Polyelectrolyte Complex for Heparin Binding Domain Osteogenic Growth Factor Delivery
Instructor Prep
concepts
Student Protocol
1. Alginate Solution Preparation
- Dissolve 200 mg of sodium alginate (non-irradiated) or 400 mg of 8 MRad irradiated sodium alginate in 10 ml double distilled water and shake for 1 hr for non-radiated alginate and 15 min for irradiated alginate. Store the alginate solution at 4 °C overnight. Filter the alginate solution with a sterile 0.2 µm syringe filter before alginate microbead fabrication.
2. Alginate Microbead Fabrication
- Disinfect the electrostatic bead generator and syringe pump with 70% ethanol and place them in a Class II Biological Safety Cabinet (Figure 1).
- Place a glass basin with a magnetic stir bar inside the bead generator.
- Set the arm electrode of the bead generator 9 cm above the basin.
- Connect the electrode cable of the bead generator to the knurled screw 2 of the arm electrode, and pour 80 ml of SrCl2 solution into the basin.
- Load 5 ml of 0.2 µm filtered alginate solution into the syringe and rubber tube. After connecting the rubber tube to the arm electrode, switch on the syringe pump at 5 ml/hr for 2 min to expel the air inside the tubing and deliver the alginate solution to the tip of the nozzle. Turn off the syringe pump.
- Next, switch on the encapsulator and then, the syringe pump to commence microbead generation. Set the alginate flow rate at 5 ml/hr and the voltage at 5.8 kV on the encapsulator. Discard the microbeads generated during the first two minutes (or the initial 0.5 ml alginate solution pumped out of the syringe), as these microbeads tend to be irregularly sized and shaped.
- Collect subsequent microbeads in 0.2 M strontium chloride solution. Turn off both the syringe pump and the encapsulator (in that order) after pumping the pre-planned volume of alginate solution. Repeat this for subsequent batches of microbead fabrication. Upon completion, turn off the syringe pump first, followed by the encapsulator.
- Store the microbeads in 20 ml of 0.2 M strontium chloride solution at 4 °C overnight to complete cross linking and stabilize the gel.
3. Size Measurement of Alginate Microbeads
- Collect 0.5 ml of alginate microbeads with a plastic pipette and place it on a glass slide. View the microbeads under an optical microscope at 10X magnification. Take ten images of the microbeads with a microscope CCD camera. Save the images with scale bar (500 µm) in TIFF format at resolution 2,048 x 1,536.
- Using the length tools in ImageJ, measure the size of the microbeads and scale bar (Figure 2). Convert the microbeads length from pixel to micrometer.
- Click on the line tools and draw a line across the middle of the alginate bead.
- Click "Analyze" on the menu bar and select "Measure". A pop-up window will appear.
- Repeat steps 3.2.1-3.2.2 to measure all alginate beads within the image. Measure the scale bar on the image.
- Convert the diameter of the alginate bead to the actual length by using the formula:length of alginate bead/length of scale bar x 500 µm. For example, 1.420 (diameter measured by ImageJ) / 2.657 (scale bar length measured by ImageJ) * 500 µm = 267 µm.
- Consider the average size of 100 microbeads (mean ± standard deviation) as the representative size of each batch of microbeads.
4. Sterilization
- Collect the microbeads using a 100 µm nylon strainer and wash the beads with double distilled water.
- Using a spatula, transfer all the microbeads made from 0.1 ml alginate solution into a 2 ml microcentrifuge tube and cover with gauze to prevent drying.
- Finally, sterilize the microbeads by autoclaving using liquid mode (115 °C, 15 min) or in accordance with manufacturer's specifications. Add 1.5 L of distilled water to the chamber to prevent beads from drying.
5. Protamine and Heparin Coating
- Inside the BSL-2 hood, incubate the sterile microbeads with 1 ml of 2 mg/ml protamine solution (sterilized using a 0.2 µm syringe filter) for 1 hr at room temperature.
- After incubating the microbeads for 1 hr (Step 5.1), collect 150 µl of the protamine solution for the micro bicinchoninic acid (microBCA) test (section 6).
- Wash the protamine coated microbeads twice with double distilled water. Spin down using a bench top centrifuge at 200 x g for 3 min at room temperature. After centrifugation, aspirate the water using a syringe.
- Incubate protamine coated microbeads with 1 ml of 0.5 mg/ml heparin solution (sterilized using a 0.2 µm syringe filter) for 30 min to create polyelectrolyte complex (PEC).
- After incubating the protamine coated microbeads for 30 min (Step 5.4), collect 400 µl of the heparin solution to determine heparin content (section 7).
- After incubation, wash off the unbound heparin from the PECs by washing twice with double distilled water.
6. Protamine Content
- Perform the microBCA test according to manufacturer instructions. Briefly, add 150 µl protamine solution (collected before and after incubation with microbeads) into a 96 well plate. Add 150 µl microBCA working solution.
- Use albumin solution (0, 0.5, 1, 2, 5, 10, 20, 40 and 200 µg/ml) as calibration standards.
- Incubate the mixture for 60 min at 60 °C. Measure the absorbance with a spectrophotometer at 562 nm.
- Use the standard curve to determine the protamine concentration of each unknown sample according to manufacturer's instructions.
- Determine the protamine content of the microbeads by subtracting the total amount of protamine in the coating solution (before incubation with microbeads) from the amount of protamine remaining in the coating solution (after incubation with microbeads).
7. Heparin Content
- Prepare the 10 ml of working solution by dissolving 4 mg toluidine blue and 20 mg sodium chloride in 0.01 N hydrochloric acid.
- Add 400 µl of sample (from step 5.5) to the working solution at a ratio of 2:3 and vortex for 30 sec.
- Add 600 µl of n-hexane (equivalent volume to the working reagent solution) and vortex the mixture to extract the toluidine blue heparin complex.
- Aspirate 200 µl of the aqueous phase by syringe after phase separation.
- Measure the amount of un-extracted toluidine blue contained in the aqueous phase using a spectrophotometer at 631 nm.
- Prepare heparin standard solutions of 0-20 µg/ml.
- Plot the 631 nm reading of each heparin standard vs. heparin concentration in µg/ml. Use the standard curve to determine the heparin concentration of each sample.
8. Confocal Image of Layer-by-layer Structure
- Fabricate protamine, heparin and NELL-1/BMP-2 fluorescent analog CF-405 protamine (blue), CF 594 heparin (red) and FITC labeled NELL-1/FITC labeled (green) BMP-2 + heparin + protamine according to manufacturer's technical datasheet.
- Coat 100 µg microbeads with 300 µl of fluorescent analog (coating method as described in 5.3-5.6) CF-405 protamine (blue) (2 mg/ml, 1 hr incubation), CF 594 heparin (red) (0.5 mg/ml, 30 min) and FITC labeled NELL-1/FITC labeled (green) BMP-2 (1.5 mg/ml, overnight). Wash the microbeads twice with distilled water to eliminate the unbound fluorescent protamine, heparin and NELL-1/BMP-2.
- Observe the layer-by-layer structure (Figure 3) by using a confocal microscope at 10X magnification.7
9. BMP-2 and NELL-1 Uptake and Release
- Load 13.3 µl of 1.5 mg/ml of BMP-2 or NELL-1 solution on 100 µg of PEC. Incubate PEC at 4 °C under 30 rpm shaking for 10 hr.
- Immerse the microbeads in 1 ml of phosphate buffered saline (PBS) at 37 °C with constant shaking (30 rpm).
- Collect 1 ml of the supernatant and replace it with 1 ml PBS after 1, 3, 6, 10 and 14 days.
- Evaluate the uptake and release efficiency of BMP-2 using the ELISA method according to the manufacturer's protocol. Evaluate the uptake and release efficiency of NELL-1 using the carboxybenzoyl quinoline-2-carboxaldehyde (CBQCA) protein assay method according to the manufacturer's protocol.
- Determine cumulative release at time (t):
Cumulative release at time (t) = Release at time (t) + Previous release at time (t-1). - Plot cumulative release of BMP-2 and NELL-1 against time.
10. In Vitro Bioactivity of NELL-1
Note: The bioactivity of NELL-1 released from PEC was assessed by measuring its ability to increase the expression of alkaline phosphatase (ALP) in rabbit bone marrow stem cells (rBMSC).
- Seed 20,000 rBMSCs per well in a 24-well plate and allow them to grow for one day with 1 ml of Dulbecco's Modified Eagle's Medium (DMEM) + 10% Fetal bovine serum (FBS) at 37 °C and 5% CO2.
- After 24 hr, replace the medium with 1 ml of an osteogenic medium (DMEM supplemented with 10% FBS, 2% penicillin streptomycin, 50 µg/ml ascorbic acid, 10 mmol/L beta-glycerophosphate, and 10-8 mol/L dexamethasone) for 7 days at 37 °C and 5% CO2.
- Place 300 µg PEC-NELL-1 (from step 8.2) and PEC inside cell culture inserts (TC insert) to keep the PECs separate from the cells (this avoids the washout of PEC microbeads during the osteogenic medium change). Place TC insert into the 24 well plate for 14 days.
- Once every three days, aspirate 1 ml of the osteogenic medium by placing a needle outside the TC insert, and replace with 1 ml of fresh osteogenic medium.
- After 7 and 14 days of incubation, determine ALP activity with an ALP assay kit in accordance with the kit manufacturer's protocol.
- Lyse cells with assay buffer containing 0.1% TritonX-100 at 4 °C for 10 min. Scrape off adhered cells using a cell scraper. Incubate cell suspension at 4 °C under agitation for at least 60 min.
- Centrifuge the cell suspension at 2,500 x g at 4 °C for 10 min. Collect the supernatant for the ALP assay.
- Add 50 µl of serially diluted alkaline phosphatase standard solution from 200 to 0 ng/ml to the wells of a 96 well plate. The final amounts of alkaline phosphatase standard are 10, 5, 2.5, 1.2, 0.6, 0.3, 0.15, and 0 ng/well.
- Add the supernatant from step 10.5.2 (50 µl/well) and dilute with dilution buffer.
- Add 50 µl of p-nitrophenyl phosphate (pNPP) substrate solution into each well. Mix the reagents by gently shaking the plate for 30 sec. Incubate the mixture for 30 min in the dark. Measure the absorbance at 405 nm by plate reader.
- Calculate ALP activity using the calibration curve.
- Determine protein content using the microBCA protein assay kit according to manufacturer's instructions. Normalize the ALP activity by dividing ALP activity by protein content.
11. Cell Viability
- Incubate 200 mg of PEC-NELL-1 with 1 ml of DMEM +10% FBS at 37 °C for 24 hr for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
- Seed 2,000 rBMSCs per well (in 100 µl of DMEM with 10% FBS) in a 96 well plate and incubate for 1 day at 37 °C, 5% CO2.
- Replace DMEM + 10% FBS with 100 µl of PEC-NELL-1/PEC extract and incubate at 37 °C, 5% CO2.
- After 1 day or 3 days of incubation, add 10 µl of 5 mg/ml MTT solution and further incubate at 37 °C, 5% CO2 for 4 hr in darkness.
- Add 100 µl DMSO solution to each well to dissolve formazan crystals.
- Determine the absorbance at 570 nm using a microplate reader.
- Calculate the relative growth rate:
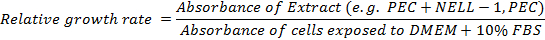
12. Packaging into Scaffold and BMP-2 & NELL-1 Loading
- Pack the PECs into the pores of a bioresorbable medical grade Polycaprolactone – tri-calcium phosphate (mPCL-TCP) scaffold using a sterilized spatula inside a BSL-2 chamber.
- Add 1.5 mg/ml solution of BMP-2 or NELL-1 onto the mPCL-TCP scaffold packed with PEC and incubate overnight at 4 °C.
Polyelectrolyte Complex for Heparin Binding Domain Osteogenic Growth Factor Delivery
Learning Objectives
In our carrier, protamine was chosen as a substitute of poly-L-lysine as it has similar chemical properties and it is FDA approved as an antidote of heparin. Optical microscope results showed that the non-irradiated microbeads were spherical in shape with a diameter of 267 ± 14 µm. (0.35 mm nozzle, flow rate of 5 ml/hr & 5.8 kV). The majority of the irradiated microbeads are of teardrop shape. The diameter measured on the round portion of the irradiated microbeads was 212 ± 30 µm (0.35 mm nozzle, flow rate of 4 ml/hr & 6 kV). (Figure 4).
Confocal images of the PEC microbeads reveal layer-by-layer coating of CF-405 labeled protamine (blue), CF594-labeled heparin (red) and FITC-BMP-2/FITC-NELL-1 (green). The results indicate that the PECs can bind with positively charged BMP-2 and negatively charged NELL-1 via the heparin binding domain (Figure 5). This suggests that the interaction between the PECs and the osteogenic growth factors is not charge dependent.
To prove that the PECs can uptake and release BMP-2, we used an ELISA assay to determine the amount of BMP-2 remaining after incubation and the amount of BMP-2 in the PBS on Day 1, 3, 6, 10 and 14. However, a similar approach does not work well with the NELL-1 protein, since heparin blocks the antibody binding site, significantly reducing the signal. Therefore, the CBQCA protein assay was used to determine the difference between PEC-NELL-1 and PEC. From the cumulative release curve, PECs not only show a higher NELL-1 uptake efficiency compared to BMP-2 but also release it much slower than BMP-2 (NELL-1: 20% vs. BMP-2: 25%) (Figure 6). This suggests that PECs bind more tightly with NELL-1 than BMP-2.
From the MTT assay, PEC-NELL-1 is not cytotoxic (Figure 7). The result matches with the Alamar Blue assay result in a previous study7. Heparin neutralizes the positive charge of protamine which plays an important role in maintaining the biocompatibility of PEC.
To determine whether NELL-1 release from PEC affects long-term osteogenic differentiation, the expression level of an osteogenic marker, alkaline phosphatase (ALP), was investigated by a colorimetric assay. NELL-1 release from PEC increases the ALP activity of rabbit bone marrow stem cells by 2.2 fold at day 14 compared to the PEC control group (Figure 8). BMP-2 shows maximum increase of ALP activity (3.75 fold) on day 7. Both PEC-BMP-2 and PEC-BMP-2 + extra BMP-2 show decrease of activity on day 9.
ALP activity with BMP-2 in the medium without PEC is shown in Figure 8C. Although 70% of the growth factor remains on the PEC, the ALP activity of PEC BMP-2 group is equivalent to the free BMP-group. In vivo, growth factor must be delivered by the carrier to avoid washout. From our rodent and porcine model, we can lower the dose of BMP-2 by 20-fold and 6-fold, respectively. The reduction of does not only reduce unwanted side effects but also cuts down the cost of growth factor usage.

Figure 1: Electrostatic bead generator and syringe pump set up in BSL-2 biosafety cabinet. (A) Nozzle secured on nozzle holder. (B) Big basin for strontium chloride solution. (C) Electrode cable. (D) Hose delivers alginate solution. (E) Nozzle holder and arm. (F) Electrodes supply the potential difference to regulate the bead size. (G) Magnetic stirrer. (H) 5 ml syringe. (I) Syringe pump to regulate alginate flow. (J) Agitator control knob to control stirring speed. (K) Voltage control knob to regulate the potential difference between 0-10 kV. Please click here to view a larger version of this figure.
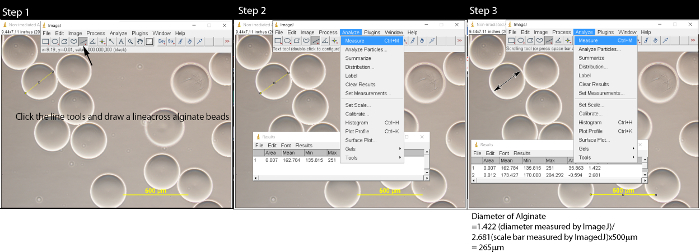
Figure 2: Measuring alginate microbeads with ImageJ software. After opening the image file by clicking File → Open, follow Step 1: click the line tool and draw a line on the alginate microbeads. In Step 2, click Analyze on the menu bar and a pop-up window will appear. Repeat Step 1 and Step 2 until all microbeads are measured. In Step 3, click the line tool, draw a line across the scale bar and measure the length of the scale bar. Convert the microbeads length to µm by using formula: length of alginate/length of Scale bar x 500 µm. Please click here to view a larger version of this figure.
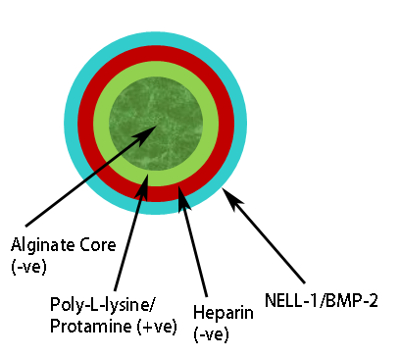
Figure 3: Schematic representation of Polyelectrolyte Complex. Alginate core (dark green) positive charge protamine layer (pale green), negative charge heparin layer (red), osteogenic growth factor, e.g., BMP-2/NELL-1 on outermost layer (pale blue). Please click here to view a larger version of this figure.
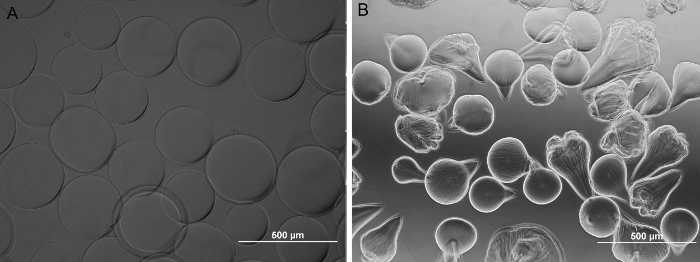
Figure 4: Bright field images of alginate microbeads. (A) Non-irradiated. (B) 8M Rad irradiated. Non-irradiated alginate microbeads are spherical and the 8M Rad irradiated counterpart is teardrop shaped. Magnification 100X. (Scale bar = 500 µm.) Please click here to view a larger version of this figure.
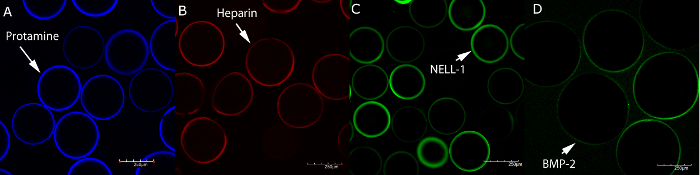
Figure 5: Confocal laser scanning microscopy images of alginate microbeads incubated with fluorescent analogues of protamine, heparin, NELL-1 and BMP-2. (A) CF 405 Protamine (blue), (B) CF 594 Heparin (red), (C) FITC NELL-1 (green), and (D) FITC BMP-2 (green). Microbeads remain spherical even after incubation with fluorescent analogues. Scale bar = 250 µm. Please click here to view a larger version of this figure.
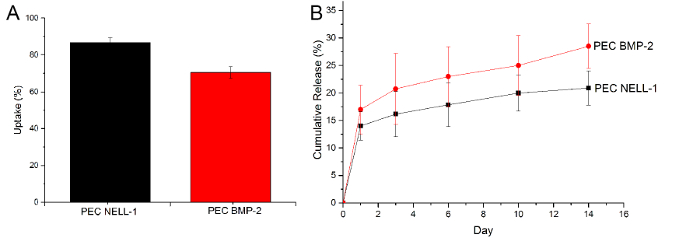
Figure 6: Uptake and release of BMP-2 and NELL-1. (A) Uptake of NELL-1 protein (black) BMP-2 (red), (B) Cumulative release curve of BMP-2 (red) and NELL-1 (black) from PEC carrier. Results are presented as mean ± standard deviation. Please click here to view a larger version of this figure.
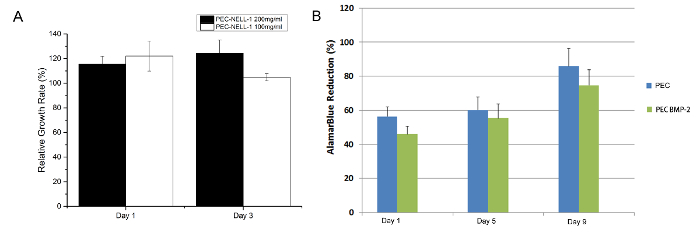
Figure 7: Cytotoxicity Assay. (A) MTT assay of rabbit bone marrow stems cell with protamine based PEC NELL-1 200 mg/ml extract (black), PEC-NELL-1 100 mg/ml extract (white) at Day 1 and 3. (B) Alamar Blue assay of rabbit bone marrow stems cell with PEC BMP-2 (blue), PEC (green) Experiments were performed in triplicate and results are presented as mean ± standard deviation. Please click here to view a larger version of this figure.
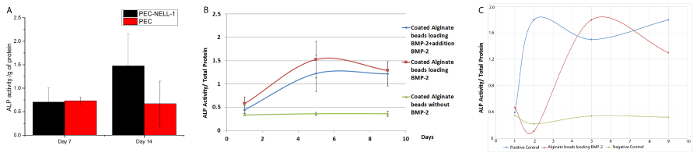
Figure 8: Bioactivity Assay for BMP-2 and NELL-1 release from PEC carrier. (A) ALP activity of rabbit bone marrow stem cell incubated with PEC-NELL-1 (black), PEC (red). ALP activity was measured as absorbance at 405 nm. 2.2 fold increase in ALP activity was observed after incubation with PEC-NELL-1 at Day 14. (B) ALP activity of rabbit bone marrow stem cell incubated with PEC-BMP-2 (red) PEC-BMP-2 + addition of BMP-2 (green) and PEC (blue). Increase in ALP activity after incubation with PEC-BMP-2 and PEC-BMP-2 + addition of BMP-2 at Day 7, ALP activity drops on Day 9. (C) ALP activity of rabbit bone marrow stem cell incubated with PEC-BMP-2 (red) BMP-2 (blue) and Medium (green). Results are presented as mean ± standard deviation. Please click here to view a larger version of this figure.
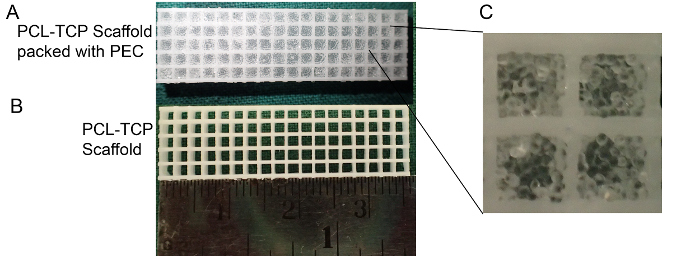
Figure 9: Polycaprolactone Tri-calcium phosphate (PCL-TCP) scaffold packed with PEC carrier. (A) Polycaprolactone Tri-calcium phosphate (PCL-TCP) scaffold (pore size 1,300 µm) packed with PEC carrier. (B) PCL-TCP scaffold. (C) High magnification: PEC maintains its shape after packing. Please click here to view a larger version of this figure.
List of Materials
| Life Science Acrodisc 25mm Syring Filter w/0.2 µm Supor Membrane | PALL | PN4612 | Sterile protamine, heparin solution by ultrafiltration |
| 24 well plate | Cell Star | 662160 | |
| 96 well plate Nuclon Delta Surface | Thermo Fisher Scientific | 167008 | |
| (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide), MTT | Sigma Aldrich | M5655 | Measure cytotoxicity of PEC-NELL-1 |
| Acetone | Fisher Scientific | A/0600/17 | Precipitate CF-405 Labelled protamine |
| Alamar Blue | Invitrogen, Life Technologies | DAL 1025 | Measure cytotoxicity of PEC-BMP-2 |
| Alkaline Phosphatase Assay (ALP) assay kit | Anaspec | AS-72146 | |
| Ammonium Chloride | Merck | Art 1145 | Stop reagent in FITC labelling |
| Anhydrous Dimethyl Sulfoxide (DMSO) | Invitrogen, Life Technologies | D12345 | Solvent for fluorescent isothiocyanate I |
| Dimethyl Sulfoxide (DMSO) | Sigma Aldrich | Dissolve formazan | |
| Autoclave | Hirayama | HU-110 | Sterilize alginate beads by steam |
| Beta-glycerophosphate | Sigma Aldrich | G9422 | |
| BMP-2 (Infuse Bone Graft Large II Kit) | Medtronic Sofarmor Danek, Memphis TN, USA | 7510800 | Osteogenic Growth Factor, dialysis is needed to remove stabilizer component that interferes with FITC coupling |
| Carboxybenzoyl quinoline-2-Carboxaldehyde (CBQCA) | Thermo Fisher Scientific | A-6222 | To quantify NELL-1 protein |
| Cell Strainer (100µm) | BD Science | 352360 | Hold PEC for ALP assay |
| Cell Scraper 290mm Bladewide 20mm | SPL Life Science | 90030 | Detach the cell from 24 well plate |
| CF 405S, Succinimidyl Ester | Sigma Aldrich | SCJ4600013 | Blue fluorescent dye for protamine labelling |
| CF 594, Hydrazide | Sigma Aldrich | SCJ4600031 | Deep red fluorescent dye for heparin labelling |
| Centrifuge | Beckman Coulter | Microfuge 22R | |
| Confocal Microscope | Olympus | FV1000 | |
| Dexamethasone | Sigma Aldrich | D4902 | Component of osteogenic growth medium |
| Dextran Desalting Columns | Pierce (Thermo Scientific) | 43230 | |
| DMEM | Gibco | 12320 | |
| BMP-2 Quantikine ELISA Kit | R&D System | DBP200 | Determine BMP-2 release |
| Fetal Bovine Serum FBS | Hyclone | SV30160.03 | |
| Fluoescein Isothiocyananate, Isomer I | Sigma Aldrich | F7250 | Green fluorescent dye for NELL-1 and BMP-2 labelling |
| ThinCert Cell Culture Inserts, For 24 Well plates, Sterile |
Greiner | 662630 | Prevents PEC wash out when changing osteogenic medium |
| Havard Appartus Syringe Pump (11 plus) | Havard Apparatus | 70-2208 | |
| n-Hexane (>99%) | Sigma Aldrich | 139386 | |
| Heparin | Sigma Aldrich | H3149 | Binds with osteogenic growth factor with heparin binding domain |
| Hydrochloric acid (37%) | Merck | 100317 | Highly Corrosive |
| Incubator | Binder | C8150 | |
| MicroBCA Protein Assay kit | Thermoscientific | 23235 | |
| Microplate Reader | Tecan | Infinite M200 | For ALP and microBCA assays |
| NELL-1 | Aragen Bioscience Morgan Hill, CA, USA | N/A | Osteogenic growth factor, keep at -80˚C |
| Nisco cell encapsulator | Nisco Engineering Inc | Encapsulation unit VAR V1 | |
| Fluorescent Microscope | Olympus | IX71 | |
| mPCL-TCP Scaffold (Pore size is 1.3mm) | Osteopore | PCL-TCP 0/90 | Hold PEC for in vivo study |
| Penicillin-Streptomycin 10,000 unit/ml, 100ml | Hyclone Cell Culture | SV30010 | Antibiotic |
| 10X Phosphate Buffered Saline (PBS) | Vivantis | PB0344-1L | 10x Solution, Ultra Pure Grade |
| Poly-L-Lysine MW 15,000-30,000 | Sigma Aldrich | P2568 | Polycation |
| Protamine Sulfate salt, from Salmon | Sigma Aldrich | P4020 | Polycation |
| Shaker | Labnet | S2025 | |
| Snakeskin Dialysis Tubing 3,500 MWCO 22mm x 35 feet | Thermo Fisher Scientific | 68035 | Remove unreacted FITC by dialysis |
| Sodium Chloride | Merck | 1.06404.1000 | |
| Sodium Hydroxide | Qrec | S5158 | |
| Sodium Bicarbonate | US Biological | S4000 | Buffer |
| Sodium carbonate | Sigma Aldrich | S7795-500G | Buffer |
| Strontium Chloride Hexahydrate | Sigma Aldrich | 255521 | Crosslinker for alginate |
| Spatula | 3dia | ||
| 5ml syringe | Terumo | 140425R | Diameter of syringe affects the flow rate |
| 75cm2 Cell Culture Flask Canted Neck | Corning | 730720 | |
| Toluidine Blue | Sigma Aldrich | 52040 | Heparin assay |
| Trypsin 1X | Hyclone Cell Culture | SH30042.01 | |
| Sodium alginate | Novamatrix (FMC Biopolymer, Princeton, NJ) | Pronova UPMVG | Core material of microbeads |
Lab Prep
During reconstructive bone surgeries, supraphysiological amounts of growth factors are empirically loaded onto scaffolds to promote successful bone fusion. Large doses of highly potent biological agents are required due to growth factor instability as a result of rapid enzymatic degradation as well as carrier inefficiencies in localizing sufficient amounts of growth factor at implant sites. Hence, strategies that prolong the stability of growth factors such as BMP-2/NELL-1, and control their release could actually lower their efficacious dose and thus reduce the need for larger doses during future bone regeneration surgeries. This in turn will reduce side effects and growth factor costs. Self-assembled PECs have been fabricated to provide better control of BMP-2/NELL-1 delivery via heparin binding and further potentiate growth factor bioactivity by enhancing in vivo stability. Here we illustrate the simplicity of PEC fabrication which aids in the delivery of a variety of growth factors during reconstructive bone surgeries.
During reconstructive bone surgeries, supraphysiological amounts of growth factors are empirically loaded onto scaffolds to promote successful bone fusion. Large doses of highly potent biological agents are required due to growth factor instability as a result of rapid enzymatic degradation as well as carrier inefficiencies in localizing sufficient amounts of growth factor at implant sites. Hence, strategies that prolong the stability of growth factors such as BMP-2/NELL-1, and control their release could actually lower their efficacious dose and thus reduce the need for larger doses during future bone regeneration surgeries. This in turn will reduce side effects and growth factor costs. Self-assembled PECs have been fabricated to provide better control of BMP-2/NELL-1 delivery via heparin binding and further potentiate growth factor bioactivity by enhancing in vivo stability. Here we illustrate the simplicity of PEC fabrication which aids in the delivery of a variety of growth factors during reconstructive bone surgeries.
Procedure
During reconstructive bone surgeries, supraphysiological amounts of growth factors are empirically loaded onto scaffolds to promote successful bone fusion. Large doses of highly potent biological agents are required due to growth factor instability as a result of rapid enzymatic degradation as well as carrier inefficiencies in localizing sufficient amounts of growth factor at implant sites. Hence, strategies that prolong the stability of growth factors such as BMP-2/NELL-1, and control their release could actually lower their efficacious dose and thus reduce the need for larger doses during future bone regeneration surgeries. This in turn will reduce side effects and growth factor costs. Self-assembled PECs have been fabricated to provide better control of BMP-2/NELL-1 delivery via heparin binding and further potentiate growth factor bioactivity by enhancing in vivo stability. Here we illustrate the simplicity of PEC fabrication which aids in the delivery of a variety of growth factors during reconstructive bone surgeries.
