Efficient Derivation of Retinal Pigment Epithelium Cells from Stem Cells
Instructor Prep
concepts
Student Protocol
1. Directed Differentiation of Stem Cell-derived RPE
NOTE: All incubation steps are carried out at 37 °C in 5% CO2
- Maintain the hES or hiPS lines (feed daily) in maintenance media (MM; Table 1). Once the lines have reached sufficient standards of quality control, maintain them on a layer of mouse embryonic feeder cells (MEFs) seeded at a density of 250,000/well of a six well plate. Xeno-free cultures can also be generated following the protocol established by Tucker et al.40.
- Allow the stem cell colonies to reach confluency.
- Switch to differentiation media with Nicotinamide (DM/NIC; Table 1); feed daily.
- After three weeks in culture, switch to differentiation media with nicotinamide and either Activin-A or IDE-1 (DM/NIC/AA or DM/NIC/IDE1; Table 1) to enhance RPE differentiation.
- After five weeks in culture, switch back to DM/NIC. Daily feedings are no longer necessary once the pH indicators in the media stop turning yellow the day after feeding.
- Wait for islets of pigmented cells to appear that are large enough to be cut and removed (See Figures 2D-F and 3A).
2. Isolating Pigmented Islets
- Coat the wells of a 24-well plate with a matrix that mimics the natural cell environment (xeno-free is preferable).
NOTE: Cornea blades and sharp, fine-tipped forceps are very useful for picking. The entire sheet of differentiated cells can detach while picking. Avoid this by working carefully and initially picking colonies in the center of the dish. - Fill as many wells of the 24-well plate with differentiation media (DM; Table 1) as needed. This decision will depend on the number of pigmented colonies collected.
- In a cell culture hood with a dissecting scope, manually cut out pigmented islets. Chop up each islet at the bottom of the original plate using a scalpel in about 2-6 pieces and grab the pigmented parts with sharp forceps.
- Transfer 1-2 pigmented pieces into each 24-well.
- Do not change media for 3-5 days until cells adhere, then start changing media three times per week. (If they do not adhere after this time, the likelihood is good that they never will).
- Expand cells for 3-4 weeks in differentiation media (DM; Table 1) — An image of a typical culture is shown in Figure 3B. Cells may still not fill the entire well but waiting longer does not seem to help. Feed cells three times per week.
- After about 3 weeks, manually remove clusters of white cells in clumps if necessary with sharp forceps. Do not do this step unless it is necessary – it is easy to introduce contaminants. It is ideal to try to obtain purest populations of RPE from the original pigmented colonies in step 1.6.
3. Passaging Stem Cell-derived RPE
- Detach cells with 200 µl with a cell dissociation solution (preferably not trypsin—a reagent that can be inactivated through dilution is preferable) for 5-8 min at 37 °C, and inactivate it by diluting with DM. Use a 200 µl pipette to wash off all cells and to resuspend them (If the selected well contains only a few cells, consider pooling the cells from two 24-wells).
- Centrifuge (800 x g for 5 min), discard supernatant, and resuspend in 3 ml DM media.
- Re-plate cells from one (or two) 24-wells into one matrix coated 6-well in 3 ml of DM per well (1:5 expansion).
- Expand for 1-2 weeks until the cells are ~90% confluent; an image of fully differentiated pure culture is shown in Figure 3C.
- Detach cells with 1 ml cell dissociation solution for about 5-8 min at 37 °C, inactivate it by dilution with DM, use 1,000 µl pipette to wash off all cells, and resuspend them.
- Spin down (800 x g for 5 min), discard supernatant, and resuspend in 18 ml DM media.
- Re-plate cells from one 6-well each into six matrix coated 6-wells in 3 ml of DM per well (1:6 expansion) or one-coated 75cm2 flask (1:7.5 expansion).
- Repeat steps 3.4–3.8 as necessary until enough cells are obtained.
NOTE: Try to collect as many colonies as possible for expansion rather than planning to amplify the cultures via passaging since each passage induces RPE dedifferentiation.
Efficient Derivation of Retinal Pigment Epithelium Cells from Stem Cells
Learning Objectives
The steps outlined in this manuscript, as depicted in Figure 1, can be used to readily generate RPE from stem cells as previously reported10,12. After maintaining the iPS lines for several weeks, pigmented colonies begin to appear in the colonies after 5-7 weeks (7 week old cultures are shown in Figure 2A-C). These colonies can continue to grow for weeks as the cultures are maintained. Once reaching sufficient sizes, as shown in Figure 2D-F (8 week old cultures), they can be manually excised as illustrated in Figure 3A. Careful excision to avoid contamination with non-RPE cells will greatly facilitate the generation of sufficiently pure RPE cultures (Figure 3B-C).

Figure 1: Schematic depicting iPS-RPE derivation. Naive stem cells are cultured in maintenance media until reaching confluency. At day 0 differentiation media (DM) lacking bFGF but containing nicotinamide is added (DM/NIC). The cells are fed daily with this media for three weeks. At the end of week three the DM media is supplemented with either recombinant Activin A (DM/NIC/AA) or IDE-1 (DM/NIC/IDE1) to enhance RPE specification and the cells are fed with this media for two weeks. During this treatment pigmented colonies begin to appear, these enlarge over the next few weeks and by week 8 can be manually removed and transferred to new plates for expansion in DM. (See Table 1 for specific media components) Please click here to view a larger version of this figure.
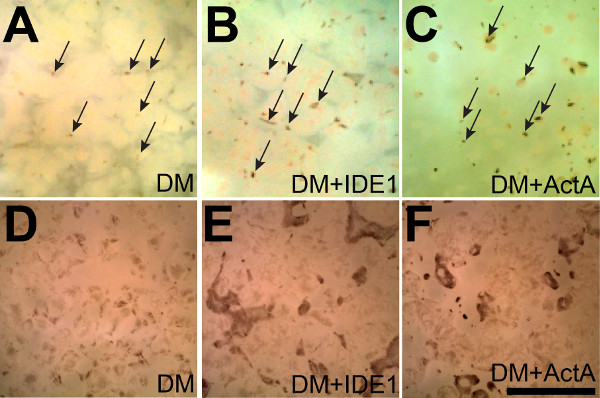
Figure 2: Activin A and IDE-1 enhance the yield of iPS-RPE. (A) Small pigmented colonies begin to appear after seven weeks in culture spontaneously between non-RPE cells in a single sheet that is adherent to the bottom of a 6-well plate (some are marked with arrows). (B and C) Supplementation with IDE-1 or Activin A results in the appearance of even more pigmented colonies (some are marked with arrows in A-C) demonstrating that supplementation with either Activin A or IDE-1 enhances RPE differentiation. (D-F) After 8 weeks the effects of supplementation with IDE-1 or Activin A are even more pronounced. Both the numbers and sizes of the pigmented islets are larger after directed differentiation. Scale bar = 5 mm
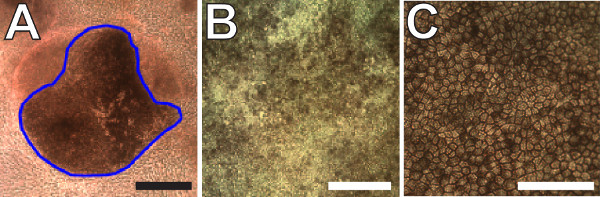
Figure 3: Expansion and terminal differentiation of pigmented iPS-RPE. (A) Representative image of a pigmented iPS-RPE colony that is large enough to excise. Non-RPE cells surround the colony in the bottom of a 6-well plate. The blue outline marks the region that would be excised. (B) Image of post-confluent immature iPS-RPE cells in culture after the first expansion step. (C) Two month old terminally differentiated iPS-RPE cells. Note the presence of obvious cell boundaries and homogenous levels of pigmentation demonstrating advanced differentiation. Scale bars = 100 µm
List of Materials
| Name of Material/ Equipment (A-Z) | Company | Catalog Number | Comments/Description |
| Corneal knife | Surgipro | SPOI-070 | knife x 1 |
| DMEM/F-12, HEPES | Life Technologies | 11330-032 | 500 mL x 4 |
| Dulbecco's Phosphate-Buffered Saline, 1X w/out Ca or Mg | VWR | 45000-434 | 500 mL x 6 |
| Fetal Bovine Serum, Regular (Heat Inactivated) | VWR | 45000-736 | 500 mL x 1 |
| FGF-Basic (AA 10-155) Recombinant Human Protein | Life Technologies | PHG0021 | 100 ug x 1 |
| IDE-1 | Stemgent | 04-0026 | 2 mg x 1 |
| Knockout DMEM | Life Technologies | 10829-018 | 500 mL x 1 |
| KnockOut Serum Replacement | Life Technologies | 10828-028 | 500 mL x 1 |
| L-Glutamine 200 mM | Life Technologies | 25030-081 | 100 mL x 1 |
| MEM Non-Essential Amino Acids Solution 100X | Life Technologies | 11140-050 | 100 mL x 1 |
| Nicotinamide | Sigma-Aldrich | N0636-100G | 100 g x 1 |
| Penicillin-Streptomycin (10,000 U/mL) | Life Technologies | 15140-148 | 20 mL x 1 |
| Recombinant Human/Murine/Rat Activin A | PeproTech | 120-14E | 10 ug x 2 |
| Synthemax-T Surface 6 Well Plates | Corning | 3877 | Case(12) x 1 |
| TrypLE-Express Enzyme (1X), no phenol red | Life Technologies | 12604-021 | 500 mL x 1 |
| Vacuum Filter/Storage Bottle System, 0.1µm pore, 500mL | Corning | 431475 | Case(12) x 1 |
Lab Prep
No cure has been discovered for age-related macular degeneration (AMD), the leading cause of vision loss in people over the age of 55. AMD is complex multifactorial disease with an unknown etiology, although it is largely thought to occur due to death or dysfunction of the retinal pigment epithelium (RPE), a monolayer of cells that underlies the retina and provides critical support for photoreceptors. RPE cell replacement strategies may hold great promise for providing therapeutic relief for a large subset of AMD patients, and RPE cells that strongly resemble primary human cells (hRPE) have been generated in multiple independent labs, including our own. In addition, the uses for iPS-RPE are not limited to cell-based therapies, but also have been used to model RPE diseases. These types of studies may not only elucidate the molecular bases of the diseases, but also serve as invaluable tools for developing and testing novel drugs. We present here an optimized protocol for directed differentiation of RPE from stem cells. Adding nicotinamide and either Activin A or IDE-1, a small molecule that mimics its effects, at specific time points, greatly enhances the yield of RPE cells. Using this technique we can derive large numbers of low passage RPE in as early as three months.
No cure has been discovered for age-related macular degeneration (AMD), the leading cause of vision loss in people over the age of 55. AMD is complex multifactorial disease with an unknown etiology, although it is largely thought to occur due to death or dysfunction of the retinal pigment epithelium (RPE), a monolayer of cells that underlies the retina and provides critical support for photoreceptors. RPE cell replacement strategies may hold great promise for providing therapeutic relief for a large subset of AMD patients, and RPE cells that strongly resemble primary human cells (hRPE) have been generated in multiple independent labs, including our own. In addition, the uses for iPS-RPE are not limited to cell-based therapies, but also have been used to model RPE diseases. These types of studies may not only elucidate the molecular bases of the diseases, but also serve as invaluable tools for developing and testing novel drugs. We present here an optimized protocol for directed differentiation of RPE from stem cells. Adding nicotinamide and either Activin A or IDE-1, a small molecule that mimics its effects, at specific time points, greatly enhances the yield of RPE cells. Using this technique we can derive large numbers of low passage RPE in as early as three months.
Procedure
No cure has been discovered for age-related macular degeneration (AMD), the leading cause of vision loss in people over the age of 55. AMD is complex multifactorial disease with an unknown etiology, although it is largely thought to occur due to death or dysfunction of the retinal pigment epithelium (RPE), a monolayer of cells that underlies the retina and provides critical support for photoreceptors. RPE cell replacement strategies may hold great promise for providing therapeutic relief for a large subset of AMD patients, and RPE cells that strongly resemble primary human cells (hRPE) have been generated in multiple independent labs, including our own. In addition, the uses for iPS-RPE are not limited to cell-based therapies, but also have been used to model RPE diseases. These types of studies may not only elucidate the molecular bases of the diseases, but also serve as invaluable tools for developing and testing novel drugs. We present here an optimized protocol for directed differentiation of RPE from stem cells. Adding nicotinamide and either Activin A or IDE-1, a small molecule that mimics its effects, at specific time points, greatly enhances the yield of RPE cells. Using this technique we can derive large numbers of low passage RPE in as early as three months.
