Integrated Compensatory Responses in a Human Model of Hemorrhage
Instructor Prep
concepts
Student Protocol
Prior to any human procedure, the institutional review board (IRB) must approve the protocol. The protocol used in this study was approved by the US Army Medical Research and Materiel Command IRB. The protocol is designed to demonstrate the physiological responses of compensation to a progressive reduction in central blood volume similar to that experienced by individuals during ongoing hemorrhage in a controlled and reproducible laboratory setting. The laboratory room temperature is controlled at 23 – 25 ˚C.
1. Equipment Preparation
- Turn on equipment and devices requiring warm-up and calibration.
NOTE: Equipment and devices include a data acquisition system to record data at 1 Hz; two separate devices that provide noninvasive, continuous measurements of brachial artery blood pressure and arterial oxygen saturation (SpO2) using two separate infrared finger photoplethysmography cuff sensors9-11; a capnograph for measurement of end-tidal CO2 and respiratory rate; and a finger pulse oximeter to acquire peripheral pulsatile arterial waveforms for measuring Compensatory Reserve. - Synchronize all of the instruments with internal clocks by adjusting the time stamp on each instrument to match a laboratory master clock that will be used to mark time during the experiment.
2. Subject Preparation
- Instruct the subject to avoid caffeine, alcohol, and strenuous exercise 24 h prior to testing, and to avoid eating at least 2 h prior to the protocol in the event that hemodynamic decompensation induces nausea.
- Prior to initiation of the protocol, have the physician perform a medical screening exam to ensure the subject meets minimal health requirements, and ensures the absence of exclusion criteria (nicotine use, hypertension, autonomic dysfunction, or history of syncopal episodes). Since pregnancy is an exclusion criterion for participation, require female participants to take a standard urine pregnancy test on the day of the study.
NOTE: For the safety of the subject, the study physician is certified in advanced life support, and is present during the study. A fully-equipped 'crash cart' is immediately available to support the subject's airway, respiration, and circulation in the event of loss of consciousness or an acute cardiac arrhythmia taking place during the LBNP procedure. - Inform the subject about the procedure, and obtain written consent to participate in the study.
NOTE: Explain to the subject that the goal of the study is to apply LBNP until the onset of cardiovascular decompensation (presyncope). Explain that there are cardiovascular parameters that define this point, and LBNP will be terminated when these cardiovascular parameters are observed. Inform the subject that they may also experience symptoms typically associated with presyncope during the LBNP procedure. Instruct the subject to notify the investigator if these symptoms occur and LBNP will immediately be terminated. - Place the neoprene LBNP skirt on the subject. Ensure that the skirt is snug around the waist and torso in order to create an air-tight seal.
- Instruct the subject to lay supine on the bed of the LBNP chamber while straddling a stationary post to secure the torso in place during LBNP. Instruct the subject to relax the lower body during LBNP exposure. Secure the subject into the LBNP chamber by sliding the bed into the chamber and attaching the neoprene skirt to the chamber opening to create an air-tight seal.
NOTE: The LBNP chamber provides the capability of accurately (within 0.1 mmHg) controlling the internal pressure from 0 to -100 mmHg either manually or with a computerized profile. The chamber includes an adjustable saddle to secure the subject's body position. Clear plexiglass windows allow for visualization of the subject's legs. An adjustable aluminum waist board allows for an air-tight seal to be created by a neoprene skirt worn by the subject and the LBNP chamber at the level of the iliac crest (Figure 1). - Place electrocardiogram (ECG) electrodes on the right and left humoral-clavicular joints, and on the right and left lower ribs (total of 4) in a modified lead II configuration (Figure 1) for continuous measurement of heart rate.
- Position the subject's arms on the arm rests, adjusted so that the hands are supported at heart level. Using appropriate size finger cuffs, place an infrared finger photoplethysmography device on the left and right middle fingers for continuous noninvasive beat-to-beat measurement of blood pressure.
- Attach the finger cuffs to the pressure monitors. Calibrate the devices and record blood pressure according to the manufacturer instructions.12 Enter subject information (age, sex, height, and weight) to enable the appropriate assumptions for calculation (estimation) of stroke volume, cardiac output and peripheral vascular resistance by the Modelflow algorithm if desired.13,14
- Place the finger pulse oximeter on the right index finger for continuous measurement of compensatory reserve1,12 (Figure 2).
- Place a nasal cannula on the subject and instruct the subject to breathe through the nose to assure sensitive reflections in inspiration and expiration. Nasal air sampling will allow the subject to talk freely for self-reporting of developing symptoms. Connect the nasal cannula to the capnograph for the continuous measurement of respiration and end tidal CO2.
3. Performing the LBNP Protocol
- Start data recording by clicking the "Start" button on the data acquisition system. Record baseline data for 5 min. Initiate the first level of central hypovolemia by turning on the vacuum motor and setting negative pressure to -15 mmHg, and hold this pressure for 5 min. Figure 3 outlines the protocol.
- Increase the LBNP to -30 mmHg, and hold this pressure for 5 min.
- Increase the LBNP to -45 mmHg, and hold this pressure for 5 min.
- Increase the LBNP to -60 mmHg, and hold this pressure for 5 min.
- Increase the LBNP to -70 mmHg, and hold this pressure for 5 min.
- Continue to increase LBNP levels by -10 mmHg every 5 min until the end of the protocol (5 min at -100 mmHg LBNP) or the point of hemodynamic decompensation. Terminate the LBNP by pressing the pressure release button on the LBNP chamber.
NOTE: Hemodynamic decompensation is identified by a precipitous fall in systolic arterial pressure below 80 mmHg, or the subject reporting presyncopal symptoms such as grey-out (loss of color vision), tunnel vision, sweating, nausea or dizziness (Figure 4). - Continue recording data on the data acquisition system during 10 min after the cessation of LBNP (postLBNP recovery).
- Stop recording data at the end of the 10-min recovery period by clicking the "Stop" button on the data acquisition system.
- Detach all instrumentation from the subject and remove the subject from the LBNP chamber. Ask the subject to sit after stepping down from the LBNP platform to ensure they are symptom-free before leaving the laboratory. The study is now complete.
- Download data files from the acquisition system for extraction of the Compensatory Reserve Index (CRI), Mean Arterial Pressure (MAP), heart rate, and SpO2 values. 1,15,16
Integrated Compensatory Responses in a Human Model of Hemorrhage
Learning Objectives
The LBNP procedure causes a reduction in air pressure around the lower torso and legs. As this vacuum is progressively increased, blood volume shifts from the head and upper torso to the lower body to create a state of central hypovolemia. The progressive reduction in central blood volume (i.e., LBNP) produces significant alterations in the features of the arterial waveform measured with the infrared finger photoplethysmograph (Figure 5). The Compensatory Reserve Index (CRI) is calculated from the recorded arterial pulse wave using a unique machine learning algorithm which analyzes changes in wave form characteristics to calculate an estimated compensatory reserve (Figure 6).1,15,16 Each continuous noninvasive photoplethysmograph waveform (represented as the monitored 'Patient's Arterial Waveform') is the input to calculate an estimate of an individual's compensatory reserve (represented as the 'CRI Estimate') based on comparison to a large 'library' of reference waveforms (represented as the 'Algorithm Waveform Library') generated from progressive levels of central hypovolemia.
In this experiment, a subject was exposed to LBNP until the onset of hemodynamic decompensation which occurs when the body is no longer able to compensate for the hypovolemia. The values for mean arterial pressure, heart rate, SpO2, and CRI plotted against time (i.e., progressive reductions in central blood volume caused by increasing levels of LBNP) are shown in Figure 7. The results of the experiment show that changes in mean arterial pressure, heart rate, and SpO2 occur during the later phases of hemorrhage (i.e., >15 min into the protocol for heart rate and >25 min for mean arterial pressure and SpO2) while CRI decreases early and progressively throughout the multiple steps of LBNP.
Tolerance to reduced central blood volume is defined as the time from the start of the experiment to decompensation. In this example, tolerance was approximately 27.5 min at a level of -70 mmHg LBNP. Based on previous experiments that were designed to equate the magnitude of actual blood loss with LBNP,8 the equivalent blood loss that our subject was able to tolerate was estimated at approximately 1.2 L.
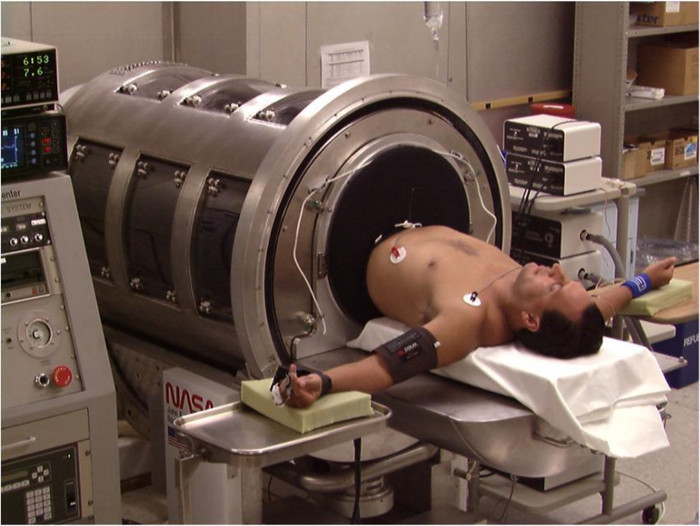
Figure 1: LBNP Chamber. A subject is shown in a supine position on the bed of the LBNP chamber. The neoprene skirt around the subject's waist is used to create an airtight seal within the LBNP chamber. Previously published in Cooke et al.17 Please click here to view a larger version of this figure.

Figure 2: Compensatory Reserve Monitoring Device. The device consists of a noninvasive finger pulse oximeter that transmits pulse oximeter and waveform data via a USB connection to a compensatory reserve monitor. The monitor unit contains an algorithm which calculates a value for compensatory reserve known as the Compensatory Reserve Index (CRI)1,12. Data are recorded at each heart beat and displayed on the monitor and stored on a memory card. Please click here to view a larger version of this figure.
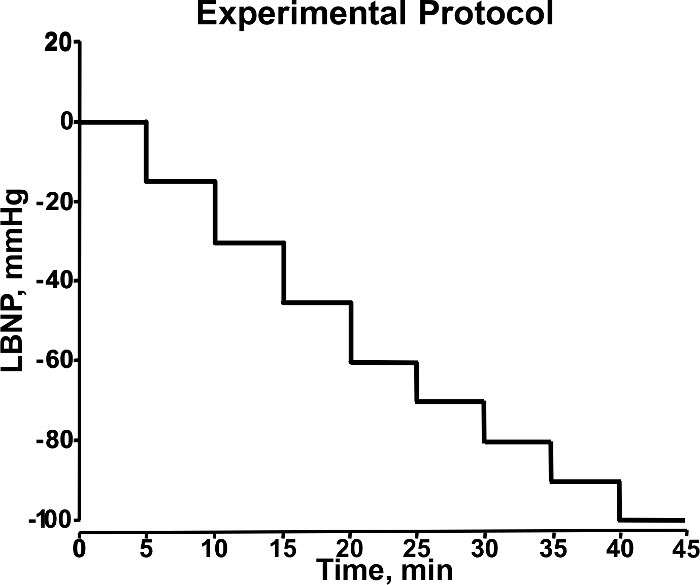
Figure 3. Stepwise Changes in LBNP During Experiment. During the experimental protocol, LBNP (mmHg) is adjusted in a stepwise manner (5 min/level) to induce progressive central hypovolemia. This diagram shows LBNP increasing from 0 to -100 mmHg during 40 min of an experimental protocol. Modified from Convertino et al.18 Please click here to view a larger version of this figure.
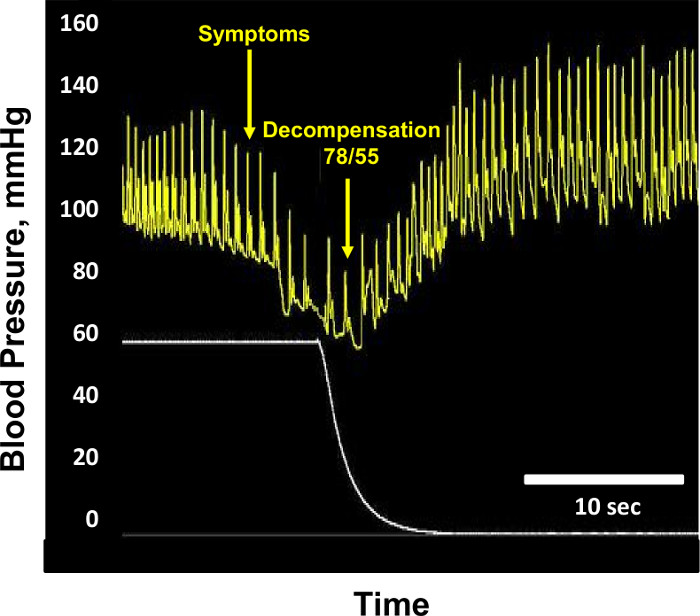
Figure 4: Hemodynamic Decompensation. Sample blood pressure (mm Hg, yellow tracing) and lower body negative pressure (mmHg, white tracing) recordings are shown from a subject at the point of hemodynamic decompensation. At the point of decompensation, blood pressure is 78/55 mmHg, and lower body negative pressure is -60 mmHg. Blood pressure returns to normal after cessation of lower body negative pressure. Modified from Convertino et al.1 Please click here to view a larger version of this figure.
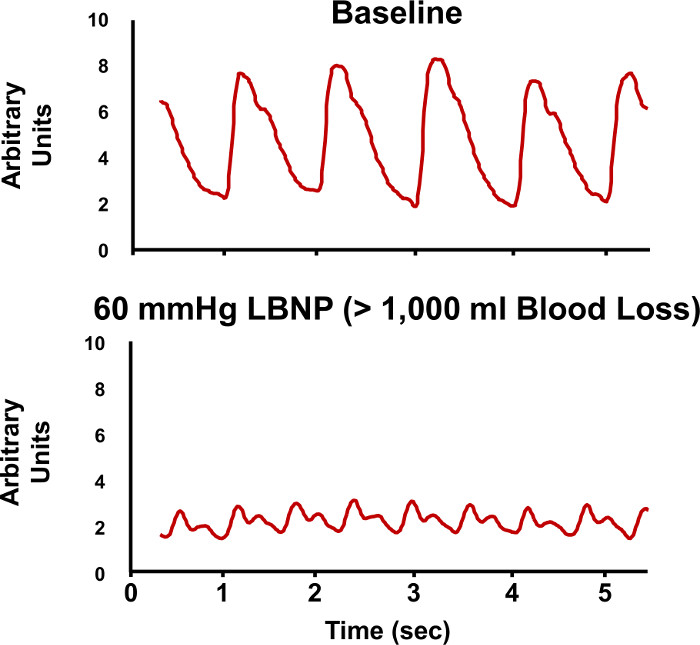
Figure 5. Arterial Waveforms During LBNP. Sample recordings of arterial pressure waveforms are shown during baseline (upper tracing) and during -60 mmHg lower body negative pressure (LBNP, lower tracing). The changes in the characteristic features of the arterial waveforms are evaluated to estimate compensatory reserve. Please click here to view a larger version of this figure.
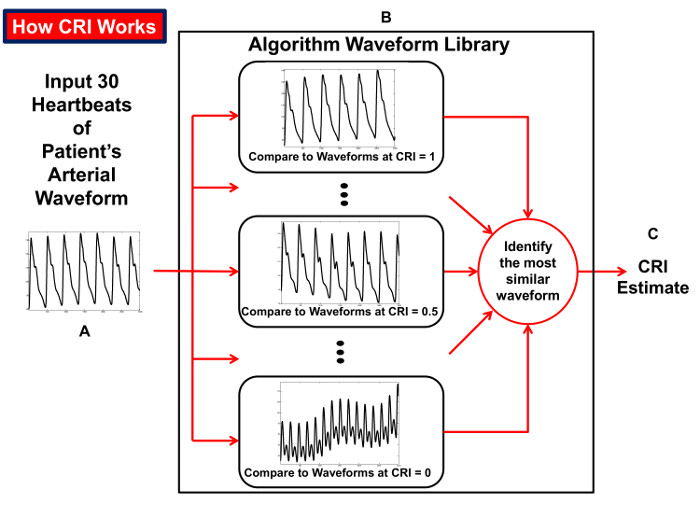
Figure 6: How the CRI is Calculated. Diagram illustrating the process of the compensatory reserve index (CRI) algorithm that compares beat-to-beat arterial blood pressure waveform tracings over an interval of 30 heartbeats (A) to a 'library' of waveforms (B) collected from humans exposed to progressive reductions in central blood volume for generation of an estimated CRI value (C). Reproduced from Convertino et al.15 Please click here to view a larger version of this figure.
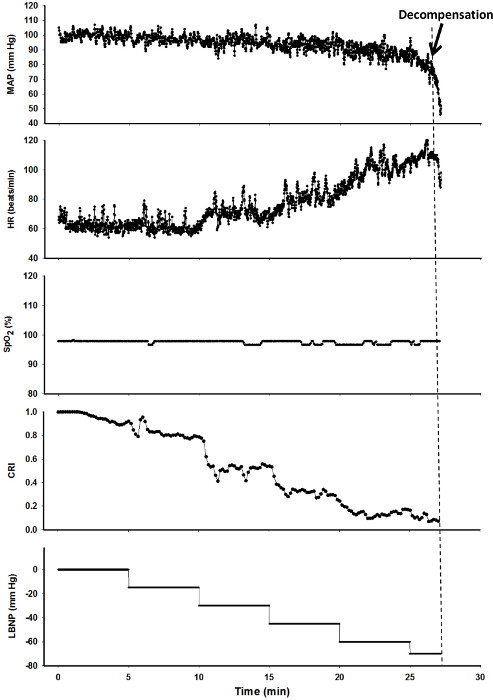
Figure 7. Sample Results of an LBNP Experiment. Values of Mean Arterial Pressure (MAP, mmHg), Heart Rate (HR, beats/min), arterial oxygen saturation (SpO2, %), Compensatory Reserve Index (CRI) and Lower Body Negative Pressure (LBNP, mmHg) are shown for one subject during an LBNP experiment. The dashed line represents the onset of cardiovascular decompensation, Please click here to view a larger version of this figure.
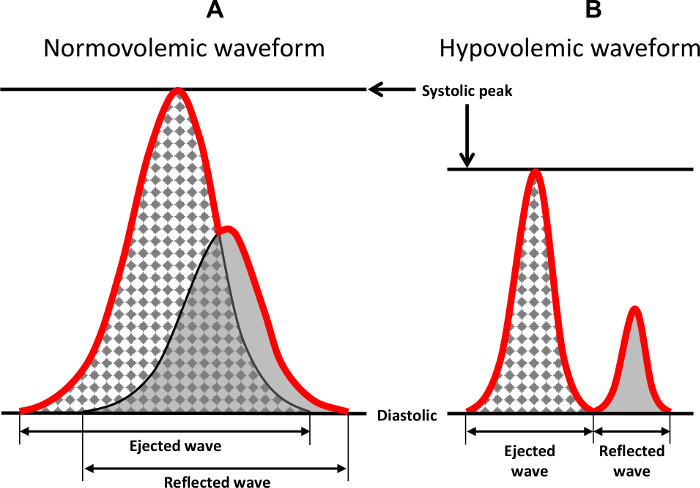
Figure 8: Characteristic Features of the Arterial Waveform. Two wave forms are shown that demonstrate the characteristic features of the arterial ejected and reflected waveforms during normovolemia and hypovolemia. The red line indicates the integrated waveform that is recorded and observed in a tracing. Previously published in Convertino et al.1 Please click here to view a larger version of this figure.
List of Materials
| Dynamic Research Evaluation Workstation (DREW) data acquisition syetem | NA | NA | Custom Built by ISR personnel. The DREW allows for time synchronization of both digital and analog signal data collection from up to 16 independent instruments with a sampling rate of 1000 Hz. |
| Finometer | Finapress Medical Systems (FMS) | Model 1 | Device that provides non-invasive, continuous measurements of brachial artery blood pressure and arterial oxygen saturation (SpO2) using two separate infrared finger photophlethymography cuff sensors. |
| BCI Capnocheck Plus | Smith Medical PM Inc. | 9004 | Capnograph used to measure end tidal CO2 and respiration rate |
| CipherOX | Flashback Technologies Inc. | R200 | Investigational device used to calculate Compensatory Reserve Index (CRI) |
| Nonin 9560 Pulse Oximeter | Nonin | 9560 | finger pulse oximeter |
| Lower Body Negative Pressure Chamber (LBNP) | NASA | 79K32632-1 | Custom Chamber built by NASA |
| ECG Biotach | Gould | 13-6615-65 | Electrocardiograph for measuring ECG |
| Nasal CO2 Sample Line | Salter Labs | REF 4000 | Latex free nasal cannula for sampling expired air |
Lab Prep
Hemorrhage is the leading cause of trauma-related deaths, partly because early diagnosis of the severity of blood loss is difficult. Assessment of hemorrhaging patients is difficult because current clinical tools provide measures of vital signs that remain stable during the early stages of bleeding due to compensatory mechanisms. Consequently, there is a need to understand and measure the total integration of mechanisms that compensate for reduced circulating blood volume and how they change during ongoing progressive hemorrhage. The body's reserve to compensate for reduced circulating blood volume is called the 'compensatory reserve'. The compensatory reserve can be accurately evaluated with real-time measurements of changes in the features of the arterial waveform measured with the use of a high-powered computer. Lower Body Negative Pressure (LBNP) has been shown to simulate many of the physiological responses in humans associated with hemorrhage, and is used to study the compensatory response to hemorrhage. The purpose of this study is to demonstrate how compensatory reserve is assessed during progressive reductions in central blood volume with LBNP as a simulation of hemorrhage.
Hemorrhage is the leading cause of trauma-related deaths, partly because early diagnosis of the severity of blood loss is difficult. Assessment of hemorrhaging patients is difficult because current clinical tools provide measures of vital signs that remain stable during the early stages of bleeding due to compensatory mechanisms. Consequently, there is a need to understand and measure the total integration of mechanisms that compensate for reduced circulating blood volume and how they change during ongoing progressive hemorrhage. The body's reserve to compensate for reduced circulating blood volume is called the 'compensatory reserve'. The compensatory reserve can be accurately evaluated with real-time measurements of changes in the features of the arterial waveform measured with the use of a high-powered computer. Lower Body Negative Pressure (LBNP) has been shown to simulate many of the physiological responses in humans associated with hemorrhage, and is used to study the compensatory response to hemorrhage. The purpose of this study is to demonstrate how compensatory reserve is assessed during progressive reductions in central blood volume with LBNP as a simulation of hemorrhage.
Procedure
Hemorrhage is the leading cause of trauma-related deaths, partly because early diagnosis of the severity of blood loss is difficult. Assessment of hemorrhaging patients is difficult because current clinical tools provide measures of vital signs that remain stable during the early stages of bleeding due to compensatory mechanisms. Consequently, there is a need to understand and measure the total integration of mechanisms that compensate for reduced circulating blood volume and how they change during ongoing progressive hemorrhage. The body's reserve to compensate for reduced circulating blood volume is called the 'compensatory reserve'. The compensatory reserve can be accurately evaluated with real-time measurements of changes in the features of the arterial waveform measured with the use of a high-powered computer. Lower Body Negative Pressure (LBNP) has been shown to simulate many of the physiological responses in humans associated with hemorrhage, and is used to study the compensatory response to hemorrhage. The purpose of this study is to demonstrate how compensatory reserve is assessed during progressive reductions in central blood volume with LBNP as a simulation of hemorrhage.
