A Simple Guide Screw Method for Intracranial Xenograft Studies in Mice
Instructor Prep
concepts
Student Protocol
1. Cell lines
- U87MG glioma cells are cultured in large tissue culture flasks with DMEM-F12 supplemented with 5% fetal bovine serum (FBS).
- Cells are harvested by washing flasks twice with warm Phosphate Buffered Saline (PBS) and incubating them at 37°C for 5 minutes with 10 ml of PBS containing 0.25% trypsin and 0.05% EDTA. Once cells are lifted, they are placed into a 50 ml tube containing 10 ml of culture media and centrifuged (300 X g for 4 min).
- Following the wash, cells are resuspended at a concentration of 10 x 106/ml in culture media, which allowed for an inoculation of 50,000 cells/5 μl.
- Cells are kept on ice until intracranial injection.
2. Guide screw intracranial bolting
This procedure can be carried out several days prior to the injection of cells. All procedures described here have been carried out under strict sterile conditions.
- Mice (BALB/c nu/nu female; 5-6 weeks; approximately 18g) are numbered for identification purposes, weighed and anesthetised with an intraperitoneal (IP) injection of a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg).
- The skin is wiped down with an iodine solution and a small incision (2-3 mm) is made along the right side of the midline and anterior to the interaural line. This exposes the coronal and sagittal sutures of the skull. The bregma is positioned at the junction of these two sutures.
- The guide screw entry point is then marked at a point 2.5 mm lateral and 1 mm anterior to the bregma. This point is located directly above the caudate nucleus1.
- A 1 x 1 mm deep hole is drilled with a sterile hand held twist drill through the skull to the dura.
- A sterilised guide screw that will extend 1.6 mm below the skulls surface into the dura is then bolted into the hole with a screw driver until flush with the skull (Figure 1A).
- A sterile stylet or screw dummy is then placed into the central hole of the guide screw to close the opening (Figure 1B).
- The wound is closed with Vetbond Tissue Adhesive (n-butyl cyanoacrylate) and the mice are given an intraperitoneal injection of reversine (small animals) (0.1 ml/kg) and carprofen (5 mg/kg/100 μl) for analgesia. Mice are then allowed to recover on a warming mat (36°C) which can take up to 20 minutes. Mice are frequently monitored and observed during this recovery time until they are fully conscious.
3. Intracranial cellular engraftment
- A sterile cuffed Hamilton syringe is prepared by applying a small plastic ring to the needle tip allowing only 2 mm of the needle to extend below the guide screw outlet (Figure 1B). The final inoculation point is therefore 3.5 mm below the skull surface.
- Four days following the guide screw surgery, the mice are again anesthetized as above (2.1) and a small incision is made over the guide screw to remove the stylet.
- The cuffed Hamilton syringe is then filled with 5 μl of well mixed cells taking precaution not to create or draw up any air bubbles.
- The syringe is secured to the perfusion pump and the needle is inserted into the guide screw (Figure 1C). The cells are then infused at a rate of 30 μl per hour.
- The automated apparatus (Figure 1D) allowed us to inject up to 10 animals at a time at a constant flow rate. Cells can also be injected manually by using the cuffed hamilton syringe or alternatively the syringe can be cuffed by a 200 μl pipette tip that has been trimmed by 3 mm to allow the needle tip to extend beyond the guide screw by 2 mm. The injection must then be performed at a constant steady pace.
- When infusion is complete, the syringe is carefully removed and the stylet is replaced into the guide screw. The wound is glued closed and the mice are given recovery medications as before (2.7) and allowed to recover on a warming mat.
4. Therapeutic challenge
- Four days after the U87MG cell inoculation, mice are weighed and randomly divided into control and treatment groups.
- The control group is given an intra-peritoneal (IP) injection of PBS (100 μl), while the treatment group is given an IP injection of AMG102 (100 μg in 100 μl of PBS).
- Injections are repeated every second day for 14 days, a total of 6 injections (Figure 2).
- After the final injection, mice are monitored daily and weighed every second day.
- When mice began to display significant neurological dysfunctions (balance disturbance, paralysis), dehydration, or more than 10% weight loss or moribund, they were humanely euthanised. These humane end-point days were then recorded on the Kaplan-Meier survival curve.
5. Evaluation of therapeutic efficiency
- The Kaplan-Meier survival curve was generated using GraphPad Prism 4.03 for Windows; GraphPad Software, San Diego California USA, www.graphpad.com3. Deaths or euthanizations were marked as end-point events and scored as 1. Animals that remained alive at the end of the experiment were recorded as non-events and scored as 0.
6. Representative results:
Control animals began to show signs of neurological disturbance and weight loss 23 days after the initial inoculation of cells. This was significantly delayed in the AMG102 treated group to 35 days. Indeed by day 35, 77% (n = 7/9) of the control group had been euthanized. The Kaplan-Meier survival curve clearly demonstrates the significant increase in survival in response to AMG102 treatment (Figure 3). By day 70, 88% of control mice had been euthanized (n = 8/9) compared to 37% (n = 3/8) of AMG102 treated group. Histological analysis of the brains confirmed that all 9 animals in the control group developed tumors compared to only 3 of the 8 AMG102 treated mice (Figure 4).
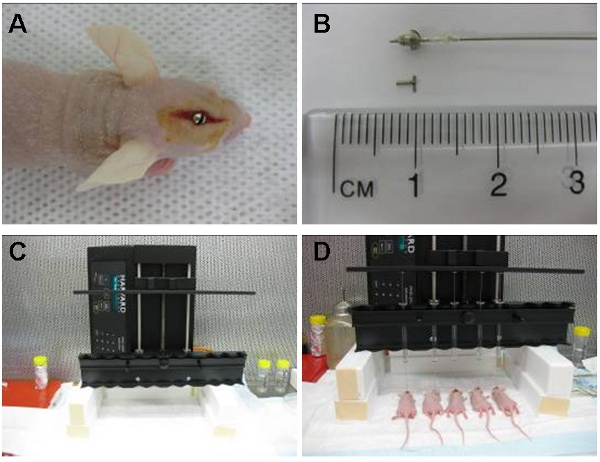
Figure 1: Image (A) shows intracranial guide screw positioned in an area located directly above the caudate nucleus. The cuffed hamilton syringe (pre-loaded with cells) is then placed inside the guide screw so that 2 mm of needle extends beyond the guide and the cells are injected 3.5 mm inside the caudate nucleus. The syringe is later removed and replaced by a stylet (B). The automatic infusion device (C) can inject up to 10 animals at a time. Shown here are five simultaneous injections (D).
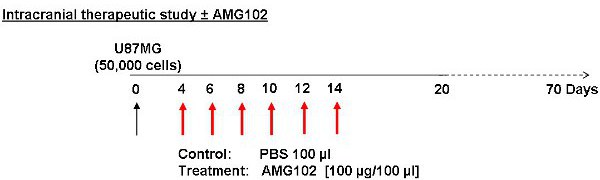
Figure 2: Schematic of study protocol. Cells were injected on day 0 with treatment commencing on day 4. Control animals received an injection of PBS (100 μl) IP while the treatment group received an injection of AMG102 (100 μg/100 μl) IP. These injections were given every second day for 10 days. A total of 6 injections were given. Animals were then monitored for 70 days for signs of neurological and physical disturbances.
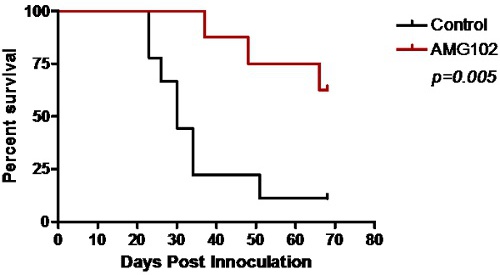
Figure 3: Kaplan-Meier survival curve comparing control and AMG102 treated mice. Significant survival was obtained following treatment with AMG102 (p=0.005).
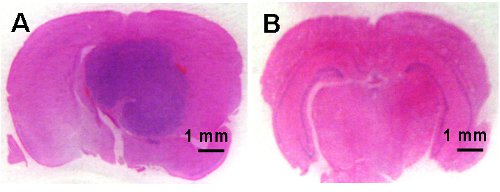
Figure 4: Microphotographs of coronal brain sections demonstrating tumor development in the control animals (A) and no brain tumor development in the AMG102 treated animals (B). Scale bar is 1 mm.
A Simple Guide Screw Method for Intracranial Xenograft Studies in Mice
Learning Objectives
List of Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| DMEM/F12 | Invitrogen | 12634010 | |
| FCS | Bovogen | SFBS | |
| Trypsin | Invitrogen | 15050065 | |
| EDTA | Sigma | E6758 | |
| Balb/c nu/nu mice | ARC Perth | ||
| Screw Holder | Plastics One | SD-1 | |
| Drill Holder | Plastics One | DH-1 | |
| Drill Bit | Plastics One | D#60 | |
| Screw guide (metal) | Plastics One | C212SG | |
| Screw Dummy (stylus) | Plastics One | C212SD | |
| Hamilton Syringe (10 μl 26g cemented needle) | Grace Division Discovery Science | 80075 | |
| PHD 22/2000 infusion pump | Harvard Apparatus | 70-2003 | Optional |
| Vetbond | 3M | 1469SB | |
| Thermo pad | Harvard Apparatus | 340390 |
Lab Prep
The grafting of human tumor cells into the brain of immunosuppressed mice is an established method for the study of brain cancers including glioblastoma (glioma) and medulloblastoma. The widely used stereotactic approach only allows for the injection of a single animal at a time, is labor intensive and requires highly specialized equipment. The guide screw method, initially developed by Lal et al.,1 was developed to eliminate cumbersome stereotactic procedures. We now describe a modified guide screw approach that is rapid and exceptionally safe; both of which are critical ethical considerations. Notably, our procedure now incorporates an infusion pump that allows up to 10 animals to be simultaneously injected with tumor cells.
To demonstrate the utility of this procedure, we established human U87MG glioma cells as intracranial xenografts in mice, which were then treated with AMG102; a fully human antibody directed to HGF/scatter factor currently undergoing clinical evaluation2-5. Systemic injection of AMG102 significantly prolonged the survival of all mice with intracranial U87MG xenografts and resulted in a number of complete cures.
This study demonstrates that the guide screw method is an inexpensive, highly reproducible approach for establishing intracranial xenografts. Furthermore, it provides a relevant physiological model for validating novel therapeutic strategies for the treatment of brain cancers.
The grafting of human tumor cells into the brain of immunosuppressed mice is an established method for the study of brain cancers including glioblastoma (glioma) and medulloblastoma. The widely used stereotactic approach only allows for the injection of a single animal at a time, is labor intensive and requires highly specialized equipment. The guide screw method, initially developed by Lal et al.,1 was developed to eliminate cumbersome stereotactic procedures. We now describe a modified guide screw approach that is rapid and exceptionally safe; both of which are critical ethical considerations. Notably, our procedure now incorporates an infusion pump that allows up to 10 animals to be simultaneously injected with tumor cells.
To demonstrate the utility of this procedure, we established human U87MG glioma cells as intracranial xenografts in mice, which were then treated with AMG102; a fully human antibody directed to HGF/scatter factor currently undergoing clinical evaluation2-5. Systemic injection of AMG102 significantly prolonged the survival of all mice with intracranial U87MG xenografts and resulted in a number of complete cures.
This study demonstrates that the guide screw method is an inexpensive, highly reproducible approach for establishing intracranial xenografts. Furthermore, it provides a relevant physiological model for validating novel therapeutic strategies for the treatment of brain cancers.
Procedure
The grafting of human tumor cells into the brain of immunosuppressed mice is an established method for the study of brain cancers including glioblastoma (glioma) and medulloblastoma. The widely used stereotactic approach only allows for the injection of a single animal at a time, is labor intensive and requires highly specialized equipment. The guide screw method, initially developed by Lal et al.,1 was developed to eliminate cumbersome stereotactic procedures. We now describe a modified guide screw approach that is rapid and exceptionally safe; both of which are critical ethical considerations. Notably, our procedure now incorporates an infusion pump that allows up to 10 animals to be simultaneously injected with tumor cells.
To demonstrate the utility of this procedure, we established human U87MG glioma cells as intracranial xenografts in mice, which were then treated with AMG102; a fully human antibody directed to HGF/scatter factor currently undergoing clinical evaluation2-5. Systemic injection of AMG102 significantly prolonged the survival of all mice with intracranial U87MG xenografts and resulted in a number of complete cures.
This study demonstrates that the guide screw method is an inexpensive, highly reproducible approach for establishing intracranial xenografts. Furthermore, it provides a relevant physiological model for validating novel therapeutic strategies for the treatment of brain cancers.
