Trypsin Digest Protocol to Analyze the Retinal Vasculature of a Mouse Model
Instructor Prep
concepts
Student Protocol
1. Retinal Preparation
- Enucleate the mouse eye. Using one hand, open the eye lids so that the eye is visible. With the other hand, using a curved forceps (curved facing upwards), apply pressure on superior and inferior aspects of the orbit until the globe protrudes. Gently close forceps at the posterior aspect of the eye and lift in a continuous motion to enucleate the eye.
- Fix eye with 10% neutral buffered formalin for at least 24 hr.
- Place eye in PBS solution in a small Petri dish.
- Dissect out retina carefully under a microscope, taking care to avoid inducing large tears. Make an initial cut in the cornea with dissection scissors. Then, while holding the cut area with straight forceps, use scissors to cut along the border of the cornea and sclera removing the cornea. Using fine curved and straight forceps, carefully peel the sclera and choroid away from the retina towards the optic nerve. This step requires patience as quickly freeing the retina can lead to tears. Remove the lens and any excess iris and debris from the retina 16.
2. Water Washes
- Place retina in a 24-well dish and cover with water (approximately 500 μl, filtered with at least a 45 μm filter).
- Change water every 30 min-1 hr, at least 4-5 times.
- Leave overnight in water with gentle shaking at room temperature.
Note: The washing steps are very important as they help separate the neural retinal layers from the blood vessels.
3. Trypsin Digest
- Remove water and incubate retina in a solution of 3% trypsin (Difco 1:250) in 0.1 M Tris buffer (pH 7.8) at 37 °C with gentle to no shaking. Trypsin digest is completed when the tissue begins showing signs of disintegration (approximately 1.5 hr).
Note: Avoid rapid shaking as this can damage the vasculature. - Carefully, pipette out trypsin into a separate well using a microscope to avoid touching the retina. This excess trypsin can be used later, when manipulating the vessels under the microscope, in step 4.3.
4. Separating the Vasculature
- Add filtered water to the retina. Then, attempt to peel off the internal limiting membrane with forceps, using scissors if necessary. Shake with mild agitation for 5 min. Carefully, pipette out the water under the microscope to avoid damaging the retina.
- Repeat water washes (for 5 min each) to help free the network of vessels from the adherent retinal tissue. Water washes are completed when there is little to no debris remaining in the water after wash.
- Careful manipulations under a dissection microscope are now needed to further free up the network of vessels. Below are several useful techniques, which may be used in sequence or individually to achieve the desired outcome based on technical preference:
- Using a 200 μl pipette, carefully pipette water up and down adjacent to vessels, blowing water at vessels, causing gentle agitation.
- Using 200 μl pipette, pipette the trypsin up and down to help coat walls of the pipette. Then, CAREFULLY pipette the entire vessel network up and down once to help break down non-vascular tissue.
Note: Centering the pipette tip at the optic nerve rather than the edges of the vascular network can also prevent the vasculature from sticking to the tip. - Gently lift vasculature with fine forceps out of water to dislodge non-vascular components.
- If the above steps do not work, add trypsin to the retina to help break down the tissue, and incubate at 37 °C for several minutes. Then, remove trypsin and repeat steps 4.1-4.3.
Note: Make sure to coat all instruments that will come into contact with the vessels in trypsin (e.g. pipette tip, forceps). This will help avoid adhesion of vasculature to the equipment.
5. Visualizing the Vasculature
- Place a drop of water onto a clean slide. Using a 200 μl pipette or forceps, transfer the digested vessels into the water. Make sure the vascular flat-mount is unfolded. The surface tension of the water helps to unfold the now diaphanous vascular network. If the tissue does not unfold, add additional water and gently shake the slide to facilitate unfolding.
- Remove excess water very slowly by pipetting or using a paper towel.
- Allow slides to dry completely.
- Stain slides via appropriate protocol with either PAS/hematoxylin or H&E stain.
Note: PAS/hematoxylin is useful for identifying vessel wall and cellular nuclei; H&E is useful for demonstration of RBC as well. - Dehydrate and mount (Permount mounting medium).
Trypsin Digest Protocol to Analyze the Retinal Vasculature of a Mouse Model
Learning Objectives
The final product of a successful procedure is a flat-mount of the entire network of the mouse retinal vasculature, with the architecture maintained, stained with either PAS/hematoxylin or H&E, as shown in Figures 2-4. Clear differentiation of endothelial cells and pericytes can be seen as shown in Figure 3. In the retina, the nuclei of endothelial cells are oval or elongated and lie entirely within the vessel wall. Pericyte nuclei are small, spherical, stain densely and generally have a protuberant position along the capillary wall.
Trypsin digest is a common procedure to analyze vascular pathology in diabetic animal models. Pathologic vascular lesions such as microaneurysms, pericyte ghosts (evidence of pericyte loss), acellular capillaries and capillary degeneration have been described. Figure 4 shows an example of capillary degeneration seen in a db/db mouse, a model for type II diabetes. Although the procedure was optimized for mouse retinas, similar results have also been obtained in rat retinas as shown in Figure 5. It’s important to note that rat retinas are much larger, and a 12-well plate was used instead of a 24-well plate. The vasculature network of the rat retina is also less fragile than the mouse counterpart.
It is extremely crucial to remove as much of the non-vascular tissue as possible. Any remnants will lead to non-specific staining and lead to poor visualization of the vasculature. Figure 6 represents an example of unsuccessful removal of the entire non-vascular tissue in step 4. Likewise, it is also important to have the retina be unfolded when mounting so as to improve visualization of the vasculature. Figure 7 represents unsuccessful unfolding of the vascular flat-mount in step 5.1.
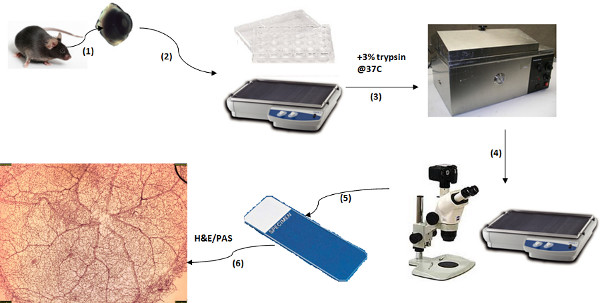
Figure 1. Schematic of Trypsin Digest Protocol. (1) Retinal Preparation: isolate retina from eye; (2) Water washes: Wash in filtered water overnight; (3) Trypsin Digest: Incubate in 3% trypsin at 37 °C for 1-1.5 hr; (4) Separating the vasculature: isolate vasculature via series of water washes and dissection under microscope; (5) Move vasculature to slide and let dry; (6) Visualizing the vasculature: stain slide with PAS or H&E, dehydrate and mount. Click here to view larger figure.
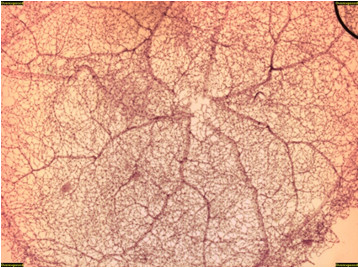
Figure 2. Control db/m mouse trypsin digest (H&E, 20x). This figure is an overview of the retinal vascular network showing normal vascular architecture.
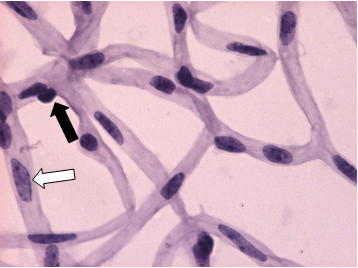
Figure 3. Control db/m mouse trypsin digest (H&E, 600x). This is a magnified view of the retina in Figure 2 showing normal vascular architecture. Endothelial cell (white arrow) and pericyte (black arrow) are highlighted.
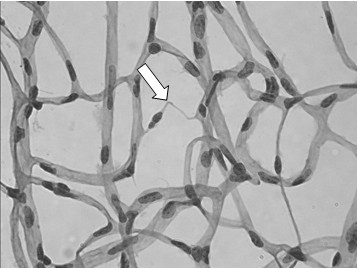
Figure 4. Diabetic db/db mouse trypsin digest (H&E, 500x). By 52 weeks, db/db mice begin to develop vascular pathology such as capillary degeneration (white arrow).
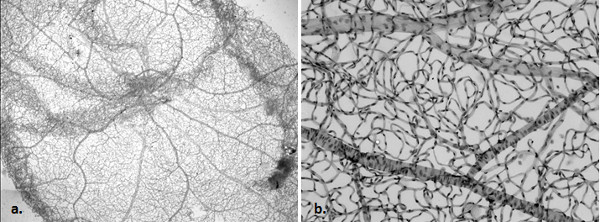
Figure 5. Normal rat retina trypsin digest, (PAS, 20x [a], 100x [b]). An overview and magnified image of a normal rat vascular network.
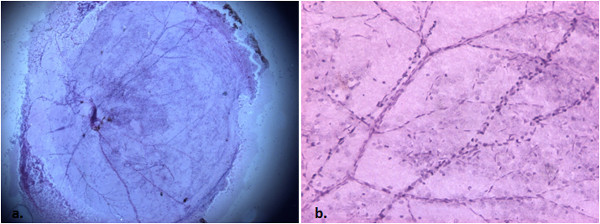
Figure 6. Control C57/Bl6 mouse trypsin digest, non-vascular tissue intact (H&E, 20x [a], 100x [b]). With poor removal of the non-vascular tissue, there is non-specific staining impairing visualization of vessels.
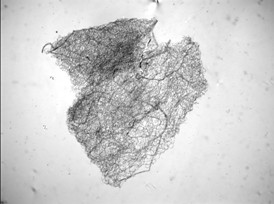
Figure 7. Control db/m mouse trypsin digest, poor mounting (H&E, 20x). If the retina is not adequately unfolded, there is inadequate visualization of the vascular network.
List of Materials
| REAGENTS | |||
| 10% neutral buffered formalin | Fischer Scientific | SF100-4 | |
| Trypsin (1:250) | Amresco | 0458-50G | Make 3% trypsin in 0.1 M Tris |
| TRIZMA base | Sigma | T1503-1KG | Create 0.1 M Tris Buffer (pH 7.8 @ 37 °C) |
| TRIZMA | Sigma | T3253-500G | |
| Steritop sterile vacuum bottle | Millipore | SCGPS05RE | Create filtered water |
| EQUIPMENT | |||
| Dissecting microscope | Any microscope that allows good visualization of the retina is adequate. (10x is sufficient with working distance of 24 mm) | ||
| Straight scissors | Fine Science Tools | 15024-10 | Cutting edge: 8 mm; Tip diameter: 0.2 mm; 10 cm |
| Straight Forceps (Inox) | Dumont #5 | 11252-20 | Biologie tip; 0.05 x 0.02 mm; 11 cm |
| Curved Forcepts (Dumostar) | Dumont #7 | 11297-10 | Biologie tip; 0.07 x 0.04 mm; 11.5 cm |
| Dubnoff Metabolic Shaker Incubator | Precision Scientific | BDG59442 | Shaker optional |
| 24-well dish | Falcon | 353047 | |
| Microscope Slides | VWR | 82027-132 |
Lab Prep
Trypsin digest is the gold standard method to analyze the retinal vasculature 1-5. It allows visualization of the entire network of complex three-dimensional retinal blood vessels and capillaries by creating a two-dimensional flat-mount of the interconnected vascular channels after digestion of the non-vascular components of the retina. This allows one to study various pathologic vascular changes, such as microaneurysms, capillary degeneration, and abnormal endothelial to pericyte ratios. However, the method is technically challenging, especially in mice, which have become the most widely available animal model to study the retina because of the ease of genetic manipulations 6,7. In the mouse eye, it is particularly difficult to completely remove the non-vascular components while maintaining the overall architecture of the retinal blood vessels. To date, there is a dearth of literature that describes the trypsin digest technique in detail in the mouse. This manuscript provides a detailed step-by-step methodology of the trypsin digest in mouse retina, while also providing tips on troubleshooting difficult steps.
Trypsin digest is the gold standard method to analyze the retinal vasculature 1-5. It allows visualization of the entire network of complex three-dimensional retinal blood vessels and capillaries by creating a two-dimensional flat-mount of the interconnected vascular channels after digestion of the non-vascular components of the retina. This allows one to study various pathologic vascular changes, such as microaneurysms, capillary degeneration, and abnormal endothelial to pericyte ratios. However, the method is technically challenging, especially in mice, which have become the most widely available animal model to study the retina because of the ease of genetic manipulations 6,7. In the mouse eye, it is particularly difficult to completely remove the non-vascular components while maintaining the overall architecture of the retinal blood vessels. To date, there is a dearth of literature that describes the trypsin digest technique in detail in the mouse. This manuscript provides a detailed step-by-step methodology of the trypsin digest in mouse retina, while also providing tips on troubleshooting difficult steps.
Procedure
Trypsin digest is the gold standard method to analyze the retinal vasculature 1-5. It allows visualization of the entire network of complex three-dimensional retinal blood vessels and capillaries by creating a two-dimensional flat-mount of the interconnected vascular channels after digestion of the non-vascular components of the retina. This allows one to study various pathologic vascular changes, such as microaneurysms, capillary degeneration, and abnormal endothelial to pericyte ratios. However, the method is technically challenging, especially in mice, which have become the most widely available animal model to study the retina because of the ease of genetic manipulations 6,7. In the mouse eye, it is particularly difficult to completely remove the non-vascular components while maintaining the overall architecture of the retinal blood vessels. To date, there is a dearth of literature that describes the trypsin digest technique in detail in the mouse. This manuscript provides a detailed step-by-step methodology of the trypsin digest in mouse retina, while also providing tips on troubleshooting difficult steps.
