following protocol complies with all the animal care guidelines of Yale University.
1. Flies
- Place 10-15 newly emerged flies in fresh standard culture vials at 25 °C and 60% relative humidity in a 12:12 h light-dark cycle.
- Use flies when 3-7 days old.
2. Chemosensory stimuli
- Obtain chemosensory stimuli of the highest available purity. Store them as recommended by the vendor until use.
- Dissolve chemosensory stimuli and dilute to the desired concentrations in water or another desired, non-toxic solvent such as paraffin oil. Stir the prepared solutions for a minimum of 1 h in the case of dissolved solid compounds.
3. Glass stimulus capillary
- Pull a glass capillary to hold the stimulus from a borosilicate glass capillary (100 mm length, 1 mm outer diameter, 0.58 mm inner diameter) using a pipette puller instrument. Aim to achieve a tip diameter between 3 µm and 10 µm.
- Fill the glass capillary with the preferred stimulus solution using a microloader pipette tip. Take care to avoid bubbles, which can be removed by gentle tapping.
- If the stimulus crystallizes at the tip, clean or replace the glass stimulus capillary.
4. Reference and recording electrodes
- Use tungsten rods (127 µm diameter and 76.2 mm length) for both reference and recording electrodes. Sharpen the reference and recording electrodes to approximately 1 µm diameter at the tip (the shapes of these electrodes are depicted in Delventhal et al.36) by dipping them repeatedly for several seconds in either a 10% KNO3 (~1 M) solution or a 10% KOH (1.8 M) solution.
NOTE: This solution requires current (0.3-3 mA) to facilitate this process.
5. Preparation of the fly for base recording
- Draw a single fly from the vial into an aspirator. Withdraw the aspirator and trap the animal by placing a finger over the end.
- Expel the fly into a 200 µL plastic pipette tip. Keeping the end of the aspirator in the pipette tip, use the end to push the fly forward, headfirst, toward the narrow end of the pipette tip.
- Trim the pipette tip at each end (i.e., anterior and posterior to the animal) using a razor blade.
- Use either clay or a small piece of cotton to push the fly forward further, until half of the head protrudes from the end of the trimmed pipette tip. Use forceps to gently push until the labellum at the front of the head is exposed.
- Fix the trimmed pipette tip onto a glass microscope slide using clay (Figure 1).
- Under the stereomicroscope, position the labellum laterally on a cover slip so that one lobe, along with its 31 taste sensilla, is exposed (Figure 1). The cover slip keeps the labellum in place.
6. Electrophysiology rig
- Select a room for the rig setup that has stable temperature and relative humidity (<70%) and is isolated from sources of electrical and mechanical noise, such as refrigerators and centrifuges.
- Place the microscope on the center of an antivibration table.
- Secure a manual micromanipulator to the antivibration table (Figure 2).
- Attach a stainless-steel shaft that holds the tungsten reference electrode to the manual micromanipulator (Figure 2).
- Connect motorized manipulators-one with a holder for the recording electrode probe and a second one with a holder connected to a stainless-steel shaft for the glass stimulus capillary-to the same table using stands (Figure 2).
- Connect the recording electrode probe to an Intelligent Data Acquisition Controller (IDAC) system or another amplifier/digitizer system.
- Link this IDAC system to the computer at the workstation.
- Ground the manual and motorized manipulators to the same location within the rig.
- Install appropriate acquisition software for the IDAC system on the computer. Ensure that the digital acquisition drivers are compatible with the operating system (e.g., Windows XP-7, -8, or -10) on the computer.
7. Recording from taste sensilla
- Place the preparation slide on the microscope stage with a low magnification (e.g., 10x) objective in position. Move the stage until the labellum is in focus at the center of the field of view at both low-magnification and high-magnification (e.g., 50x) objectives.
- Insert the reference electrode into the eye using the low magnification objective. To insert the reference electrode, target the eye on the side of the fly opposite the side with the recording electrode, for example, if the recording electrode is approaching from the right, place the reference electrode in the left eye. Utilize a manual micromanipulator for precise insertion.
- Bring the tip of the glass stimulus capillary into focus at the center of the field of view of both low-magnification and high-magnification objectives using a motorized micromanipulator (Figure 3).
- Under low magnification, bring the recording electrode close to the labellum using a second motorized micromanipulator.
- Under high magnification, insert the recording electrode into the base of a taste sensillum using the motorized micromanipulator until the sound of neuronal firing activity from the audio output of the IDAC system is heard.
- Once a stable signal has been established, start recording the signal using the software that comes with the IDAC system (Figure 4A-D). To start recording, press the Start recording button.
- Bring the tip of the stimulus glass capillary to cover the tip of the taste sensillum using the motorized manipulator.
- To end the stimulus, remove the glass stimulus capillary from the sensillum using the motorized manipulator.
- Mark the start and end of the stimulation manually using a pedal. The pedal is connected to the IDAC, and its communication with the software is facilitated through the IDAC to mark the start/end of the stimulus.
8. Analysis
- Use the different functions of the software that comes with the IDAC system to sort spike populations by amplitude (when possible) and analyze response dynamics.
- To count spikes, left click on the recording of interest, bringing up a window to select from. Choose To Spikes, initiating another window named Convert waves to spikes. Enter a name in the New field and press the OK button.
- Entering the name in the New field in step 8.1.1 leads to the Amplitude Histogram view. Choose the amplitude to count, then close this view. Left click to add a counter.
- Examine the spikes manually to confirm conclusions based on analysis with software.
NOTE: The software also allows the exportation of data in different formats for further analysis.
Representative Results
Figure 4A shows spontaneous spikes that arise from a sensillum. They fall into two classes based on amplitude, with the larger spikes deriving from the neuron that is sensitive to bitter compounds and the smaller spikes from the neuron that responds to sugars. The relationship between spike amplitude and functional specificity has been corroborated by genetic experiments4,14,37,38,39 .
Figure 4B shows the response of the bitter-sensitive neuron of the S5 sensillum to the odor of DEET; this response occurred without any contact between the DEET solution and the sensillum. One spike appears to be of intermediate amplitude and may result from the superposition of two smaller spikes, one from the sugar neuron and one from the mechanosensory neuron.
Figure 4C shows the ON response that occurs following contact between a bitter stimulus and the I1 sensillum, followed by an OFF response that occurs following the termination of contact. Not that the magnitude of the OFF response, measured in spikes/s, is greater than the ON response.
Figure 4D shows an ON and an OFF response to the bitter compound berberine from an I1 sensillum of another species of fly, Drosophila virilis. One of the advantages of this technique is that it can be performed on other species, including mosquitoes, without the need to introduce any transgenes into them.
Figure 4E illustrates two problems that can occur during recording. First, if the labellum is not properly secured it can move, producing spikes from the mechanosensory neuron of the sensillum. Second, the recording electrode can become dislodged from the sensillum, often as a result of movement of the labellum. In this case, contact is lost and the electrode must be re-inserted to record spikes.
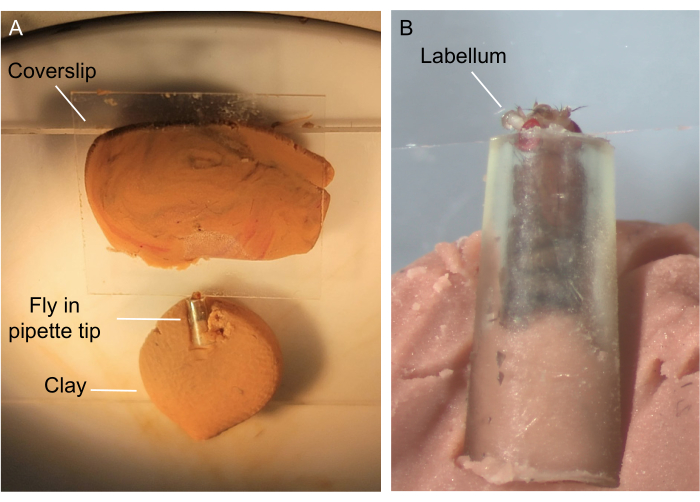
Figure 1: Fly preparation for base recording. A trimmed pipette tip containing a female fly with the labellum securely placed on a glass coverslip. (A) Both the pipette tip and the coverslip are held securely on mounds of clay. (B) Higher magnification of labellum resting on a coverslip. Please click here to view a larger version of this figure.
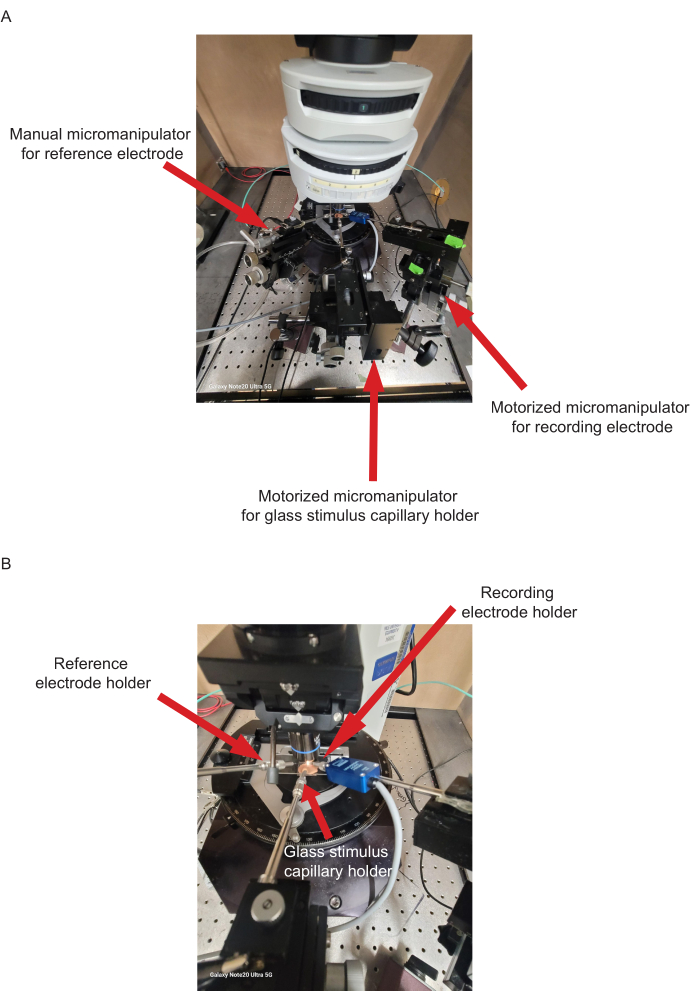
Figure 2: Electrophysiology rig setup. (A) An overview showing the positions of a manual micromanipulator for the reference electrode, a motorized micromanipulator for the glass stimulus capillary, and a motorized micromanipulator for the recording electrode. (B) Overview showing holders for the reference electrode, glass stimulus capillary, and recording electrode. Please click here to view a larger version of this figure.
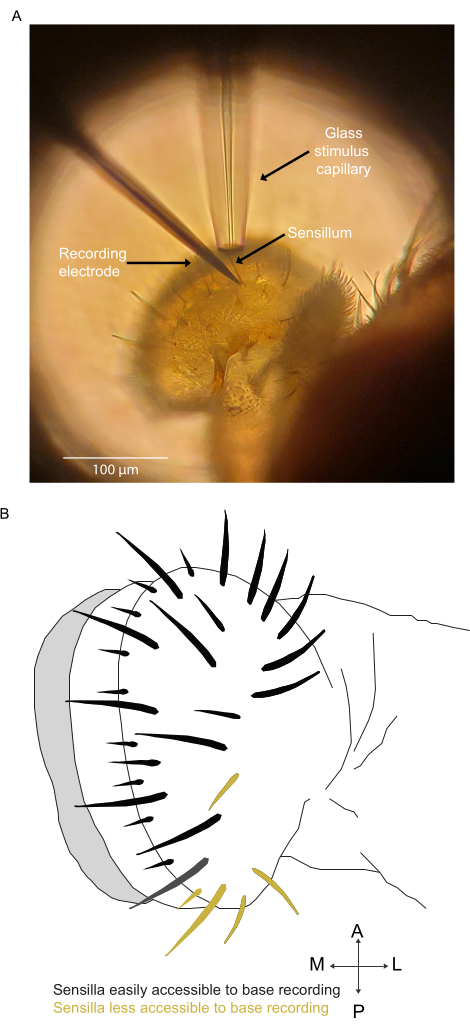
Figure 3: The labellum and sensilla. (A) The labellum under an inverted microscope. Close-up of the labellum showing the taste sensilla of one lobe of the labellum. It also shows the stimulus glass capillary close to one of the large sensilla known as L2 (Large type 2) and a recording electrode in the base of this sensillum. (B) Accessibility of labellar sensilla. The labellum showing sensilla that are easily accessible to base recording and sensilla that are less accessible due to their position in the preparation that we generally use. Abbreviations: A = anterior; M = medial; P = posterior; L = lateral. Please click here to view a larger version of this figure.
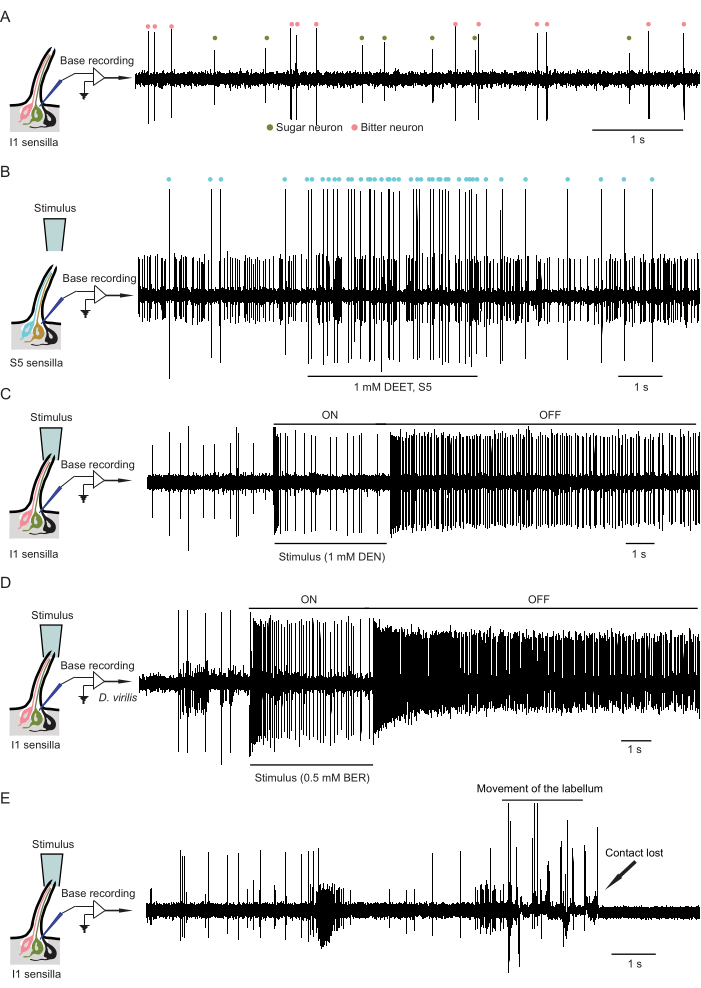
Figure 4: Example traces of spontaneous spikes, bitter neuron responses, and suboptimal recording. (A) Example trace of spontaneous spikes. The spikes are from a bitter-sensitive neuron (red dots) and a sugar-sensitive neuron (green dots) in an I1 sensillum. In the drawing of the sensillum, the bitter, sugar, and mechanosensory neurons are colored red, green, and black, respectively. (B) Example trace of the response of the bitter neuron (red dots) in an S5 sensillum to the vapor of 1 mM DEET. Note that the small amplitude spikes are from the sugar neuron and mechanosensory neurons, which in this example trace are difficult to distinguish by amplitude. Abbreviation: DEET = N,N-diethyl-meta-toluamide. (C) Example trace of ON and OFF responses from a bitter neuron in I1 sensilla to 1 mM denatonium benzoate. Spikes are observed before (spontaneous firing), during (ON response), and after (OFF response) contact with the stimulus. Abbreviation: DEN = denatonium benzoate. (D) Example trace of ON and OFF responses from a bitter neuron in I1 sensilla of D. virilis to 0.5 mM berberine chloride. Spikes are observed before, during, and after contact. Abbreviation: BER = berberine chloride. (E) Example trace of a suboptimal recording. The contact was lost in the middle of the experiment due to the movement of the labellum. Please click here to view a larger version of this figure.
Discussion
In recordings from some types of sensilla, it can be challenging to differentiate the spikes of different neurons. For example, the sugar neurons and mechanosensory neurons of S and I sensilla produce spikes of similar amplitudes, making it difficult to distinguish them4,14. We find that the use of a very sharp tungsten recording electrode reduces the firing of the mechanosensory neuron, as does the judicious placement of the recording electrode. Insertion of the recording electrode into the collar of the sensillum socket (i.e. into the base but not deep) often results in reduced mechanosensory stimulation. Additionally, we find that if a recording has a high level of firing of the mechanosensory neuron, withdrawing the recording electrode and placing it in a different position relative to the sensillum often leads to a lower level of firing.
Ensuring the stability of the recording electrode throughout experiments is another critical issue (see Figure 4E). Mechanical disturbances or shifts in the electrode position can adversely impact the quality of recordings. Investing time and effort in thorough preparation, as specified in the protocol described above, is key to preventing frustration that may result from labellum movement.
We note one other issue we have observed with certain compounds that produce both ON and OFF responses from I sensilla of the I-a class14. Sometimes, a second delivery of a stimulus elicits the OFF but not the ON response. The reason for this reduction in ON response is unknown.
Another challenge encountered in base recording is that not all sensilla are easily accessible for recording because of their position on the labellum. Of the 31 labellar sensilla, only 26 are convenient to record from, presenting a limitation to the technique, as illustrated in Figure 3B. However, in principle, these sensilla (I8, I9, I10, L9, and S10) can be made accessible by rotation of the Olympus microscope mechanical XY stage.
Finally, we emphasize the importance of investing effort into fly preparation. A well-prepared fly is much more likely to yield reliable and high-quality recordings throughout the course of the experiment. Additionally, sucrose can be used as a positive control to ensure the quality of recording; it elicits a response from all labellar sensilla.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Zina Berman for support, Lisa Baik for comments on the manuscript, and other members of the Carlson laboratory for discussion. This work was supported NIH grant K01 DC020145 to H.K.M.D; and NIH grants R01 DC02174, R01 DC04729, and R01 DC011697 to J.R.C.
Materials
| Microscope | Olympus | BX51WI | equipped with a 50X objective (LMPLFLN 50X, Olympus) and 10X eyepieces. |
| Antivibration Table | TMC | 63-7590E | |
| motorized Micromanipulators | Harvard Apparatus and Märzhäuser Micromanipulators | Micromanipulator PM 10 Piezo Micromanipulator | |
| manual Micromanipulators | Märzhäuser Micromanipulators | MM33 Micromanipulator | |
| Magnetic stands | ENCO | Model #625-0930 | |
| Reference and recording Electrode Holder | Ockenfels Syntech GmbH | ||
| Stimulus glass capillary Holder | Ockenfels Syntech GmbH | ||
| Universal Single Ended Probe | Ockenfels Syntech GmbH | ||
| 4-CHANNEL USB ACQUISITION CONTROLLER , IDAC-4 | Ockenfels Syntech GmbH | ||
| Stimulus Controllers | Ockenfels Syntech GmbH | Stimulus Controller CS 55 | |
| Personal Computer | Dell | Vostro | Check for compatibility with digital acquisition system and software |
| Tungsten Rod | A-M Systems | Cat#716000 | |
| Aluminum Foil and/or Faraday Cage | Electromagnetic noise shielding | ||
| Borosilicate Glass Capillaries | World Precision Instruments | 1B100F-4 | |
| Pipette Puller | Sutter Instrument Company | Model P-97 Flaming/Brown Micropipette Puller | |
| Stereomicroscope | Olympus | VMZ 1x-4x | For fly preparation |
| p200 Pipette Tips | Generic | ||
| Microloader tips | Eppendorf | E5242956003 | |
| 1 ml Syringe | Generic | ||
| Crocodile clips | |||
| Power Transformers | STACO ENERGY PRODUCTS | STACO 3PN221B | Assembled from P1000 pipette tips, flexible plastic tubing, and mesh |
| Modeling Clay | Generic | ||
| Forceps | Generic | ||
| Plastic Tubing | Saint Gobain | Tygon S3™ E-3603 | |
| Standard culture vials | Archon Scientific | Narrow 1-oz polystyrene vails, each with 10 mL of glucose medium, preloaded with cellulose acetate plugs | |
| Berberine chloride (BER) | Sigma-Aldrich | Cat# Y0001149 | |
| Denatonium benzoate (DEN) | Sigma-Aldrich | Cat# D5765 | |
| N,N-Diethyl-m- toluamide (DEET) | Sigma-Aldrich | Cat# 36542 |
References
- Joseph, R. M., Carlson, J. R. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet. 31 (12), 683-695 (2015).
- Montell, C. Drosophila sensory receptors-a set of molecular Swiss Army Knives. Genetics. 217 (1), 1-34 (2021).
- Dweck, H. K. M., Carlson, J. R. Molecular logic and evolution of bitter taste in Drosophila. Curr Biol. 30 (1), 17-30 (2020).
- Weiss, L. A., Dahanukar, A., Kwon, J. Y., Banerjee, D., Carlson, J. R. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 69 (2), 258-272 (2011).
- He, Z., Luo, Y., Shang, X., Sun, J. S., Carlson, J. R. Chemosensory sensilla of the Drosophila wing express a candidate ionotropic pheromone receptor. PLoS Biol. 17 (5), e2006619 (2019).
- Ling, F., Dahanukar, A., Weiss, L. A., Kwon, J. Y., Carlson, J. R. The molecular and cellular basis of taste coding in the legs of Drosophila. J Neurosci. 34 (21), 7148-7164 (2014).
- Falk, R., Bleiser-Avivi, N., Atidia, J. Labellar taste organs of Drosophila melanogaster. J Morphol. 150 (2), 327-341 (1976).
- Hiroi, M., Meunier, N., Marion-Poll, F., Tanimura, T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J Neurobiol. 61 (3), 333-342 (2004).
- Shanbhag, S. R., Park, S. K., Pikielny, C. W., Steinbrecht, R. A. Gustatory organs of Drosophila melanogaster: fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 304 (3), 423-437 (2001).
- Siddiqi, O., Rodrigues, V. Genetic analysis of a complex chemoreceptor. Basic Life Sci. 16, 347-359 (1980).
- Clyne, P. J., Warr, C. G., Carlson, J. R. Candidate taste receptors in Drosophila. Science. 287 (5459), 1830-1834 (2000).
- Koh, T. W., et al. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 83 (4), 850-865 (2014).
- Sánchez-Alcañiz, J. A., et al. An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing. Nat Commun. 9 (1), 4252 (2018).
- Dweck, H. K. M., Carlson, J. R. Diverse mechanisms of taste coding in Drosophila. Sci Adv. 9 (46), (2023).
- Chyb, S., Dahanukar, A., Wickens, A., Carlson, J. R. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci U S A. 100, 14526-14530 (2003).
- Dahanukar, A., Lei, Y. T., Kwon, J. Y., Carlson, J. R. Two Gr genes underlie sugar reception in Drosophila. Neuron. 56 (3), 503-516 (2007).
- Delventhal, R., Carlson, J. R. Bitter taste receptors confer diverse functions to neurons. Elife. 5, e11181 (2016).
- Dweck, H. K. M., Talross, G. J. S., Luo, Y., Ebrahim, S. A. M., Carlson, J. R. Ir56b is an atypical ionotropic receptor that underlies appetitive salt response in Drosophila. Curr Biol. 32 (8), 1776-1787 (2022).
- Hiroi, M., Marion-Poll, F., Tanimura, T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 19 (9), 1009-1018 (2002).
- Jeong, Y. T., et al. An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron. 79 (4), 725-737 (2013).
- Jiao, Y., Moon, S. J., Montell, C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 104 (35), 14110-14115 (2007).
- Jiao, Y., Moon, S. J., Wang, X., Ren, Q., Montell, C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 18 (22), 1797-1801 (2008).
- Kim, S. H., et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A. 107 (18), 8440-8445 (2010).
- Lacaille, F., et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2 (7), e661 (2007).
- Lee, Y., et al. Gustatory receptors required for avoiding the insecticide L-canavanine. J Neurosci. 32 (4), 1429-1435 (2012).
- Lee, Y., Kim, S. H., Montell, C. Avoiding DEET through insect gustatory receptors. Neuron. 67 (4), 555-561 (2010).
- Lee, Y., Moon, S. J., Montell, C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 106 (11), 4495-4500 (2009).
- Lee, Y., Moon, S. J., Wang, Y., Montell, C. A Drosophila gustatory receptor required for strychnine sensation. Chem Senses. 40 (7), 525-533 (2015).
- Meunier, N., Marion-Poll, F., Rospars, J. P., Tanimura, T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 56 (2), 139-152 (2003).
- Moon, S. J., Köttgen, M., Jiao, Y., Xu, H., Montell, C. A taste receptor required for the caffeine response in vivo. Curr Biol. 16 (18), 1812-1817 (2006).
- Moon, S. J., Lee, Y., Jiao, Y., Montell, C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Current Biology. 19, 1623-1627 (2009).
- Rimal, S., et al. Mechanism of acetic acid gustatory repulsion in Drosophila. Cell Rep. 26 (6), 1432-1442 (2019).
- Shim, J., et al. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat Commun. 6, 8867 (2015).
- Xiao, S., Baik, L. S., Shang, X., Carlson, J. R. Meeting a threat of the Anthropocene: Taste avoidance of metal ions by Drosophila. Proc Natl Acad Sci U S A. 119 (25), e2204238119 (2022).
- Zhang, Y. V., Ni, J., Montell, C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 340 (6138), 1334-1338 (2013).
- Delventhal, R., Kiely, A., Carlson, J. R. Electrophysiological recording from Drosophila labellar taste sensilla. J Vis Exp. (84), e51355 (2014).
- Marella, S., et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 49 (2), 285-295 (2006).
- Thorne, N., Amrein, H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J Comp Neurol. 506 (4), 548-568 (2008).
- Wang, Z., Singhvi, A., Kong, P., Scott, K. Taste representations in the Drosophila brain. Cell. 117 (7), 981-991 (2004).

