Implementation of a Permeable Membrane Insert-based Infection System to Study the Effects of Secreted Bacterial Toxins on Mammalian Host Cells
Instructor Prep
concepts
Student Protocol
1. Bacterial Culture
- Generate isogenic mutant strains to be used for the study of the specific secreted factor of interest21.
Note: The GASM1T1 5448 strains utilized for these experiments included WT GAS', a sagAΔcat SLS-deficient mutant (abbreviated in text as ΔsagA), and a sagA complemented strain (abbreviated in text as ΔsagA+sagA)21. - Prepare overnight liquid cultures of bacterial strains of interest. Grow GAS M1T1 5448 strains in -10 ml of Todd-Hewitt broth at 37 °C for 16-20 hr prior to infecting human cells.
- Centrifuge overnight bacterial cultures (2,400 rcf for 10 min) and resuspend in fresh bacterial medium.
Note: Resuspension in the same volume as the overnight cultures were grown in (5-10 ml) generally produces a desirable initial optical density. - Normalize bacterial cultures to equal starting concentrations by diluting the re-suspended cultures in additional bacterial medium until the same optical density at 600 nm (OD600) is obtained for all strains to be tested (OD600 of approximately 1.0 is recommended for convenience).
Note: In these studies, stationary phase bacterial cultures were used to infect host cells immediately following normalization. However, as some strains of bacteria exit stationary phase nonsynchronously it may be more appropriate to begin host infections with log phase cultures if this is found to be the case for the strains of interest. - Prior to further analysis, perform a colony counting assay to determine the colony forming units (CFU) per milliliter present in the starting cultures so that the multiplicity of infection (MOI), or ratio of bacteria per host cell, can be determined.
- Prepare 1 ml serial dilutions of bacterial cultures that have been normalized to the desired optical density in phosphate buffered saline (PBS) or appropriate bacterial growth medium (Todd-Hewitt broth was used in these studies). Prepare dilutions ranging from 10-1 to 10-6 to produce at least one set of agar plates with countable colonies (30-300 colonies). Perform all dilutions in triplicate.
- Transfer 100 µl of each serial dilution onto an agar plate containing appropriate medium for the bacterial species being analyzed.
Note: Todd-Hewitt agar was used in these studies. - Use a glass or plastic bacterial spreader to evenly distribute the 100 µl aliquot across the entire surface of the agar plate, and continue spreading until the liquid has been absorbed into the agar. Perform under flame using sterile technique.
- Incubate the agar plates at 37 °C overnight or until countable colonies appear.
- Count the colonies that grow on each plate and calculate the CFU/ml in the original culture by the following formula: number of colonies x inverse of the serial dilution / culture volume plated in milliliters.
e.g. (200 colonies x 1/10-5 dilution) / 0.1 ml plated = 2.0 x 108 CFU/ml
2. Host Cell Culture
- Obtain an appropriate host cell line for the desired analyses.
Note: HaCaT human epithelial keratinocytes29, a kind gift from V. Nizet, were used for these studies.- Maintain HaCaT cells in 100 mm culture dishes in Dulbecco's Modified Eagle's Medium (DMEM) with 10% heat inactivated fetal bovine serum (FBS). Incubate at 37 °C with 5% CO2.
3. Host Cell Infection with Permeable Membrane Insert System
- Plate cells in appropriate tissue culture dishes and allow them to grow until they reach 90% confluency.
Note: The HaCaT cells used in these studies typically reach confluency within 2-3 days after plating.- For lysate collection (e.g. signaling analysis and adenosine triphosphate (ATP) determination assays), plate HaCaT cells in 6 well dishes at a seeding density of approximately 3 x 105 cells/well.
- For immunofluorescence imaging experiments, plate cells on sterile glass coverslips within 6 well dishes at a seeding density of approximately 3 x 105 cells/well.
- For ethidium homodimer membrane permeabilization and lactate dehydrogenase (LDH) release assays, plate HaCaT cells in 24 well dishes at a seeding density of approximately 5 x 104 cells/well.
- Immediately prior to treatment, wash cells with 1x sterile PBS.
- Apply fresh growth medium to cells. For the conditions described here, apply 2 ml medium per well for 6 well plates or 0.5 ml medium per well for 24 well plates. If pharmacological treatments are being tested, mix with fresh medium and apply during this step.
Note: Some assays may require alternative media. For example, as phenol red and high serum concentrations interfere with the LDH release assay, phenol red-free DMEM supplemented with 1% bovine serum albumin (BSA) (w/v), 2 mM L-glutamine, and 1 mM sodium pyruvate was utilized for these experiments. - After fresh medium has been applied, carefully place a sterile 0.4 µm permeable membrane insert in each well. Perform this step in a laminar flow hood, and use sterile forceps to transfer the permeable membrane insert into each well.
- Apply fresh cell culture medium to the upper chamber of each well according to the manufacturer's instructions. For the conditions described here, apply 1 ml medium per well for 6 well plates or 0.1 ml medium per well for 24 well plates.
- Apply an appropriate volume of normalized bacterial cultures (see section 1) to the upper chamber of the permeable membrane insert system. Use bacterial medium for uninfected control treatments. For the results described here, add 25 µl of the normalized cultures per chamber for 24 well plates and add 100 µl of the cultures per chamber for 6 well plates.
Note: In these studies, wild-type or mutant GAS was applied to host cells at a MOI of 10. MOI was calculated based on the final eukaryotic cell numbers (e.g. 2 x 106 cells/well), such that 10 times as many bacteria were added per host cell at the beginning of the infection period (e.g. 2 x 107 CFU per well). Appropriate MOI will vary with different bacterial species and with the desired follow-up analyses.
Note: In addition to using bacterial medium for uninfected controls, other useful controls for certain applications of this technique may include heat-killed bacteria or spent bacterial medium for comparison. - Incubate infected cells at 37 °C with 5% CO2.
4. Sample Collection
- To collect cell culture medium, host cell lysates, or glass coverslips with intact cells for follow-up analyses, begin by carefully removing the permeable membrane insert with sterile forceps.
- For Assessment of Secreted Host Protiens:
- Collect the medium from the lower chamber into 1.5 ml tubes. Avoid disturbing the monolayer during collection.
- Centrifuge samples at 14,000 rcf for 10 min at 4 °C to remove cellular debris.
- Remove all but 50 µl of the supernatant to a fresh 1.5 ml tube and store at -20 °C or use immediately.
- For Assessment of Host Cell Lystates:
- Gently aspirate the medium above the monolayer of host cells. Avoid disturbing the monolayer, as this may result in sample loss.
- Rinse cells once with 1x PBS.
- Gently aspirate PBS. Immediately apply an appropriate volume of ice-cold lysis buffer to achieve the desired protein concentration, and incubate the samples on ice for 15 min. Apply between 200 µl and 350 µl of lysis buffer per well of each 6 well plate to achieve protein concentrations between 0.5 and 1.5 mg/ml.
Note: The lysis buffer used in these studies was composed of deionized water, 1% (v/v) Nonidet P40 Substitute, a phosphatase inhibitor cocktail, and a protease inhibitor cocktail. Specific reagents are listed in the Materials and Equipment section. - Use a cell scraper to detach the cells from the plate surface of each well and pipette the entire contents of each well into a 1.5 ml tube.
- Centrifuge samples at 14,000 rcf for 20 min at 4 ˚C.
- To assess soluble lysate components, remove the supernatant to a fresh tube and store at -20 ˚C or use immediately.
- To assess nuclear or other insoluble lysate components, reserve the pellet and store at -20 ˚C or use immediately.
- For Assessment by Immunofluorescence Imaging:
- Aspirate medium and wash cells in 1x cold PBS.
- Fix cells overnight in 4% paraformaldehyde (PFA) solution in PBS (w/v).
Note: In these studies, 1 ml of PFA was added per well of each 6 well plate.
5. Suggested Applications
- Host Signal Transduction Analysis by SDS-Page and Western Blotting
- Collect host cell lysates as described in section 4.3.
- Determine the protein concentration of each sample lysate by bicinchoninic acid (BCA) assay or equivalent using protein standards30.
- Normalize protein concentrations between samples prior to loading and running the samples on a 4-15% polyacrylamide gel or appropriate alternative31.
- Normalize sample concentrations by pipetting the same protein amount from each sample (e.g. 20 µg) into a new 1.5 ml tube and adding variable volumes of lysis buffer (buffer volume = total volume – sample volume added) to each tube to make the total volume (e.g. 50 µl) and final protein concentration equivalent across samples.
- Transfer samples to a polyvinylidene fluoride (PVDF) membrane (or equivalent). For the results described here, transfer samples at 25 volts for 2 hr before increasing the voltage to 70 volts for an additional 45 min. Use transfer buffer composed of 200 mM Tris base, 1.5 M glycine and 20% methanol (v/v) in deionized water.
- Block membranes in 5% BSA + 0.1% Tween 20 in Tris buffered saline (TBS). Adjust accordingly based on manufacturer recommendations for the antibody to be used.
- Incubate membranes with primary antibodies overnight at 4 °C. Use at manufacturer recommended dilution.
- Wash membranes for 1.5 hr in TBS+0.1% Tween 20; refresh buffer every 10-15 min.
- Incubate with Horseradish Peroxidase (HRP)-conjugated secondary antibody at a dilution of 1:5,000 for 1.5 hr at room temperature.
- Wash membranes for 1.5 hr in TBS + 0.1% Tween 20; refresh buffer every 10 – 15 min.
- Incubate membranes with chemiluminescence detection reagent prior to developing on film according to the manufacturer's instructions. Other detection methods may be used as desired.
- Host Cell Immunofluorescence Staining and Imaging
- Fix coverslips containing host cells as described in section 4.4.
- Remove PFA solution and wash coverslips containing treated cells two times in PBS, aspirating between washes.
Note: PFA is highly toxic and must be disposed of appropriately. - Block the coverslips for 2 hr at room temperature in PBS with 1% (w/v) normal goat serum, 2% (v/v) Triton X-100, and 0.5% (v/v) Tween 20.
- Wash coverslips with PBS for 30 min, refreshing the buffer every 10 min.
- Incubate the coverslips with primary antibody at a 1:50 ratio (or as recommended by manufacturer) in blocking solution overnight at 4 °C.
- Wash the coverslips for 1.5 hr in PBS, refreshing the buffer every 30 min.
- Incubate the coverslips for 2 hr at room temperature in secondary antibody (Goat anti-rabbit IgG AlexaFluor488 was used for these studies) using a 1:200 ratio of antibody to blocking solution.
- Wash coverslips for 1 hr, refreshing the buffer every 20 min.
- Apply desired stains.
Note: These studies utilized DAPI (nuclear stain) and rhodamine-phalloidin (actin stain) at ratios of 1:1,000 in blocking solution. - Incubate coverslips with nuclear and actin stains for 30 min at room temperature.
- Wash coverslips in PBS for 30 min at room temperature, refreshing the buffer every 10 min.
- Mount the coverslips on glass slides using mounting medium.
- Allow coverslips to set on the slides overnight prior to sealing and imaging. For the results described here, collect images on a fluorescence microscope using a standard 20X objective. Use ImageJ and microscope imaging software to process the captured images.
- Ethidium Homodimer Cell Death Assay
- Aspirate medium from each well and wash cells twice with sterile PBS.
- Apply 4 µM ethidium homodimer-1 in PBS.
- Incubate cells at room temperature for 30 min in the dark.
- Determine the level of fluorescence using a plate reader set to 528 nm excitation and 617 nm emission with a cutoff value of 590 nm.
- Add 0.1% (w/v) Saponin to each well following the initial reading and allow the plate to incubate for an additional 20 min at room temperature (with rocking) before reading the plate a second time at the same settings.
- Determine percent membrane permeabilization individually for each well by dividing the initial fluorescence reading (post-treatment) by the second fluorescence reading (post-Saponin).
- ATP Determination Assay
- Collect lysates as described in section 4.3 above.
- Normalize protein levels in the lysates via BCA assay or similar30.
- Determine cellular ATP levels using a luminescence-based ATP determination kit or equivalent according to the manufacturer's instructions.
- Normalize treated values to the corresponding uninfected control samples.
- LDH Release Assay
- Following infection, collect supernatants as described in section 4.3.
- Evaluate LDH release using a cytotoxicity detection kit for LDH release according to the manufacturer's instructions.
Implementation of a Permeable Membrane Insert-based Infection System to Study the Effects of Secreted Bacterial Toxins on Mammalian Host Cells
Learning Objectives
The permeable membrane insert-based infection system protocol developed for the study of secreted bacterial factors is detailed in Figure 1. This system relies on the separation of bacteria and host cells via a porous membrane to assess the effects of secreted bacterial factors, in this case Streptolysin S (SLS), on host responses such as host membrane integrity, cellular viability, cellular signal transduction, and secreted host cell factors. Figure 2 provides representative Western Blot data demonstrating that this system may be used to assess changes in the activation of contact-independent host signaling proteins. Specifically, the representative data show significantly enhanced p38 MAPK activation in the presence of SLS-producing GAS strains. This system can also be applied to visualize the effects of secreted bacterial factors on host protein localization by immunofluorescence microscopy (Figure 3). The data show SLS-dependent activation of the key inflammatory mediator Nuclear Factor kappa B (NFκB), which translocates from the cytoplasm to the nucleus upon activation21. The results in Figures 2 and 3 indicate that both SLS-dependent inflammatory signaling responses do not require direct contact between the bacteria and host cells. As previous experiments have already demonstrated SLS-dependent p38 and NFκB activation in direct infection models21, similar levels of p38 or NFκB activation among the three GAS strains in the permeable membrane insert-based system would have indicated that the response required direct contact between the bacteria and host cells. Figure 4 demonstrates that this infection system may be applied to assess toxin-dependent changes in host cytotoxicity via ethidium homodimer and LDH release assays. This is evidenced by the significant increase in both membrane permeabilization and in the release of LDH from host cells exposed to GAS strains containing SLS compared to uninfected cells or cells exposed to the SLS-deficient strain. These changes in cytotoxicity are not immediate, as significant effects are not evident until 12 hr post infection in the case of membrane permeabilization and 16 hr in the case of LDH release. The representative data illustrate the importance of selecting appropriate time points and infection conditions for the evaluation of these host responses. In addition to allowing for the assessment of host signaling changes and cytotoxicity, the permeable membrane insert-based infection system is applicable to the study of metabolic changes such as accurate determination of host ATP levels by preventing bacterial contamination of host cell lysates (Figure 5). These data show a significant loss of keratinocyte ATP in response to GAS infection by 16 hours post infection, with enhanced ATP loss in the presence of SLS. These results are consistent with the observed toxin-dependent increases in host stress-response signaling and cytotoxicity.
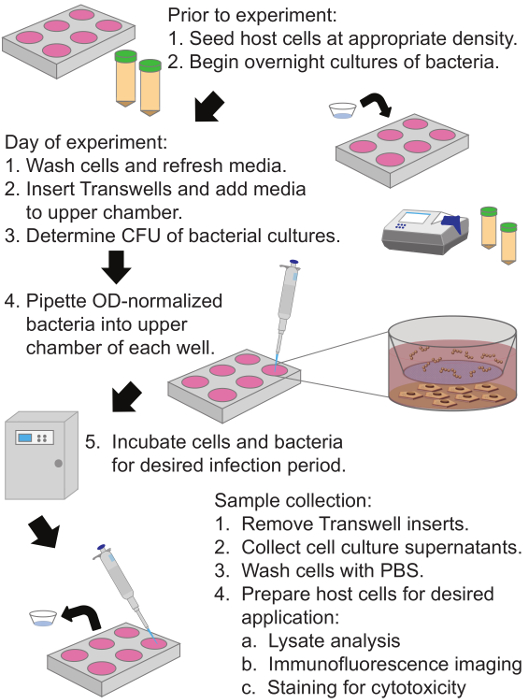
Figure 1: Diagram of Permeable Membrane Insert-Based Infection System Protocol to Assess the Effects of Secreted Bacterial Factors on Host Cells. Human keratinocytes are plated in the bottom compartment and grown to 90% confluence. A collagen coated membrane with 0.4 µm pores separates the upper and lower chambers, and bacteria are added to the upper chamber of the permeable membrane insert system for the desired infection period. Cell culture supernatants and host cells may be collected following the infection period and utilized for a variety of analyses. Please click here to view a larger version of this figure.
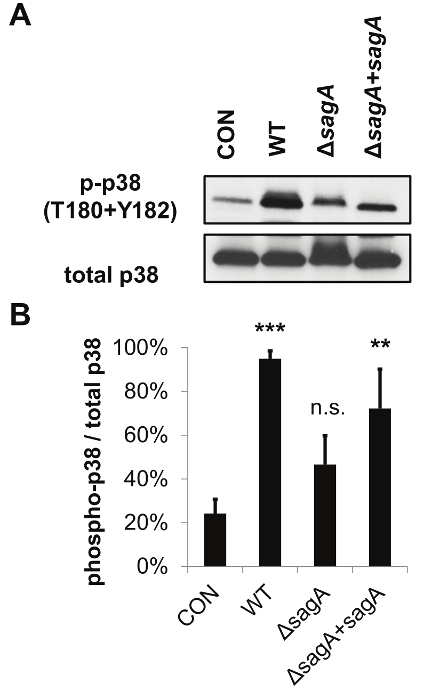
Figure 2: The Permeable Membrane Insert-Based Infection System can be Utilized for Host Cell Lysate Analysis by SDS-PAGE and Western Blotting. The representative data demonstrate that SLS enhances activation of the p38 MAPK pathway in infected keratinocytes. (A) HaCaTs were infected with GAS for 7 hr via the permeable membrane insert infection system (at MOI = 10) and lysates were assessed for activation of p38. (B) Densitometry from three independent Western Blots was performed to quantify the relative activation of p38 in response to GAS'infection. Averages from three biological replicates are shown, with error bars representing standard deviation. Relative activation of p38 is represented as phosphorylated/total protein level. Statistical significance was determined compared to uninfected cells. The overall p-value was determined by ANOVA (p = 0.0063). Dunnett's tests were performed post hoc to compare each condition to the corresponding uninfected control mean. *, p = 0.01-0.05; **, p = 0.001-0.01; ***, p = 0.0001-0.001; ****, p <0.0001. This figure has been modified from Flaherty et al. 201521. Please click here to view a larger version of this figure.
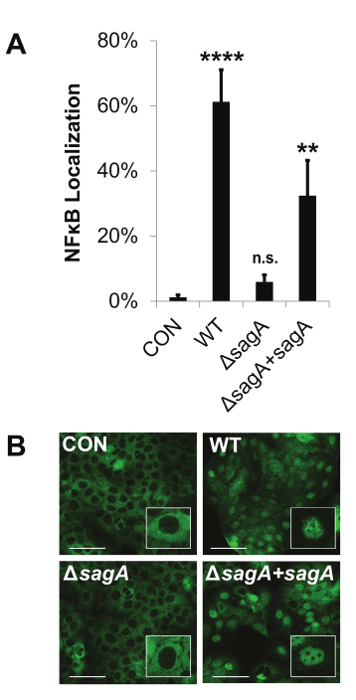
Figure 3: The Permeable Membrane Insert-Based Infection System Allows for the Visualization of Host Signaling Changes by Immunofluorescence Microscopy. The representative data show that Streptolysin S enhances pro-inflammatory signaling through activation of NFκB. HaCaT human keratinocytes were infected with GAS at an MOI of 10 for 8 hr using the permeable membrane insert-based infection system. (A and B) Nuclear localization of NFκB was assessed by immunofluorescence imaging. The percentage of nuclear localized cells was calculated by counting the number of cells in which NFκB had translocated from the cytoplasm to the nucleus for a given field and dividing that number by the total number of cells for the same field. Scale bars indicate 100 µm. (A) The average of three biological replicates are represented for each condition with error bars representing standard deviation. The overall p-value was determined by ANOVA; p <0.0001. Dunnett's tests were performed to compare each condition to the corresponding uninfected control condition. *, p = 0.01-0.05; **, p = 0.001-0.01; ***, p = 0.0001-0.001; ****, p <0.0001. This figure has been modified from Flaherty et al. 201521. Please click here to view a larger version of this figure.
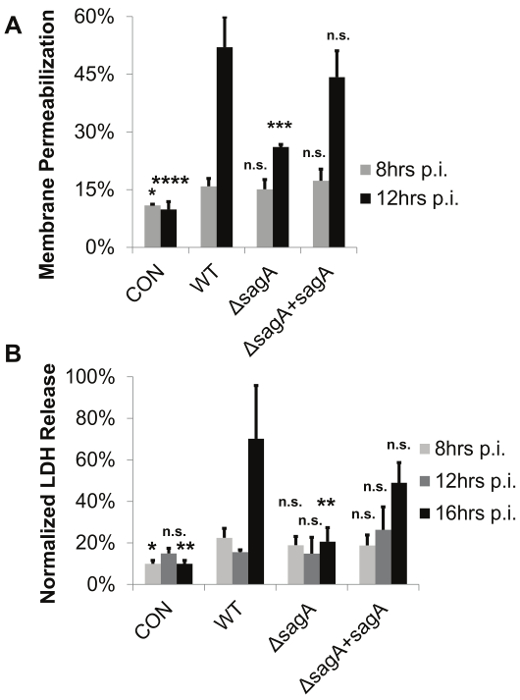
Figure 4: The Permeable Membrane Insert-Based Infection System Allows for the Determination of Bacterial-Mediated Membrane Permeabilization and Cytotoxicity in the Absence of Direct Contact between Bacteria and Host Cells. The representative data demonstrate that keratinocyte viability decreases in the presence of active SLS toxin. GAS–induced cell death was assessed in HaCaT cells in the presence of WT, SLS-deficient or sagA complemented GAS. Keratinocytes were exposed to GAS using the permeable membrane insert-based infection system for 8-16 hr at an MOI of 10. (A) Viability was assessed by ethidium homodimer assay or (B) LDH release assay. In both panels, 3 replicates are averaged and error bars represent standard deviation. Significance for each time point was determined by ANOVA (A) 8 hr, p = 0.0241; 12 hr, p <0.0001 (B) 8 hr, p = 0.0287; 12 hr, p = 0.1977; 16 hr, p = 0.0031. Dunnett's tests were performed to compare means from each condition to the wild-type infection for the corresponding time point. *, p = 0.01-0.05; **, p = 0.001-0.01; ***, p = 0.0001-0.001; ****, p <0.0001. This figure has been modified from Flaherty et al. 201521. Please click here to view a larger version of this figure.
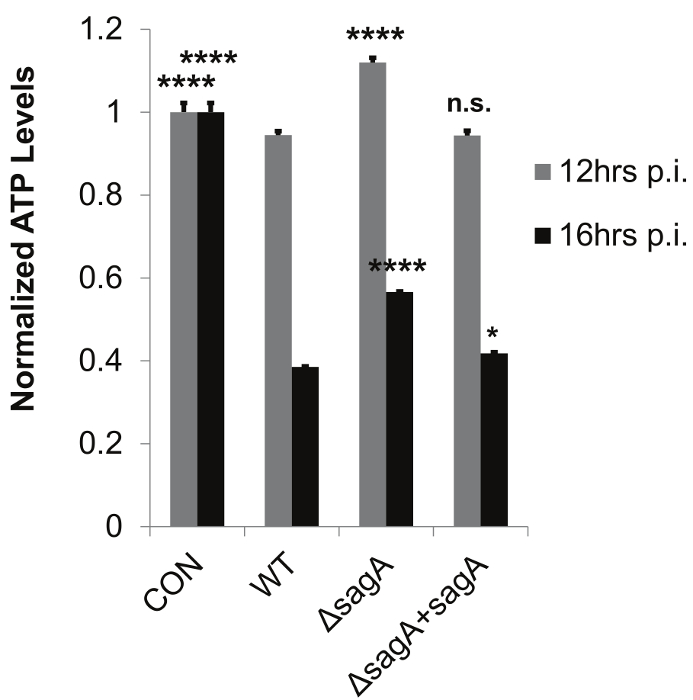
Figure 5: The Permeable Membrane Insert-Based Infection System Allows for Accurate Determination of Host ATP Levels by Preventing Bacterial Contamination of Host Cell Lysates. The representative data demonstrate SLS-dependent loss of ATP during Group A Streptococcal infection. HaCaT cells were infected with GAS for 8-16 hr using the permeable membrane insert-based infection system. Technical replicates (n = 3) from one representative biological replicate (2 x 106 cells per sample) were averaged for each condition, with error bars representing standard deviation. The overall p-values were determined by ANOVA (12 hr, p <0.0001; 16 hr, p <0.0001). Dunnett's tests were performed to compare each condition with wild-type infection for the corresponding time point. *, p = 0.01-0.05; **, p = 0.001-0.01; ***, p = 0.0001-0.001; ****, p <0.0001. This figure has been modified from Flaherty et al. 201521. Please click here to view a larger version of this figure.
List of Materials
| Todd-Hewitt broth | Acumedia | 7161A | Use appropriate broth for bacterial species of interest. |
| BioSpectrometer | Eppendorf | basic model | Any UV-Vis spectrophotometer is suitable. |
| Petri Dishes | Fisher Scientific | FB0875713 | Other brands are suitable. |
| Agar | Sigma | A1296-1kg | Other brands are suitable. |
| HaCaT human epithelial keratinocytes | Gift from lab of Victor Nizet. | ||
| Dulbecco's Modified Eagle's Medium (DMEM) | Life Technologies | 11995-073 | Use appropriate media for cell line of interest. |
| Fetal Bovine Serum (FBS) | Biowest | S162H | Use appropriate media additives for cell line of interest. |
| 10cm tissue culture dishes | Nunc | 150350 | Other brands are suitable. |
| 6 well tissue culture dishes | CytoOne | cc7682-7506 | Other brands are suitable but need to be compatible with the Corning insert. |
| 24 well tissue culture dishes | CytoOne | cc7682-7524 | Other brands are suitable but need to be compatible with the Corning insert. |
| Glass coverslips | Fisherbrand | 12-541-B | These must be autoclaved prior to use for cell plating. |
| Phosphate Buffered Saline (PBS) | Gibco | 10010-023 | |
| Phenol Red Free DMEM | Life Technologies | 31053028 | Phenol Red Free media is only required for the LDH release assay. |
| Bovine Serum Albumin (BSA) | Fisher Scientific | BP1600-100 | |
| L-glutamine | Gibco | 25030149 | Only required to supplement cell culture media for LDH release assay. |
| Sodium pyruvate | Gibco | BP356100 | Only required to supplement cell culture media for LDH release assay. |
| Collagen-coated 0.4μm Transwell inserts (6 well) | Corning | 3540 | |
| Collagen-coated 0.4μm Transwell inserts (24 well) | Corning | 3595 | |
| Forceps | Fisher | 3120018 | These must be sterilized with ethanol prior to handeling Transwell inserts. |
| Cell scrapers | Fisherbrand | 08-100-241 | These are only required for the collection of cell lysates. |
| Paraformaldehyde | Fisher Scientific | 04042-500 | TOXIC. This is only required for immunofluorescence imaging. |
| Bicinchoninic acid (BCA) assay kit | Pierce | 23227 | Other protein quantification assays may be used. |
| Tris-glycine 4-15% polyacrylamide gels | BioRad | 4561083 | Buffer system and percent polyacrylamide should be selected based on proteins of interest. |
| Electrophoresis cassette supplies | BioRad | 1658063 | Only required for Western Blotting. |
| Electrophoresis power supply | BioRad | 1645050 | Only required for Western Blotting. |
| Western blot transfer cassette | BioRad | Only required for Western Blotting. | |
| Polyvinylidene (PVDF) Membrane | EMD Millipore | IPVH00010 | Only required for Western Blotting. |
| Tween 20 | Sigma | P1379-500mL | |
| Rabbit IgG-HRP secondary antibody | Santa Cruz Biotechnology | sc2030 | The secondary antibody should be determined based on the selected method of detection. |
| Mouse IgG-HRP secondary antibody | Santa Cruz Biotechnology | sc2031 | The secondary antibody should be determined based on the selected method of detection. |
| Phospho-p38 (T180+T182) MAPK antibody | Cell Signaling | 4511 | Select appropriate primary antibodies based on proteins of interest. |
| Total p38 MAPK antibody | Cell Signaling | 8690 | Select appropriate primary antibodies based on proteins of interest. |
| Goat anti-rabbit IgG Alexafluor488 | Molecular Probes | A-11034 | Other secondary antibodies may be used for immunofluoresence imaging detection. |
| LumiGLO Detection Reagent | KPL | 54-61-00 | Only required for Western Blotting with detection on film. |
| Detection film | Biodot | BDB57 | Only required for Western Blotting with detection on film. |
| DeltaVision Nikon 90i fluoresence microscope | Nikon | Other fluorescence microscopes are suitable for these analyses. | |
| Normal goat serum | Thermo Scientific | 31873 | |
| Triton X-100 | Sigma | T9284-500mL | |
| DAPI nuclear stain | Cell Signaling | 4083 | Select stains based on specific immunofluorescence applications of interest. |
| Rhodamine-phalloidin actin stain | Molecular Probes | R415 | Select stains based on specific immunofluorescence applications of interest. |
| Fluoromount-G | Southern Biotech | 0100-01 | Only required for immunofluorescence imaging. |
| Ethidium homodimer 1 | Molecular Probes | E1169 | Only required for membrane permeabilization cytotoxicity assay. |
| Spectramax M5 Microplate Reader | Molecular Devices | Other microplate readers capable of detecting UV-vis, fluorescence and luminescence may be suitable. | |
| Saponin | Sigma | 47036-50G-F | Only required for membrane permeabilization cytotoxicity assay. |
| Molecular Probes ATP Determination Kit | Life Technologies | A22066 | Other kits are likely to be suitable. |
| Cytotoxicity Detection Kit for LDH release | Roche | 11644793001 | Other kits are likely to be suitable. |
| Nonidet P40 Substitute | Sigma | 74385-1L | Other suppliers are suitable. |
| HALT Phosphatase Inhibitor Cocktail | Thermo Scientific | 78420B | Other cocktails are likely to be suitable. |
| SIGMAFAST Protease Inhibitor Cocktail Tablets, EDTA-free | Sigma | S8830-2TAB | Other cocktails are likely to be suitable. |
Lab Prep
Many bacterial pathogens secrete potent toxins to aid in the destruction of host tissue, to initiate signaling changes in host cells or to manipulate immune system responses during the course of infection. Though methods have been developed to successfully purify and produce many of these important virulence factors, there are still many bacterial toxins whose unique structure or extensive post-translational modifications make them difficult to purify and study in in vitro systems. Furthermore, even when pure toxin can be obtained, there are many challenges associated with studying the specific effects of a toxin under relevant physiological conditions. Most in vitro cell culture models designed to assess the effects of secreted bacterial toxins on host cells involve incubating host cells with a one-time dose of toxin. Such methods poorly approximate what host cells actually experience during an infection, where toxin is continually produced by bacterial cells and allowed to accumulate gradually during the course of infection. This protocol describes the design of a permeable membrane insert-based bacterial infection system to study the effects of Streptolysin S, a potent toxin produced by Group A Streptococcus, on human epithelial keratinocytes. This system more closely mimics the natural physiological environment during an infection than methods where pure toxin or bacterial supernatants are directly applied to host cells. Importantly, this method also eliminates the bias of host responses that are due to direct contact between the bacteria and host cells. This system has been utilized to effectively assess the effects of Streptolysin S (SLS) on host membrane integrity, cellular viability, and cellular signaling responses. This technique can be readily applied to the study of other secreted virulence factors on a variety of mammalian host cell types to investigate the specific role of a secreted bacterial factor during the course of infection.
Many bacterial pathogens secrete potent toxins to aid in the destruction of host tissue, to initiate signaling changes in host cells or to manipulate immune system responses during the course of infection. Though methods have been developed to successfully purify and produce many of these important virulence factors, there are still many bacterial toxins whose unique structure or extensive post-translational modifications make them difficult to purify and study in in vitro systems. Furthermore, even when pure toxin can be obtained, there are many challenges associated with studying the specific effects of a toxin under relevant physiological conditions. Most in vitro cell culture models designed to assess the effects of secreted bacterial toxins on host cells involve incubating host cells with a one-time dose of toxin. Such methods poorly approximate what host cells actually experience during an infection, where toxin is continually produced by bacterial cells and allowed to accumulate gradually during the course of infection. This protocol describes the design of a permeable membrane insert-based bacterial infection system to study the effects of Streptolysin S, a potent toxin produced by Group A Streptococcus, on human epithelial keratinocytes. This system more closely mimics the natural physiological environment during an infection than methods where pure toxin or bacterial supernatants are directly applied to host cells. Importantly, this method also eliminates the bias of host responses that are due to direct contact between the bacteria and host cells. This system has been utilized to effectively assess the effects of Streptolysin S (SLS) on host membrane integrity, cellular viability, and cellular signaling responses. This technique can be readily applied to the study of other secreted virulence factors on a variety of mammalian host cell types to investigate the specific role of a secreted bacterial factor during the course of infection.
Procedure
Many bacterial pathogens secrete potent toxins to aid in the destruction of host tissue, to initiate signaling changes in host cells or to manipulate immune system responses during the course of infection. Though methods have been developed to successfully purify and produce many of these important virulence factors, there are still many bacterial toxins whose unique structure or extensive post-translational modifications make them difficult to purify and study in in vitro systems. Furthermore, even when pure toxin can be obtained, there are many challenges associated with studying the specific effects of a toxin under relevant physiological conditions. Most in vitro cell culture models designed to assess the effects of secreted bacterial toxins on host cells involve incubating host cells with a one-time dose of toxin. Such methods poorly approximate what host cells actually experience during an infection, where toxin is continually produced by bacterial cells and allowed to accumulate gradually during the course of infection. This protocol describes the design of a permeable membrane insert-based bacterial infection system to study the effects of Streptolysin S, a potent toxin produced by Group A Streptococcus, on human epithelial keratinocytes. This system more closely mimics the natural physiological environment during an infection than methods where pure toxin or bacterial supernatants are directly applied to host cells. Importantly, this method also eliminates the bias of host responses that are due to direct contact between the bacteria and host cells. This system has been utilized to effectively assess the effects of Streptolysin S (SLS) on host membrane integrity, cellular viability, and cellular signaling responses. This technique can be readily applied to the study of other secreted virulence factors on a variety of mammalian host cell types to investigate the specific role of a secreted bacterial factor during the course of infection.
