Preparation and Characterization of Novel HDL-mimicking Nanoparticles for Nerve Growth Factor Encapsulation
Instructor Prep
concepts
Student Protocol
NOTE: The animal studies included in all procedures have been approved by the Institutional Animal Care and Use Committee at the University of North Texas Health Science Center.
1. Preparation of NGF HDL-mimicking Nanoparticles
- Dissolve the excipients, phosphatidylcholine (PC), sphingomyelin (SM), phosphatidylserine (PS), cholesteryl oleate (CO), and D-α-tocopheryl polyethylene glycol succinate (TPGS), in ethanol to prepare stock solutions at 1 mg/mL.
NOTE: The stock solutions were aliquoted and stored at -20 °C. The cholesteryl oleate was stored in dark bottles. The PC, SM, PS, and CO stock solutions were stable for up to 6, 3, 12, and 12 months, respectively, at -20 °C. The TPGS solution was stable for at least 12 months at -20 °C. - Mix 10 µL of NGF (1 mg/mL in water) with 10 µL of protamine USP (1 mg/mL in water) in a 1.5 mL microcentrifuge tube and let it stand for 10 min at room temperature to form the complex.
NOTE: The NGF and protamine stock solutions were aliquoted and stored at -20 °C. Repeated freezing and thawing is not recommended for the NGF stock. - Add 59 µL of PC, 11 µL of SM, 4 µL of PS, 15 µL of CO, and 45 µL of TPGS to a glass vial. Mix and evaporate the ethanol under a gentle nitrogen stream for about 5 min; all excipients should form an oily, thin film at the bottom of the glass vial.
- Add 1 mL of ultrapure (type 1) water to the vial and homogenize at 9,500 rpm (8,600 x g) for 5 min at room temperature to form the prototype NPs.
- Add the complex prepared in step 1.2 to the prototype NPs and incubate at 37 °C for 30 min with stirring by using a small stirring bar in the glass vial.
- Cool the NPs down by stirring at room temperature for another 30 min. After this cools, add 106 µL of Apo A-I (1.49 mg/mL) and stir at room temperature overnight to form the final NGF HDL-mimicking NPs.
2. Characterization of NGF HDL-mimicking Nanoparticles
- Measure the particle size and zeta potential using a particle analyzer (see the Materials Table) as per the manufacturer's instructions.
- Use a cross-linked agarose gel filtration chromatography column to separate the unloaded NGF from the NGF HDL-mimicking NPs and determine the entrapment efficiency of the NGF.
- For the column preparation, transfer 15 mL of Sepharose 4B-CL suspension to a 50-mL beaker. Stir the bead suspension using a glass rod and pour some into a column (30 cm length × 1 cm diameter, with a glass frit at the bottom). Gently tap the column to get rid of bubbles. Allow the solvent to drain and the beads to settle for a few min.
- Continue to add the remaining suspension. Rinse the inside wall of column to clean the beads. Drain the solvent until the solvent level is slightly above the top of the stationary phase. Wash and condition the column with 20 mL of 1x phosphate-buffered saline (PBS, containing 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4).
- To determine the fractions containing unloaded NGF, load 200 µL of NGF solution (10 µg/mL) onto the gel filtration column (25 cm length x 1 cm diameter) and elute with 1x PBS.
- Collect a total of 12 fractions (1 mL for each fraction) and measure the concentration of NGF in each fraction using a Sandwich ELISA kit for NGF.
- Detect NGF from fractions 6 to 10 using the Sandwich ELISA kit. Obtain a chromatogram of unloaded NGF on the column. Wash the column with 20 mL of PBS.
- To determine the fractions containing NGF HDL-mimicking NPs, load 200 µL of the NPs onto the column and elute with 1x PBS. Collect a total of 12 fractions (1 mL for each fraction) and measure the intensity of particles in each fraction using the particle size analyzer. Detect the NGF NPs from fractions 2 to 4.
NOTE: The intensity is a parameter measured by the particle analyzer, which tells how many nanoparticles may exist in the test solution. The more NPs exist in solution, the higher the intensity is. By this approach, we can confirm that the column can separate NGF NPs from unloaded NGF. - To measure the entrapment efficiency of NGF, load 200 µL of NGF HDL-mimicking NPs onto the column and elute with 1x PBS. Collect a total of 12 fractions (1 mL for each fraction).
- Measure the unloaded NGF concentration from fractions 6 to 10 using the Sandwich ELISA kit.
- Add the NGF amounts measured from fractions 6 to 10 together as the unloaded NGF and calculate the entrapment efficiency of NGF using the following equation.
%EE = (1 – unloaded NGF/total NGF added into NP) x 100% Equation (1)
3. In Vitro Release of NGF HDL-mimicking Nanoparticles
- Study free NGF (10 µg/mL in water; n = 4) and NGF HDL-mimicking NPs (10 µg/mL; n = 4) in parallel.
- Pre-treat 8 dialysis tubes (molecular weight cutoff: 300 kDa) by following the manufacturer's instructions.
- Prepare 5% bovine serum albumin (BSA) in PBS as the release medium. Add 30 mL of release medium to a 50-mL centrifuge tube and warm it up to 37 °C in a shaker with a 135 rpm shaking speed. Place another 50 mL of release medium in the shaker for replacement.
- Add 400 µL of release medium into the dialysis tube and quickly add 200 µL of the tested sample to make enough volume for dialysis.
- Close the tube and quickly put the dialysis tube into the release medium (the centrifuge tube). Quickly withdraw 100 µL of the release medium from the outside dialysis tube as the sample for time 0. Immediately put the sample into -20 °C for later analysis. Add 100 µL of fresh release medium into the centrifuge tube to replace the withdrawn sample. Start the timer.
- At 1, 2, 4, 6, 8, 24, 48, and 72 h, withdraw 100 µL of the release medium and replace it with 100 µL of fresh medium. Immediately put the withdrawn samples into -20 °C for later analysis.
- After 72 h, take out all samples from -20 °C and thaw them at room temperature. Measure the NGF concentrations of the release samples using the Sandwich ELISA kit.
4. Bioactivity of NGF HDL-mimicking Nanoparticles (Neurite Outgrowth Study)
- Culture PC12 cells in RPMI-1640 medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum, 100 µg/mL streptomycin, and 100 units/mL penicillin. Maintain the cells in a humidified incubator at 37 °C and with 5% CO2.
- When the cells reach ~70-80% confluence, remove the culture medium and wash the cells with 1x PBS (approximately 2 mL per 10 cm2 culture surface area). Remove the wash solution. Trypsinize the cells by adding 0.25% Trypsin-EDTA solution (0.25% trypsin and 1 mM EDTA; 0.5 mL per 10 cm2 culture surface area) to the flask and incubate at 37 °C until most cells are detached.
- Add two volumes of culture medium and spin down at 180 x g for 5 min. Remove the supernatant and resuspend the cell pellet in 5 mL of culture medium. Filter the cells through a 22 G, ½ inch needle to break cell clusters.
- Split the cells at a ratio of 1:3 into a new cell culture flask. After incubating overnight, remove unattached PC12 cells. Allow the attached cells to remain for further growth.
- Repeat this procedure for 3 passages to select the subculture of PC12 that has a strong adhesion property. Pre-coat a 6-well plate with 800 µL/well of rat tail collagen type I (100 µg/mL).
- Trypsinize the selected PC12 cells as described in step 4.2 and filter them through a 22 G, ½ inch needle to break the cell clusters. Count the cells with a hemocytometer. Seed the cells overnight at a density of 10,000 cells/well in the pre-coated 6-well plate in step 4.5 to allow the cells to attach to the plate.
- Dilute free NGF (10 µg/mL) and NGF HDL-mimicking NPs (10 µg/mL) with the culture medium (described in step 4.1) to prepare 0.5, 1, 5, 10, 50, and 100 ng/mL concentrations. Remove 2 mL of medium from each well and replace with 2 mL of the diluted free NGF or NGF HDL-mimicking NPs. Culture for 4 days.
- On day 4, change the medium to fresh medium (described in step 4.1) containing the corresponding treatment and continue the treatment for another 3 days.
- On day 7, visualize the cells with an inverted light microscope and image each well at random spots under 10X magnification.
5. Biodistribution of NGF HDL-mimicking Nanoparticles
- Use adult BALB/c mice (male, 25-30 g) to test the tissue distribution of NGF NPs. Randomly divide the mice into three groups of 3 mice per group. Use a cone-shaped plastic restraint (e.g., decapicone) to restrain a mouse and wipe the tail with ethanol to promote vasodilation and the visibility of the vein.
- Inject 100 µL of either saline, free NGF, or NGF HDL-mimicking NPs to each group of mice (3 groups) through the tail veins at a dose of 40 µg/kg of NGF. Use a 30½ G needle attached to a 1 mL syringe.
- At 30 min after the injection, anesthetize the mice using 3% inhaled isoflurane (in oxygen at flow rate of 2 L/min). Perform a tail and toe pinch to determine the depth of anesthesia. Collect blood by cardiac puncture.
- Withdraw approximately 1 mL of blood from the heart. Euthanize each mouse by cervical dislocation.
- Place the mouse carcass in dorsal recumbency. Open the abdomen using surgical scissors and move fat and intestine aside using a cotton swab to expose the liver, spleen, and kidney. Harvest these tissues and rinse them in 1x PBS to clean the blood.
- Immediately centrifuge the blood samples at 3,400 x g and 4 °C for 5 min to obtain the plasma. Store the plasma and tissues at -80 °C until the analyses.
- To analyze the tissue samples, move the samples from -80 °C to 4 °C and suspend 100 mg of tissue sample in a 10x volume of extraction buffer (0.05 M sodium acetate, 1.0 M sodium chloride, 1% triton X-100, 1% BSA, 0.2 mM phenylmethanesulfonyl fluoride, and 0.2 mM benzethonium chloride). Homogenize at 10,000 rpm and 4 °C for 5 min.
- Sacrifice two untreated mice to collect blank plasma and tissues, as described in steps 5.3 and 5.4. Prepare NGF standard solutions using blank plasma or blank tissue homogenates.
- Determine the concentrations of NGF in the plasma and tissue homogenates using the Sandwich ELISA kit, as described above.
Preparation and Characterization of Novel HDL-mimicking Nanoparticles for Nerve Growth Factor Encapsulation
Learning Objectives
The engineering scheme of HDL-mimicking, α-tocopherol-coated NGF NPs prepared by an ion-pair strategy is shown in Figure 1. To neutralize the surface charges of NGF, protamine USP was used as an ion-pair agent to form a complex with NGF. To protect the bioactivity, prototype HDL-mimicking NPs were engineered, first using homogenization; then, the NGF/protamine complex was encapsulated into the prototype NPs. Homogenization provided sufficient energy and successfully promoted the mixing of the excipients. After a 3 min homogenization, consistent particle sizes (around 170 nm) were obtained for the prototype NPs (Figure 2). Apo A-I was incubated with the prototype NPs in different conditions, including 2 h of stirring at room temperature; 4 h of stirring at room temperature; 4 h of stirring at room temperature, followed by overnight incubation at 4 °C; and stirring at room temperature overnight. Over 26% of Apo A-I was incorporated in the NPs when stirred at room temperature overnight. To add the NGF-protamine complex, the complex was incubated with the prototype NPs for 30 min at 37 °C and then Apo A-I was added to the mixture to finish the final coating of Apo A-I on the surface of the NPs. Using the procedure described here, the final NGF HDL-mimicking NPs had particle sizes of 171.4 ±6.6 nm (n = 3), with 65.9% of NGF entrapment efficiency (Table 1). NGF HDL-mimicking NPs had a slight negative charge (Table 1). The NPs had a narrow size distribution, and incorporating NGF into the NPs did not affect the particle size (Figure 3).
To measure the entrapment efficiency of NGF, various methods were evaluated to separate unloaded NGF from NGF HLD-mimicking NPs. Unexpectedly, NGF cannot pass a separation filter (molecular weight cutoff: 100 kDa). Gel filtration columns, including Sephadex G-50, Sephadex G-100, and Sephacryl S-100, cannot separate unloaded NGF and NGF HDL-mimicking NPs, since both of them come out in the same fractions after elution. A Sepharose CL-4B column performed the separation with the optimized sample loading, elution buffer, and elution rate. As shown in Figure 4, unloaded NGF and NGF HDL-mimicking NPs were completely separated on a Sepharose CL-4B column.
A dialysis method was used to study the in vitro release of NGF HDL-mimicking NPs in PBS with 5% BSA, which was included to mimic the physiological conditions in blood. By increasing the size of the dialysis device (molecular weight cutoff: 300 kDa) and by adding PBS and BSA to the release medium, NGF did not bind with the dialysis membrane and freely passed through. As a result, the recovery of free NGF in this dialysis method was over 85% (Figure 5). NGF HDL-mimicking NPs showed a slow release profile, and about 10% of the NGF was released from the NPs over 72 h (Figure 5).
To test the bioactivity of NGF HDL-mimicking NPs, the subculture of PC12 cells that had a strong adhesion property was selected to conduct a neurite outgrowth assay. Figure 6 represents the imaging of neurite outgrowth when the cells were treated with 50 ng/mL free NGF (Figure 6A) and NGF HDL-mimicking NPs (Figure 6B). When the treatment concentration was higher than 10 ng/mL, neurite outgrowth was clearly observed by the microscope. At these high concentrations, free NGF and NGF HDL-mimicking NPs did not show a significant difference on the effect of neurite outgrowth. When the concentration of NGF was lower than 10 ng/mL, neurite outgrowth could not be observed clearly, both for free NGF and for NGF HDL-mimicking NPs. Biodistribution studies were performed to compare the in vivo behaviors of free NGF and NGF HDL-mimicking NPs. As shown in Figure 7, NGF HDL-mimicking NPs significantly increased the plasma concentration and decreased the uptake in the liver, kidney, and spleen.
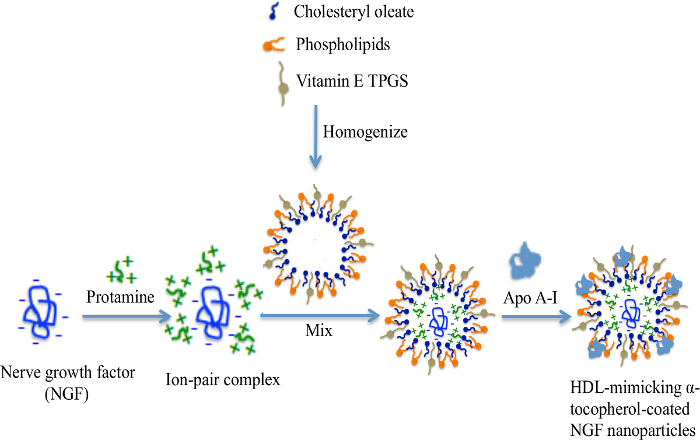
Figure 1: The engineering scheme of NGF HDL-mimicking nanoparticles prepared by an ion-pair strategy. NGF is a negatively charged hydrophilic molecule. A cationic peptide, protamine, was used to neutralize the charges and formed an ion-pair complex with NGF. Cholesteryl oleate, phospholipids, and TPGS formed self-assembly prototype NPs by homogenization. The NGF/protamine complex was incorporated into the prototype NPs. Finally, Apo A-I was coated on the NP surface after overnight incubation. This figure has been modified from Prathipati et al.16. Please click here to view a larger version of this figure.
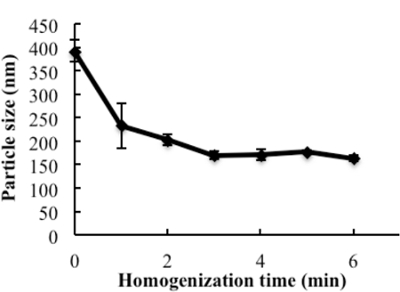
Figure 2: The influence of homogenization on the particle size of the prototype nanoparticles. The excipients, PC, SM, PS, CO, and TPGS, which were dissolved in ethanol, were added to glass vials, and the solvent was evaporated under N2 stream. 1 mL of water was added and homogenized at 9,500 rpm for different times. The particle sizes of subsequent nanoparticles were measured. Data are presented as the mean ± standard deviation (n = 4). This figure has been modified from Prathipati et al.16. Please click here to view a larger version of this figure.
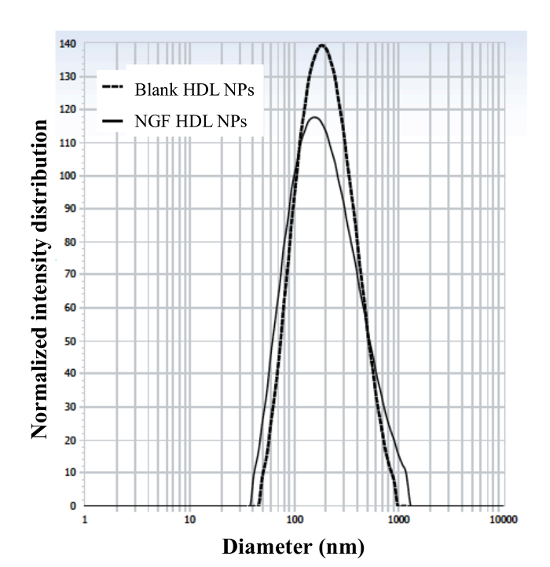
Figure 3: Particle size and size distribution of blank HDL-mimicking nanoparticles and NGF HDL-mimicking nanoparticles. The particle sizes and distributions were measured using a particle analyzer. This figure has been modified from Prathipati et al.16. Please click here to view a larger version of this figure.
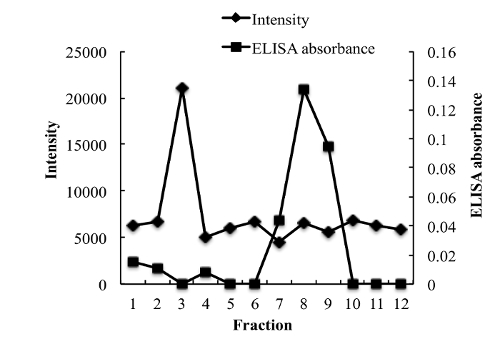
Figure 4: Chromatograms of free NGF and NGF HDL-mimicking nanoparticles on a Sepharose CL-4B column eluted by PBS. 200 µL of free NGF solution (10 µg/mL) and NGF NP solution were loaded onto the gel filtration column and eluted with 1x PBS. A total of twelve fractions (1 mL for each fraction) were collected for both samples. The NP intensity in each fraction was measured by a particle analyzer, and the concentration of NGF in each fraction was measured using a Sandwich ELISA kit. This figure has been modified from Prathipati et al.16. Please click here to view a larger version of this figure.
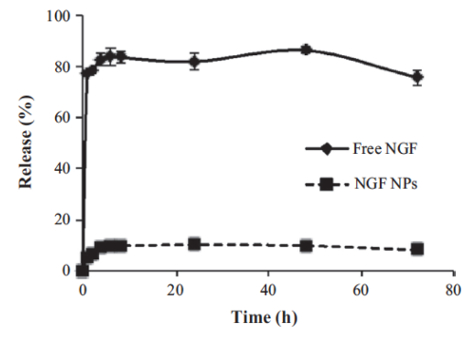
Figure 5: In vitro release of NGF HDL-mimicking nanoparticles measured by a dialysis method. PBS with 5% BSA was used as a release medium. 200 µL of free NGF solution (10 µg/mL) or NGF NPs were added to a dialysis tube supplemented with 400 µL of the release medium. The dialysis tube was put into 30 mL of pre-warmed release medium. The study was performed at 37 °C with 135 rpm shaking. At 1, 2, 4, 6, 8, 24, 48, and 72 h, 100 µL of the release medium was withdrawn and replaced with 100 µL of fresh medium. The NGF concentration in each sample was measured using a Sandwich ELISA kit. Data are presented as the mean ± standard deviation (n = 4). This figure has been modified from Prathipati et al.16. Please click here to view a larger version of this figure.
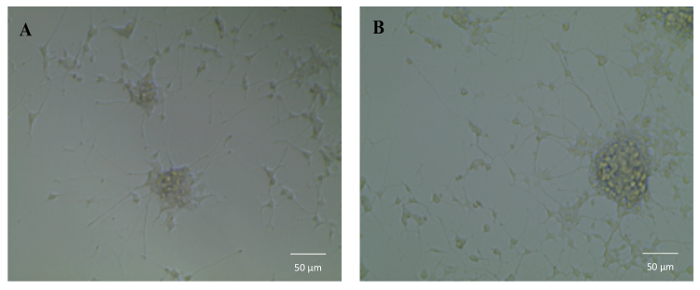
Figure 6: The influence of NGF HDL-mimicking nanoparticles on neurite outgrowth in PC12 cells. Cells were treated with 50 ng/mL free NGF (A) and NGF HDL-mimicking nanoparticles (B) for 7 days. The neurite was imaged using an inverted light microscope under 10X magnification. Please click here to view a larger version of this figure.
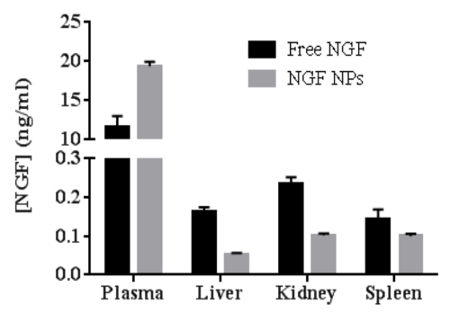
Figure 7: The comparison of biodistribution between free NGF and NGF HDL-mimicking nanoparticles in mice (n = 3). The mice were administered with 40 mg/kg of NGF by tail-vein injection and were sacrificed at 30 min after administration. The blood, liver, spleen, and kidney were collected, and the concentration of NGF in each sample was measured using a Sandwich ELISA kit. Data are shown as the mean ± standard deviation. This figure has been modified from Prathipati et al.16. Please click here to view a larger version of this figure.
| Sample | Particle size (nm) | P.I. | EE% of NGF | Zeta potential (mV) |
| NGF HDL-mimicking NPs | 171.4 ±6.6 | 0.239 ±0.01 | 65.9 ±1.4 | -12.5 ±1.9 |
Table 1: Characterization of NGF HDL-mimicking nanoparticles (n = 3). Data are shown as the mean ± standard deviation. This table has been modified from Prathipati et al.16.
List of Materials
| Recombinant Human Beta-NGF | Creative Biomart | NGF-05H | |
| L-a-Phosphatidylcholine (PC) | Avanti | 131601P | 95%, Egg, Chicken |
| Sphingomyelin (SM) | Avanti | 860062P | Brain, Porcine |
| Phosphatidylserine (PS) | Avanti | 840032P | Brain, Porcine |
| Cholesteryl oleate (CO) | Sigma | C9253 | |
| D-α-Tocopheryl polyethylene glycol succinate (TPGS) | BASF | 9002-96-4 | Vitamin E Polyethylene Glycol Succinate |
| Protamine sulfate | Sigma | P3369 | meets USP testing specifications |
| Apolipoprotein A1, Human plasma | Athens Research & Technology | 16-16-120101 | 1mg in 671 µl 10 mM NH4HCO3, pH 7.4 |
| Sepharose 4B-CL | Sigma | CL4B200 | Cross-linked agarose, gel filtration chromatography column filling material |
| Sandwich ELISA Kit for NGF | R&D system | DY008 | |
| Bovine Serum Albumin | Sigma | A2153 | |
| RPMI-1640 medium | GE Healthcare Life Science | SH30096.02 | |
| Horse serum | GE Healthcare Life Science | SH30074.03 | |
| Fetal bovine serum | Gibco | 10082147 | |
| PC12 cells | ATCC | CRL-1721 | |
| Rat tail collagen type I | Sigma | C3867 | |
| Sodium acetate | Sigma | S2889 | |
| Sodium chloride | Sigma | 31414 | |
| Triton X-100 | Sigma | T8787 | |
| Phenylmethanesulfonyl fluoride (PMSF) | Sigma | P7626 | |
| Benzethonium chloride | Sigma | B8879 | |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Homogenizer | Tekmar | T 25-S1 | |
| Delsa Nano HC particle analyzer | Beckman-Coulter | Delsa Nano HC | |
| Float-A-Lyzer G2 Dialysis Device | Spectrum Laboratories | G235036 | Molecule Cutoff 300 kDa |
| Centrifuge | Eppendoff | 5424R | |
| Polytron homogenizer | Kinematica | PT 1200C | |
| DecapiCone | Braintree Scientific Inc. | DC-M200 |
Lab Prep
The objective of this article is to introduce preparation and characterization methods for nerve growth factor (NGF)-loaded, high-density, lipoprotein (HDL)-mimicking nanoparticles (NPs). HDLs are endogenous NPs and have been explored as vehicles for the delivery of therapeutic agents. Various methods have been developed to prepare HDL-mimicking NPs. However, they are generally complicated, time consuming, and difficult for industrial scale-up. In this study, one-step homogenization was used to mix the excipients and form the prototype NPs. NGF is a water-soluble protein of 26 kDa. To facilitate the encapsulation of NGF into the lipid environment of HDL-mimicking NPs, protamine USP was used to form an ion-pair complex with NGF to neutralize the charges on the NGF surface. The NGF/protamine complex was then introduced into the prototype NPs. Apolipoprotein A-I was finally coated on the surface of the NPs. NGF HDL-mimicking NPs showed preferable properties in terms of particle size, size distribution, entrapment efficiency, in vitro release, bioactivity, and biodistribution. With the careful design and exploration of homogenization in HDL-mimicking NPs, the procedure was greatly simplified, and the NPs were made scalable. Moreover, various challenges, such as separating unloaded NGF from the NPs, conducting reliable in vitro release studies, and measuring the bioactivity of the NPs, were overcome.
The objective of this article is to introduce preparation and characterization methods for nerve growth factor (NGF)-loaded, high-density, lipoprotein (HDL)-mimicking nanoparticles (NPs). HDLs are endogenous NPs and have been explored as vehicles for the delivery of therapeutic agents. Various methods have been developed to prepare HDL-mimicking NPs. However, they are generally complicated, time consuming, and difficult for industrial scale-up. In this study, one-step homogenization was used to mix the excipients and form the prototype NPs. NGF is a water-soluble protein of 26 kDa. To facilitate the encapsulation of NGF into the lipid environment of HDL-mimicking NPs, protamine USP was used to form an ion-pair complex with NGF to neutralize the charges on the NGF surface. The NGF/protamine complex was then introduced into the prototype NPs. Apolipoprotein A-I was finally coated on the surface of the NPs. NGF HDL-mimicking NPs showed preferable properties in terms of particle size, size distribution, entrapment efficiency, in vitro release, bioactivity, and biodistribution. With the careful design and exploration of homogenization in HDL-mimicking NPs, the procedure was greatly simplified, and the NPs were made scalable. Moreover, various challenges, such as separating unloaded NGF from the NPs, conducting reliable in vitro release studies, and measuring the bioactivity of the NPs, were overcome.
Procedure
The objective of this article is to introduce preparation and characterization methods for nerve growth factor (NGF)-loaded, high-density, lipoprotein (HDL)-mimicking nanoparticles (NPs). HDLs are endogenous NPs and have been explored as vehicles for the delivery of therapeutic agents. Various methods have been developed to prepare HDL-mimicking NPs. However, they are generally complicated, time consuming, and difficult for industrial scale-up. In this study, one-step homogenization was used to mix the excipients and form the prototype NPs. NGF is a water-soluble protein of 26 kDa. To facilitate the encapsulation of NGF into the lipid environment of HDL-mimicking NPs, protamine USP was used to form an ion-pair complex with NGF to neutralize the charges on the NGF surface. The NGF/protamine complex was then introduced into the prototype NPs. Apolipoprotein A-I was finally coated on the surface of the NPs. NGF HDL-mimicking NPs showed preferable properties in terms of particle size, size distribution, entrapment efficiency, in vitro release, bioactivity, and biodistribution. With the careful design and exploration of homogenization in HDL-mimicking NPs, the procedure was greatly simplified, and the NPs were made scalable. Moreover, various challenges, such as separating unloaded NGF from the NPs, conducting reliable in vitro release studies, and measuring the bioactivity of the NPs, were overcome.
