Floral-dip Transformation of Arabidopsis thaliana to Examine pTSO2::β-glucuronidase Reporter Gene Expression
Instructor Prep
concepts
Student Protocol
A subscription to JoVE is required to view this content. Sign in or start your free trial.
JoVE Journal
Biology
Floral-dip Transformation of Arabidopsis thaliana to Examine pTSO2::β-glucuronidase Reporter Gene Expression
I. Floral-dip transformation of Arabidopsis thaliana
- Approximately 1 month prior to transformation, sow Arabidopsis seeds onto 4 to 8 pots (5 inch square pots) with approximately 10-15 plants per pot. For plants with normal fertility, 4 pots of plants per plasmid construct will be sufficient. If one has to transform mutant Arabidopsis with reduced fertility, increase the number of pots accordingly.
- The plants are grown under standard conditions (16 hr light and 8 hr dark at 22°C). If plants are grown under shorter day or lower temperature, they will take longer to flower and longer to be ready for transformation. Good plant care will ensure healthy plants, which are essential for successful transformation.
- When the plants have just bolted and begun to flower. They are now ready for transformation. To increase transformation efficiency, 3-4 days before the transformation, trim off the main inflorescence shoots as soon as they have bolted to encourage more secondary shoot formation. Water the plants the day before transformation. This ensures that the soil will not soak up too much infiltration media during floral-dipping.
- Two days prior to transformation, inoculate 40ml LB containing appropriate antibiotics (in the case of pTSO2::GUS, 50 μg/ml Kanamycin) with Agrobacterium tumefaciens strain GV3101 carrying the pTSO2::GUS construct. The pTSO2::GUS is cloned in the pBIN20 vector that possesses NPTII gene that confers resistance to Kanamycin3. Grow overnight in a shaking incubator at 28-30°C.
- The following day, transfer 8ml of the overnight culture into 400ml of LB containing 50 μg/ml Kanamycin. Grow overnight in a shaking incubator at 28-30°C.
- On the day of transformation, when the OD600 of the Agrobacterium culture reaches approximately 0.8 (OD600 between 0.5 to1 are acceptable), spin down the Agrobacterium overnight culture at for 8 minutes at 5000rpm.
- Resuspend the Agrobacterium pellet in 1 L (liter) of infiltration media (10mM MgCl2, 5% sucrose, 0.44mM 6-benzyladenine (BA), 0.3% silwet L-77, 1x Gamborg s vitamin solution and autoclaved water). The medium does not need to be autoclaved but needs to be made fresh. Pour the 1 L Agrobacterium-containing infiltration medium into a dish (such as 8"x15"x2" Pyrex glass dish).
- Invert the pot (plants won't fall off) and dip all aerial parts of the plants into the infiltration solution, hold for 5 minutes one pot at a time. While all aerial parts of the plant are immersed in the infiltration medium, avoid letting soil soak up any of the infiltration media to reduce chances of fungal growth on the soil.
- After dipping, place all dipped pots on their side on several layers of paper towel in a tray. The paper towels soak up access amount of infiltration media. Cover the tray with plastic wrap to ensure high humidity. Place the tray into plant growth chamber.
- On the following day, remove the plastic wrap and paper towels, and place pots upright. Do not water these plants for four to five more days. Afterwards, maintain the plants normally until they set seeds and collect seeds in bulk.
- Pour plates (150x15mm petri dish) with MS medium containing 50 μg/ml Kanamycin (weight 2.2g Murashige and Skoog basal salt without vitamin, dissolve in 500 ml water, pH to 5.8 with 1M NaOH, add 4 gram agar, autoclave, cool and add Kanamycin to 50 μg/ml final concentration). Plates are kept in 4°C until needed.
- To select for antibiotic-resistant transgenic plants, one needs to screen 20,000 seeds at minimum. 0.1 gram is about 5000 seeds. Weight and estimate the amount of seeds.
- Sterilize seeds by wash them in 15 ml Falcon tube with 70% ethanol for 30 seconds. Wash seeds with sterile water once. Soak the seeds for 30 seconds in a solution containing 1:10 dilution of store-bought 5% bleach (Clorox). Rinse seeds with sterile water three to six times. In sterile hood, pipette about 5000 seeds onto each MS (Kanamycin) plate, spread the seeds evenly on the plate, seal the plates with Micropore 3M tape to avoid contamination.
- Incubate the plates at 4°C in the dark for 3-4 days, then transfer the plates to a chamber (could be the same plant growth chamber). After about 14 days, transgenic seedlings stay green but the non-transgenic ones are turning pale and dying. Transfer the transgenic seedlings to soil by slowing pulling the roots out from the medium and placed them in soil.
II. Examining pTSO2::GUS expression patterns
- Harvest pTSO2::GUS transgenic seedlings and place them in cold 90% acetone in a glass scintillation vial or microfuge tubes that are kept on ice. Polystyrene microtiter plates (not polypropylene) can also be used if one analyzes a large numbers of samples.
- After all samples are harvested, place the vials at room temperature for 20 minutes. During this time make up fresh staining buffer without x-Gluc (0.2% Triton x-100, 50mM NaHPO4 Buffer pH7.2, 2mM Potassium Ferrocyanide, 2mM Potassium Ferricyanide) and place on ice.
- Remove acetone from the samples and add the staining buffer.
- Make up x- Gluc staining solution (0.2% Triton x-100, 50mM NaHPO4 Buffer pH7.2, 2mM Potassium Ferrocyanide, 2mM Potassium Ferricyanide, 2mM x-gluc). Remove the staining buffer from samples and add the staining buffer containing x-Gluc to the samples.
- Infiltrate the samples under a vacuum for 15 to 20 minutes. Release the vacuum slowly and verify that all the samples sink beneath the surface of the staining solution. If necessary, repeat infiltration until all samples sink after the vacuum is released.
- Incubate samples at 37°C overnight in the staining buffer with x-Gluc.
- Remove samples from incubator and remove staining buffer. Wash the samples in successive ethanol series (20%, 35% and 50% ethanol) at room temperature for 30 minutes each change.
- Fix the tissues in FAA fixative (50% Ethanol, 5% Formaldehyde, 10% Acetic acid, rest water) for 30 minutes at room temperature. Remove FAA and add 70% ethanol. At this point the tissues can be visualized and photographed under a dissecting microscope equipped with a digital camera. A dark blue color indicates where TSO2 is expressed (Figure 1). Alternatively, the tissues can be stored at 4°C for later viewing.
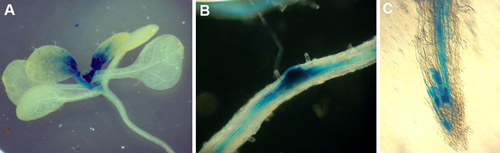
Figure 1. pTSO2::GUS expression in transgenic seedlings (A), lateral roots (B), and root tip (C). Note that TSO2 promoter is highly active in young and dividing tissues. The sporadic expression pattern shown in (C) is typical of genes expressed in specific cell cycle phases.
Representative Results
When done correctly, transformation efficiency should be approximately 0.1-0.2%. In another word, one should get 5-10 transgenic seedlings by screening every 5000 seeds. On average, about 100 transgenic lines can be obtained by transforming 4 pots of wild-type plants.
For transgenic seedlings containing the pTSO2::GUS construct3, dark blue color reflecting GUS activity is found in actively dividing cells including young leaves, shoot apex, root tip, and lateral root primordia (Figure 1). The non-uniform sporadic pattern is characteristic of cell cycle phase-specific expression.
Floral-dip Transformation of Arabidopsis thaliana to Examine pTSO2::β-glucuronidase Reporter Gene Expression
Learning Objectives
List of Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| 6-benzyladenine | Sigma | 852430 | ||
| Silwet-77 | Crompton Corp. | Toxic, wear gloves | ||
| Murashige and Skoog Basal medium (MS) | Sigma | M5519 | ||
| Gamborg’s vitamin solution 1000X | Sigma | G1019 | ||
| X-Gluc (5-bromo-4-chloro-3-indolyl β-D-glucuronide cyclohexamine salt | Biosynth | B-7300 | Toxic, light sensitive keep in the dark | |
| Micropore 3M Tape | Fisher | 19027761 |
Lab Prep
The ability to introduce foreign genes into an organism is the foundation for modern biology and biotechnology. In the model flowering plant Arabidopsis thaliana, the floral-dip transformation method1-2 has replaced all previous methods because of its simplicity, efficiency, and low cost. Specifically, shoots of young flowering Arabidopsis plants are dipped in a solution of Agrobacterium tumefaciens carrying specific plasmid constructs. After dipping, the plants are returned to normal growth and yield seeds, a small percentage of which are transformed with the foreign gene and can be selected for on medium containing antibiotics. This floral-dip method significantly facilitated Arabidopsis research and contributed greatly to our understanding of plant gene function. In this study, we use the floral-dip method to transform a reporter gene, β-glucuronidase (GUS), under the control of TSO2 promoter. TSO2, coding for the Ribonucleotide Reductase (RNR) small subunit3, is a cell cycle regulated gene essential for dNDP biosynthesis in the S-phase of the cell cycle. Examination of GUS expression in transgenic Arabidopsis seedlings shows that TSO2 is expressed in actively dividing tissues. The reported experimental method and materials can be easily adapted not only for research but also for education at high school and college levels.
The ability to introduce foreign genes into an organism is the foundation for modern biology and biotechnology. In the model flowering plant Arabidopsis thaliana, the floral-dip transformation method1-2 has replaced all previous methods because of its simplicity, efficiency, and low cost. Specifically, shoots of young flowering Arabidopsis plants are dipped in a solution of Agrobacterium tumefaciens carrying specific plasmid constructs. After dipping, the plants are returned to normal growth and yield seeds, a small percentage of which are transformed with the foreign gene and can be selected for on medium containing antibiotics. This floral-dip method significantly facilitated Arabidopsis research and contributed greatly to our understanding of plant gene function. In this study, we use the floral-dip method to transform a reporter gene, β-glucuronidase (GUS), under the control of TSO2 promoter. TSO2, coding for the Ribonucleotide Reductase (RNR) small subunit3, is a cell cycle regulated gene essential for dNDP biosynthesis in the S-phase of the cell cycle. Examination of GUS expression in transgenic Arabidopsis seedlings shows that TSO2 is expressed in actively dividing tissues. The reported experimental method and materials can be easily adapted not only for research but also for education at high school and college levels.
Procedure
The ability to introduce foreign genes into an organism is the foundation for modern biology and biotechnology. In the model flowering plant Arabidopsis thaliana, the floral-dip transformation method1-2 has replaced all previous methods because of its simplicity, efficiency, and low cost. Specifically, shoots of young flowering Arabidopsis plants are dipped in a solution of Agrobacterium tumefaciens carrying specific plasmid constructs. After dipping, the plants are returned to normal growth and yield seeds, a small percentage of which are transformed with the foreign gene and can be selected for on medium containing antibiotics. This floral-dip method significantly facilitated Arabidopsis research and contributed greatly to our understanding of plant gene function. In this study, we use the floral-dip method to transform a reporter gene, β-glucuronidase (GUS), under the control of TSO2 promoter. TSO2, coding for the Ribonucleotide Reductase (RNR) small subunit3, is a cell cycle regulated gene essential for dNDP biosynthesis in the S-phase of the cell cycle. Examination of GUS expression in transgenic Arabidopsis seedlings shows that TSO2 is expressed in actively dividing tissues. The reported experimental method and materials can be easily adapted not only for research but also for education at high school and college levels.
Tags
Floral-dip TransformationArabidopsis ThalianaPTSO2::β-glucuronidase Reporter Gene ExpressionForeign GenesModern BiologyBiotechnologyAgrobacterium TumefaciensPlasmid ConstructsArabidopsis ResearchPlant Gene FunctionReporter Geneβ-glucuronidase (GUS)TSO2 PromoterRibonucleotide Reductase (RNR)DNDP BiosynthesisS-phase Of The Cell CycleTransgenic Arabidopsis SeedlingsEducation
