Differential Scanning Calorimetry — A Method for Assessing the Thermal Stability and Conformation of Protein Antigen
Instructor Prep
concepts
Student Protocol
1. Instrument Start-up
- Switch on the differential scanning calorimeter and increase the pressure in the cells to suppress boiling of the samples as well as prevent the formation bubbles at elevated temperatures. This is typically achieved by supplying nitrogen into the system.
- Depending on the constituting material of the cell (e.g., tantalum, gold, platinum, etc.), adjust the pressure of the nitrogen gas supply according to the manufacturer's recommended pressure to avoid damaging the cell. For example, set the pressure of the nitrogen gas supply to 45 psi for the instrument used to develop this procedure, and pressure above 80 psi may damage the cell.
- Ensure that all the cleaning agent reservoirs are filled to the required volume. Cleaning agents required include detergent and water to wash and clean the cell respectively after each sample run.
- Set the temperature of the sample holding compartment to a suitable value, preferably 5 °C, to maintain the integrity of the sample prior to experiment.
2. Sample Preparation
- Dialyze the sample against the buffer that will be used as the reference for the experiment. Alternatively, elution buffer collected at the final step of protein purification (i.e. column elution) can be used.
- Determine the concentration of the protein sample using the most suitable protein concentration determination method such as Kjedahl method 33 or Lowry method 34. For objective comparison of results, use the same method consistently within the same study. The required concentration range may vary depending on the model of the instrument. For the instrument used in this protocol, the preferable working range is 0.5 – 1 mg/mL.
- Degas the sample and reference buffer in vacuum to get rid of microbubbles that can cause volume inaccuracy. This step can be skipped for newer calorimeter models.
- Using a micropipette and sterile tips in a laminar flow biocontainment cabinet, load the samples and their respective buffer in pairs into 96 well plates compatible with the instrument. Fill the first two pairs of wells with buffer and the last two pairs with water for the buffer-buffer and the water scan respectively. Buffer-buffer scans verify the suitability of the instrument prior to sample measurement (i.e. assessment of instrumentation error) as well as establish a baseline; while water scans are run to clean the cells.
- Cover the 96-well plate with a sealing film, and ensure that the wells are properly sealed before taking the plate out of the Biosafety Cabinet to avoid sample contamination.
- Place the plate in the sample holding compartment in the proper orientation.
3. Experimental Parameter Setup
Note: Depending on the instrumentation, samples can be loaded into the cell either manually using a syringe, or automatically using an autosampler. In this case (i.e. an industrial setting), an autosampler is used to save time.
- Using the acquisition software, enter the sample information in the order the plate was loaded as per section 2.4. Enter concentrations if available, otherwise, enter concentration values into analysis software prior to data analysis (section 4.2).
- Select the option that ensures cleaning of cells with detergent before every sample scan, which should be followed by multiple water rinse steps to ensure no detergent residue is left in the cells.
- Set the starting temperature of the experiment to 20 °C, but this can vary depending on prior knowledge of the sample. For known proteins, pre-determined starting temperature can be used, while a lower starting temperature can be applied for unknown samples.
- Set the final temperature of the experiment, e.g. 100 °C. The final temperature may vary depending on prior knowledge of the sample.
- Set the scan rate of the experiment, e.g. 60 °C/h, which is the typical scan rate. However, scan rate may vary depending on prior knowledge of the sample, e.g. 90 °C/h, or 120 °C/h. It is advisable to scan unknown samples at different scan rates to assess the kinetics of unfolding.
- Rescan samples to investigate the reversibility of the thermal unfolding. The unfolding of a protein is considered reversible if the enthalpy obtained for the second scan is at least 80% of the enthalpy value for the first scan.
- Set the post-experiment thermostat to 10 °C to preserve the integrity of the calorimeter's cells.
- Verify that the experiment setup parameters are correct before executing the experiment. If everything is in place, start the experiment.
4. Data Analysis
- Retrieve raw data from the experiment and select one sample at a time for analysis. Subtract reference scan, i.e. buffer, from the sample scan.
NOTE: Reference subtraction is carried out automatically by newer models of DSC instruments. - Enter sample concentration value if it was omitted as per section 3.1.
- Fit and subtract baseline from the acquired thermogram to account for differences in the heat capacities of the folded and unfolded states of the protein which is caused by the exposure of hydrophobic groups to water upon unfolding. Linear or cubic curve fitting can be applied depending on the shape of the DSC profile for the sample. For consistency, the same type of fitting has to be used during a study, e.g. real-time stability study. This step is required to process the curve for peak integration to obtain enthalpy of transition.
- Perform peak integration using non-linear least square fit. Based on product knowledge apply two-state or non-two-state model. Two-state model can be used for single cooperative thermal transitions, and for unknown proteins, apply non-two state model until further product knowledge is available. If applicable, adjust curve fitting using the iterative curve fitting function of the equipment's software until the Chi-square value remains constant.
- Obtained results will show the values for midpoint of transition temperatures (Tm), calorimetric enthalpy (ΔH), and van’t Hoff enthalpy (ΔHVH) of the sample.
Differential Scanning Calorimetry — A Method for Assessing the Thermal Stability and Conformation of Protein Antigen
Learning Objectives
The raw data from most DSC experiments are presented as a heat flux versus temperature graph, as the calorimeter actually measures the difference in the rate of heat flow into the sample solution and buffer 35. Therefore, if both cells (i.e. sample and reference cells) contain identical solutions during an experiment, the raw data from the scan should be a flat line with no observable peaks. Any peak observed can be attributed to instrumentation error (e.g. damaged or contaminated cells), which is why running buffer scans prior to sample analysis is an adequate system suitability test. Figure 1 illustrates the result of a typical buffer scan indicating that the calorimeter was in good working condition prior to sample analysis.
Figure 2 shows the raw data for a DSC experiment carried out on different lots of two protein samples. As implied earlier, the observed peaks are the differences in the heat flux of the samples and their respective buffers. Differences in sample concentration can cause variations in heat capacity recorded by the calorimeter; however, these variations are normalized during sample analysis as per section 4.2 of the procedure. Higher concentrations can also reveal additional thermodynamic domains not contributing to the transition at lower concentrations. In addition, each transition represents a thermodynamic domain that may include one or more structural domains of the protein 36. In this case, the Protein 1 has three structural domains that melt cooperatively.
Figure 3 shows the results generated from the analysis of the raw data for protein 1 and 2 presented in Figure 2, i.e., after baseline subtraction and iterative curve fitting. The resulting thermograms have been normalized for scanning rate (automatically carried out by a pre-set algorithm in the analysis software) and concentration; thus, presenting the results of the experiment in comparable heat capacity versus temperature graphs. The analysis software uses data from the Heat Capacity versus Temperature graphs, such as Tm and ΔCp, to derive other thermodynamic parameters using variations of the equations given above depending on the cooperativity of protein unfolding.
When testing unknown samples, setting the appropriate temperature range is crucial. Otherwise, incomplete thermograms may result, as illustrated in Figure 4. Although Tm from such profiles can be derived, ΔH cannot be accurately determined. Therefore, the sample must be retested with a larger temperature range to completely capture the thermal transition. Some proteins also readily form aggregates after complete denaturation, resulting in an increasing post-transition heat capacity: this often appears as an incomplete thermogram as illustrated in Figure 2B. However, retesting with a higher final temperature can help confirm whether there is an occurrence of a conformational transition at that region of the thermogram or it is merely the heat absorbing effect of protein aggregates.
Thermal stability is one of the most significant physical properties of proteins and protein-based products in the industry 37. In pharmaceuticals, it is used to determine the stability of biologics under different conditions, including formulation buffers and environmental factors such as humidity and temperature. It is also used to monitor key manufacturing step (e.g., purification and detoxification) to ensure conformational consistency between production lots. Figures 5 and 6 illustrate the use of DSC to examine the effects of chemical detoxification and storage conditions respectively on the stability and structural conformation of two different proteins. The significant differences in Tm and ∆H indicate conformational changes and protein degradation respectively. In addition, the loss of the third transition in Figure 6 further illustrates the degradation of a domain which was confirmed by a decrease in molecular weight when the samples were analyzed using Size-Exclusion Chromatography with multi-angle light scattering (SEC-MALS) (data not shown).
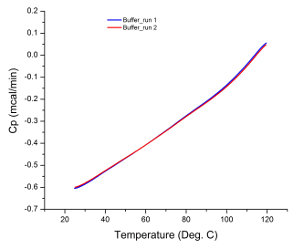
Figure 1: Buffer Scans. The similarity in gradient of each scan with no observable peaks indicates that the instrument is in good working condition and generated reproducible results. Please click here to view a larger version of this figure.
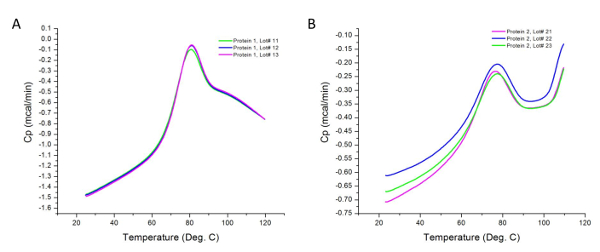
Figure 2: Raw Data Collected from DSC Experiments. These graphs are good representations of the unanalyzed (raw) data acquired after experimental runs (i.e. prior to baseline subtraction and curve fitting). Each line represents a production lot. Protein 2 tends to aggregate more readily upon heating, resulting in an increase in heat capacity above 100 °C in the post-transition region of the thermogram. Please click here to view a larger version of this figure.
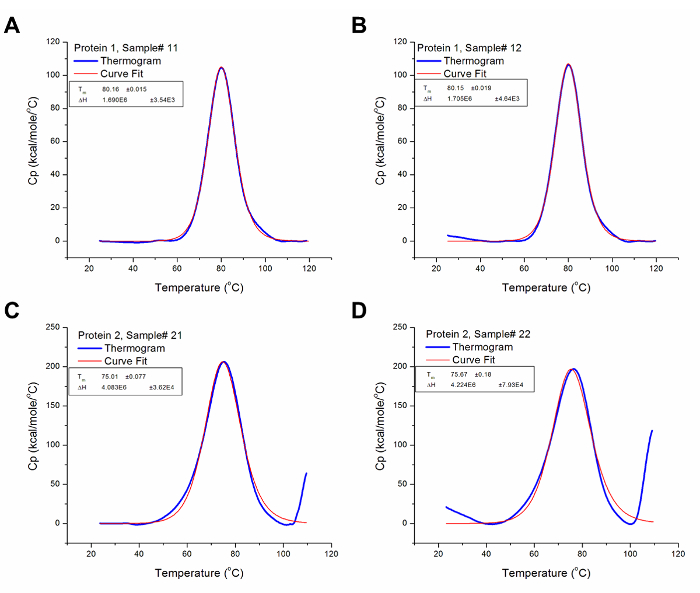
Figure 3: Analyzed DSC Data. These graphs are good representations of analyzed DSC data (i.e. after baseline subtraction and curve fitting). The blue line represents the thermogram after baseline subtraction, while the red line represents the curve with the best fit to the thermogram. (A) The Tm and ΔH for Protein 1 Sample # 12 are 80.16 °C and 1.69 x 106 cal/mol respectively. (B) The Tm and ΔH for Protein 1 Sample# 13 are 80.15 °C and 1.71 x 106 cal/mol respectively. (C) The Tm and ΔH for Protein 2 Sample # 21 are 75.01 °C and 4.08 x 106 cal/mol respectively. (D) The Tm and ΔH for Protein 2 Sample # 22 are 75.67 °C and 4.22 x 106 cal/mol respectively. Please click here to view a larger version of this figure.
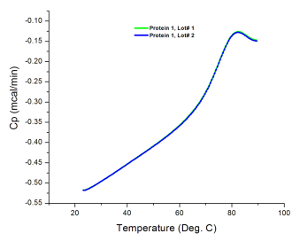
Figure 4: An Incomplete Thermogram. Raw data collect for Protein 1 analyzed at an inadequate temperature range. The final temperature of experiment was set to 90 °C which did not accommodate the entire transition profile of the protein as compared to the experiment for Figure 2A which was set to 120 °C. Please click here to view a larger version of this figure.
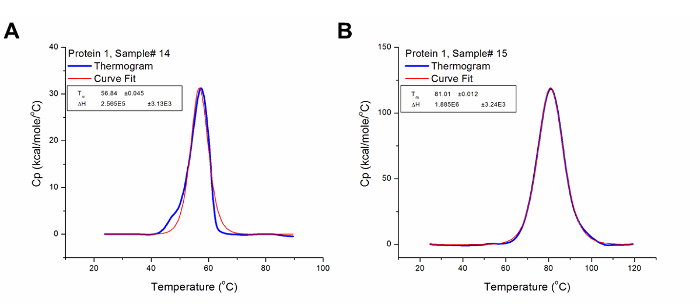
Figure 5: Analyzed Data Showing the Effect of Chemical Detoxification on the Tertiary Structure of Protein 1. (A) Protein 1 is a toxin in its native conformation and has its Tm at 56.84 °C and ΔH at 2.57 x 105 cal/mol. (B) The detoxified form of Protein 1 (i.e. toxoid) has Tm and ΔH values of 81.01 °C and 1.89 x 106 cal/mol respectively. Thus, it can be concluded that the detoxification step introduced some form of variation to the structural conformation of Protein 1 which confers greater stability (higher Tm) to its detoxified form. Please click here to view a larger version of this figure.
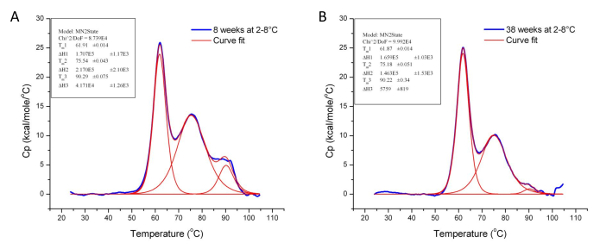
Figure 6: Analyzed Data Showing the Effect of Storage Conditions on the Conformation of Protein 3. These graphs illustrate the effect of storage temperature (2 – 8 °C) on the stability and tertiary structure of Protein 3 over 30 weeks. The Tm and ΔH values for Protein 3 at the 8th (A) and 38th week (B) of storage are given in Table 1 below. Please click here to view a larger version of this figure.
| Sample | Tm 1 (°C) | ∆H 1 (cal/mol) | Tm 2 (°C) | ∆H 2 (cal/mol) | Tm 3 (°C) | ∆H 3 (cal/mol) |
| Protein 3 stored at 2-8 °C for 8 weeks | 61.91 | 1.71 x 105 | 75.54 | 2.17 x 105 | 90.29 | 4.17 x 105 |
| Protein 3 stored at 2-8 °C for 38 weeks | 61.87 | 1.66 x 105 | 75.18 | 1.46 x 105 | 90.22 | 5.76 x 105 |
Table 1: Tm and ΔH Values for Protein 3 at the 8th and 38th Week of storage at 2 – 8 °C. Although the Tm values at both time points are similar, the difference in the ΔH values indicates that the tertiary structure of Protein 3 has degraded over 30 weeks under the specified storage condition.
List of Materials
| Differential Scanning Calorimeter | Malvern Instruments Ltd | 28428948 (Via GE Healthcare) | Has an autosampler for automated dispensing of samples into the cell to reduce human effort and errors. |
| Contrad 100 | Decon Laboratories Inc | 1504 | Dilute with water to 20% before use |
| 500µL Polypropylene round bottom 96 well plate | Canadian Life Science | ML072100 | Equivalent plates from other suppliers (e.g., VW) can also be used |
| MicroCal ThermoVac | Malvern Instruments Ltd | N/Ap | provided with the Cap VP DSC |
| Biosafety cabinet | Labconco | Logic+ – A2 | biocontainment laminar flow cabinet for sample preparation |
| Slide-A-Lyzer dialysis cassette | Thermo Scientific | 66810 or 66380 | to equilibrate the sample and buffer |
Lab Prep
Differential scanning calorimetry (DSC) is an analytical technique that measures the molar heat capacity of samples as a function of temperature. In the case of protein samples, DSC profiles provide information about thermal stability, and to some extent serves as a structural “fingerprint” that can be used to assess structural conformation. It is performed using a differential scanning calorimeter that measures the thermal transition temperature (melting temperature; Tm) and the energy required to disrupt the interactions stabilizing the tertiary structure (enthalpy; ∆H) of proteins. Comparisons are made between formulations as well as production lots, and differences in derived values indicate differences in thermal stability and structural conformation. Data illustrating the use of DSC in an industrial setting for stability studies as well as monitoring key manufacturing steps are provided as proof of the effectiveness of this protocol. In comparison to other methods for assessing the thermal stability of protein conformations, DSC is cost-effective, requires few sample preparation steps, and also provides a complete thermodynamic profile of the protein unfolding process.
Differential scanning calorimetry (DSC) is an analytical technique that measures the molar heat capacity of samples as a function of temperature. In the case of protein samples, DSC profiles provide information about thermal stability, and to some extent serves as a structural “fingerprint” that can be used to assess structural conformation. It is performed using a differential scanning calorimeter that measures the thermal transition temperature (melting temperature; Tm) and the energy required to disrupt the interactions stabilizing the tertiary structure (enthalpy; ∆H) of proteins. Comparisons are made between formulations as well as production lots, and differences in derived values indicate differences in thermal stability and structural conformation. Data illustrating the use of DSC in an industrial setting for stability studies as well as monitoring key manufacturing steps are provided as proof of the effectiveness of this protocol. In comparison to other methods for assessing the thermal stability of protein conformations, DSC is cost-effective, requires few sample preparation steps, and also provides a complete thermodynamic profile of the protein unfolding process.
Procedure
Differential scanning calorimetry (DSC) is an analytical technique that measures the molar heat capacity of samples as a function of temperature. In the case of protein samples, DSC profiles provide information about thermal stability, and to some extent serves as a structural “fingerprint” that can be used to assess structural conformation. It is performed using a differential scanning calorimeter that measures the thermal transition temperature (melting temperature; Tm) and the energy required to disrupt the interactions stabilizing the tertiary structure (enthalpy; ∆H) of proteins. Comparisons are made between formulations as well as production lots, and differences in derived values indicate differences in thermal stability and structural conformation. Data illustrating the use of DSC in an industrial setting for stability studies as well as monitoring key manufacturing steps are provided as proof of the effectiveness of this protocol. In comparison to other methods for assessing the thermal stability of protein conformations, DSC is cost-effective, requires few sample preparation steps, and also provides a complete thermodynamic profile of the protein unfolding process.
