Quantifying the Heterogeneous Distribution of a Synaptic Protein in the Mouse Brain Using Immunofluorescence
Instructor Prep
concepts
Student Protocol
This protocol does not involve experiments on live animals. Experiments involving euthanizing of animals to obtain brain samples were approved by the local animal protection authorities (Tierschutzkommission der Universitätsmedizin Göttingen) under the approval number T 10/30.
NOTE: For this protocol, 3 adult male C57BL/6 mice were used.
1. Sample Preparation
- Prepare fixative and 0.1 M phosphate buffer (PB; see Table 1).
- Fix the animal by transcardial perfusion as described in Gage et al.28. First wash out the blood with 0.9% NaCl-solution, then perfuse with 30 mL of 4% paraformaldehyde (PFA).
- Open the skull with scissors and carefully isolate the brain using a spoon with blunt edges to avoid damaging the tissue.
- Fill a 50 mL reaction tube with fixative and postfix the brain in 4% PFA at 4 °C overnight.
- Remove the fixative and wash the brain in 50 mL of 0.1 M PB on a shaker for 30 min.
- After washing, incubate the brain in a 50 mL reaction tube in 30% sucrose in 0.1 M PB for 48 h or until it sinks in the tube at 4 °C for cryoprotection.
- Trim the cryoprotected brain with a sharp blade, place it in a cryomold, and embed it with optimal cutting temperature (OCT) compound. Avoid bubbles. Orient the brain and freeze the cryomold in the -80 °C freezer.
- Mount the frozen tissue for sectioning. Equilibrate the tissue to the cryomicrotome temperature for at least 15 min before sectioning.
- Section the brain into 25 µm thick coronal slices. Touch the OCT carefully with a glass hook without touching the brain tissue. Collect 3 adjacent slices per well in a 24 well plate and store them in 0.1 M PB at 4 °C until staining.
NOTE: The protocol can be paused here for up to two weeks. Longer storage times can interfere with the tissue quality and thus influence the outcome of the experiment.
2. Immunofluorescence
- Prepare solutions including the blocking buffer, antibody buffer, washing buffer 1, and washing buffer 2 (see Table 1).
- Rinse slices once with PB to remove excess OCT.
- Remove the solution with a plastic pipette without sucking in the brain slices. Add 250 µL of fresh PB with a 1000 µL pipette.
CAUTION: Slices should not dry out, so remove and add fluids well by well.
- Remove the solution with a plastic pipette without sucking in the brain slices. Add 250 µL of fresh PB with a 1000 µL pipette.
- Remove the PB with a plastic pipette and add 250 µL of blocking buffer per well with a 1000 µL pipette. Incubate for 3 h at room temperature (RT) on the shaker.
- During the incubation time, dilute the primary antibodies in antibody buffer in a reaction tube. Use 250 µL antibody buffer per well and add the appropriate amount of antibody (see Table 2) by pipetting it directly into the solution using a 2 µL pipette. Mix the solution by gently pipetting up and down several times. Vortex shortly afterwards to ensure proper mixing.
NOTE: To determine the background fluorescence, stainings should also be performed without adding the primary antibody. For that, incubate the slice in antibody solution without primary antibodies according to the protocol. - After the incubation time, remove the blocking buffer with a plastic pipette and add 250 µL of antibody solution containing primary antibodies per well. Incubate slices with primary antibody overnight at 4 °C on a shaker.
- Next day, wash the slices with washing buffer 1 3x for 10 min at RT on a shaker.
- Remove the medium with a plastic pipette and add 300 µL of washing buffer 1 per well. Incubate at RT for 10 min. Repeat 3 times.
- During the washing steps, dilute the fluorophore-coupled secondary antibodies in antibody buffer in a reaction tube. Use 250 µL antibody buffer per well and add the appropriate amount of antibody (see Table 2) by pipetting it directly into the solution using a 2 µL pipette. Mix the solution by gently pipetting up and down several times. Vortex shortly afterwards to ensure proper mixing.
CAUTION: Because the antibodies are light-sensitive, all steps from this point on need to be performed in the dark. - After the washing steps, remove the washing buffer with a plastic pipette and add 250 µL of antibody solution containing secondary antibodies per well. Incubate the slices with secondary antibody for 90 min at RT in the dark.
- Wash the slices with washing buffer 2 3x for 10 min at RT.
- During the washing steps, dilute 4′,6-diamidino-2-phenylindole (DAPI) in 0.1 M PB in a concentration of 1:2000.
- Remove the washing buffer 2 with a plastic pipette and add 250 µL of DAPI solution per well. Incubate for 5 min at RT on the shaker.
- Remove the DAPI solution with a plastic pipette and add 500 µL of 0.1 M PB per well with a 1000 µL pipette.
- Mount slices on microscope slides.
- Place a microscope slide under the stereoscope. With a fine brush, add three separate drops of 0.1 M PB onto the slide. Place one slice per drop onto the microscope slide.
- Use the fine brush to flatten and orient the slices on the microscope slide.
- When all slices are positioned correctly, remove excess PB with a tissue and dry the slide carefully.
CAUTION: Avoid drying the brain slices completely. - Add 80 µL of embedding medium onto the slide. Carefully lower the coverslip onto the slide, thereby embedding the brain slices.
- Leave the slides to dry in the fume hood for 1-2 h (cover them to avoid light exposure) and store them in a microscope slide box at 4 °C.
NOTE: The protocol can be paused here.
3. Imaging
- After the embedding medium is completely hardened, place the microscope slide under the confocal microscope.
NOTE: Epifluorescence microscopy combined with deconvolution software should yield similar image quality. - Adjust the laser settings by increasing or decreasing the laser intensity for every channel so that few pixels are overexposed to ensure maximum distribution of grey values.
- Acquire virtual tissues of the whole brain slice for the different channels.
- In the imaging software (see Table of Materials), select the Tiles option and manually delineate the brain slice with the Tile Region Setup.
- Distribute support points throughout the tile region and adjust the focus for the different support points by pressing Verify Tile Regions/Positions….
- Adjust the settings in Acquisition Mode according to the desired resolution and file size of the resulting image and start the scan.
- When the scan is finished, use the Stitching function to process the virtual tissue. Export the file as a .tif with the function Image Export.
4. Computer-based Analysis
- Load all single channels for one image into FIJI29 by clicking File| Open.
- With the Freehand selection tool, delineate one hemisphere in the DAPI-channel. Create a mask of the selection by clicking Edit| Selection| Create mask.
- Determine the mean fluorescence intensity for the single channels (Mover and synaptophysin) by clicking Analyze| Measure.
NOTE: Make sure to select the different channels to determine the mean fluorescence intensity values for each channel. - Copy the mean fluorescence intensity for the single channels into a spreadsheet.
- Determine the mean fluorescence intensity for the single channels in an area of interest by delineating the area also with the Freehand selection tool. Use a mouse brain atlas as reference.
- Repeat steps 4.1-4.5 for all hemispheres and all areas of interest.
NOTE: Determine the values for each hemisphere separately in order to later compare the values in an area of interest to that in the hemisphere (see step 5.2).
5. Data Handling
- In case the background fluorescence is high (see Discussion), a background subtraction might be needed. For that, determine the mean fluorescence intensity for the slice processed without primary antibody against the reference protein (here: synaptophysin) and subtract that value from all values obtained for the brain regions and hemispheres.
- When the mean fluorescence intensities for the single channels for every hemisphere and every area of interest have been determined (see Table 3), calculate the ratio of Mover to synaptophysin by dividing the value for Mover by the value for synaptophysin (yellow in Table 3). Perform this action for every hemisphere and every area of interest separately.
- Divide the ratio obtained for one area of interest by the ratio obtained for the corresponding hemisphere (orange in Table 3) to determine the ratio of the area of interest to the hemisphere.
- To determine the relative Mover abundance, translate the ratio obtained in 5.2 into a percentage by determining its deviation from 1 (red in Table 3). A ratio of 1.25 would therefore give a relative Mover abundance of 25% above average, and a ratio of 0.75 would yield a relative Mover abundance of 25% below average.
Quantifying the Heterogeneous Distribution of a Synaptic Protein in the Mouse Brain Using Immunofluorescence
Learning Objectives
Representative staining patterns of different markers can be seen in Figure 1. The pattern varies depending on the distribution of the protein. Examples of five rostro-caudal levels are shown in columns (A)-(E). A representative DAPI staining is shown in the first row: DAPI adheres to the DNA of a cell and thus nuclei are stained. This results in a punctate pattern. Regions with a high cell density are brighter than regions with low cell densities. An example for a heterogeneously distributed protein can be seen in the second row. The Mover staining reveals a differential distribution throughout the brain, with bright hotspot areas and dimmer areas. In the third row, an example for the more homogeneously distributed reference marker synaptophysin is shown. An overlay of the two proteins (fourth row) shows the differential distribution of Mover (red) compared to the marker protein synaptophysin (green).
Figure 2 shows the quantification described in step 4 of the protocol. Shown are the mean fluorescence intensity values for the different channels across the hemispheres (Mover, Figure 2A; synaptophysin, Figure 2B) and across the areas of interest (Mover, Figure 2C; synaptophysin, Figure 2D). To determine the Mover abundance relative to the number of synaptic vesicles, a ratio is taken of the Mover fluorescence values to synaptophysin fluorescence values. These ratios for the areas of interest are shown in Figure 2E, and already provide an indication of the heterogeneous distribution of Mover, with areas with high and low Mover levels relative to synaptic vesicles. To additionally compensate for the inherent technical variability, the ratio in one area of interest (Figure 2E) is compared to that across the hemisphere (not shown) and translated into a percentage. This relative Mover abundance (Figure 2F) gives a measure of how much Mover is present in one area of interest relative to average.
As mentioned above, one of the major advantages of this technique is the ability to determine the abundance of the protein of interest across very small areas, even subregions and layers of areas of interest. One example of this application is shown in Figure 3, where the relative Mover abundance was determined for the different layers in the subfields of the hippocampus. The quantification in the different layers shown in Figure 3D, Figure 3F, and Figure 3H corresponds to the layers shown in Figure 3C, Figure 3E, and Figure 3G, with the corresponding colors. Within the hippocampus, Mover is heterogeneously distributed, with high Mover levels relative to synaptic vesicles in layers associated with intra-hippocampal computation (i.e., the polymorph layer of dentate gyrus [DG], stratum radiatum, lucidum and oriens of Cornu Ammonis 3 [CA3], and stratum radiatum and oriens of Cornu Ammonis 1 [CA1]), and low levels in input- and output layers (the inner and outer molecular layer of DG, the pyramidal cell layers of CA3 and CA1, and the stratum lacunosum-moleculare of CA1).
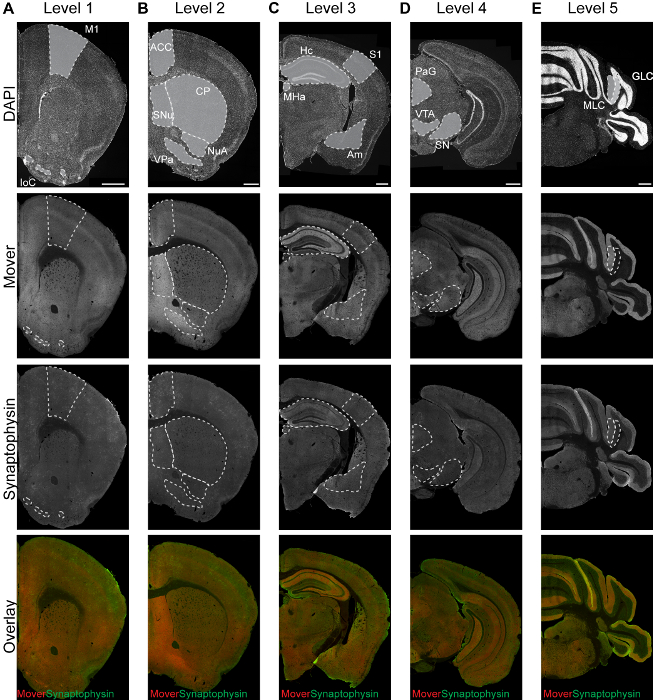
Figure 1: Representative immunofluorescence images of DAPI (first row), Mover (second row), synaptophysin (third row), and their overlay (fourth row, Mover in red, synaptophysin in green) at the 5 rostro-caudal levels (A-E). Areas of interest are shaded in grey in the upper row of panels. M1, primary motor cortex; IoC, islands of Calleja; ACC, anterior cingulate cortex; SNu, septal nuclei; VPa, ventral pallidum; NuA, nucleus accumbens; CP, caudate putamen; S1, primary somatosensory cortex; Hc, hippocampus; Am, amygdala; MHa, medial habenula; PAG, periaqueductal grey; SN, substantia nigra; VTA, ventral tegmental area; MLC, molecular layer of the cerebellum; GLC, granular layer of the cerebellum. Scale bar = 500 µm. This figure has been modified from Wallrafen and Dresbach1. Please click here to view a larger version of this figure.
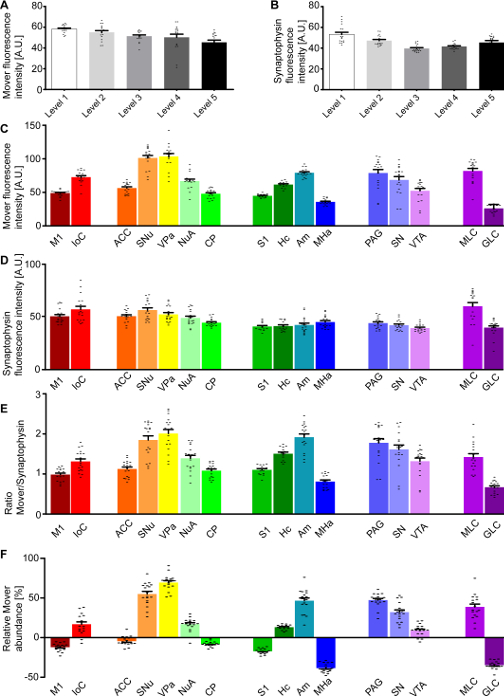
Figure 2: Quantification of the Mover distribution across the 5 rostro-caudal levels. Mean fluorescence intensity of the Mover signal (A) and the synaptophysin signal (B) at the different levels. Mean fluorescence intensity of the Mover signal (C) and the synaptophysin signal (D) at the 16 manually delineated brain regions. (E) Ratios of Mover and synaptophysin in the 16 brain areas of interest. (F) Quantification of the relative Mover abundance, comparing Mover/synaptophysin ratio at the respective region to the ratio of the corresponding hemisphere. M1, primary motor cortex; IoC, islands of Calleja; ACC, anterior cingulate cortex; SNu, septal nuclei; VPa, ventral pallidum; NuA, nucleus accumbens; CP, caudate putamen; S1, primary somatosensory cortex; Hc, hippocampus; Am, amygdala; MHa, medial habenula; PAG, periaqueductal grey; SN, substantia nigra; VTA, ventral tegmental area; MLC, molecular layer of the cerebellum. Black dots represent single data points. Bars show the mean ± standard error of the mean (SEM). This figure has been modified from Wallrafen and Dresbach1. Please click here to view a larger version of this figure.
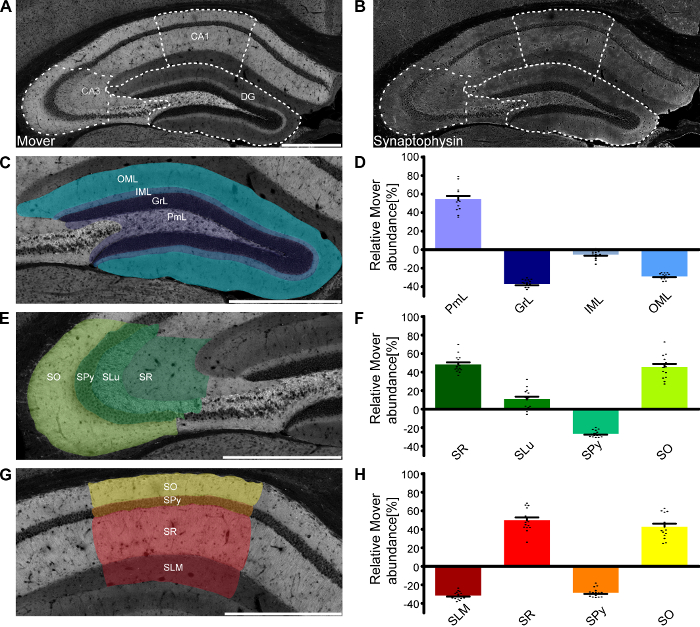
Figure 3: Mover distribution in the mouse hippocampus. Immunofluorescence stainings of coronal slices of the mouse hippocampus. Overview of the hippocampus showing the heterogeneous Mover expression pattern (A) and the corresponding Synaptophysin staining (B). The three regions of interest (DG, Figure 3C; CA3, Figure 3E; CA1, Figure 3G) are delineated with white dotted lines. (D,F,H) Quantification comparing the ratio in the respective layers to the ratio of the corresponding hemisphere. The colors in the bar graphs correspond to the respective shading in panels C, E, and G. Mover expression is high in levels associated with intra-hippocampal computation (i.e., the polymorph layer of DG, stratum radiatum, lucidum and oriens of CA3, and stratum radiatum and oriens of CA1), and low in the main input- and output layers (the inner and outer molecular layer of DG, the pyramidal cell layers of CA3 and CA1, and the stratum lacunosum-moleculare of CA1). OML, outer molecular layer; IML, inner molecular layer; GrL, granular layer; PmL, polymorph layer/hilus; SO, stratum oriens; SPy, stratum pyramidale; SLu, stratum lucidum; SR, stratum radiatum; SLM, stratum lacunosum-moleculare. Scale bar = 500 µm. Black dots represent single data points. Bars show the mean ± SEM. This figure has been modified from Wallrafen and Dresbach1. Please click here to view a larger version of this figure.
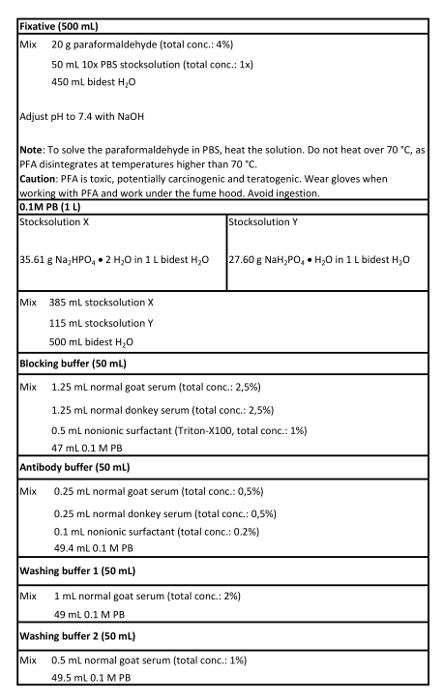
Table 1: Solutions used in this protocol.
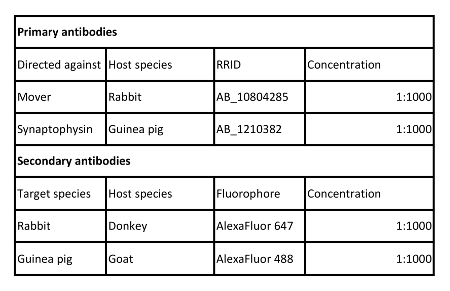
Table 2: Antibodies used in this protocol.
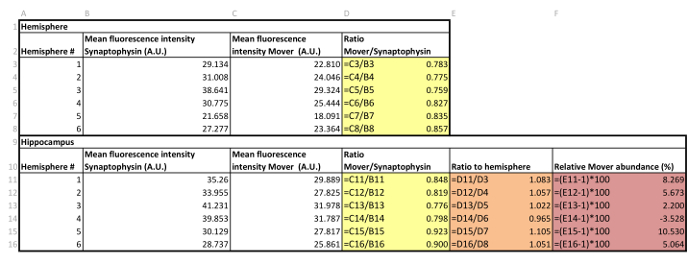
Table 3: Example of data handling.
List of Materials
| 1.5 mL reaction tubes | Eppendorf | 30120094 | |
| 50 mL reaction tubes | Greiner Bio-One | 227261 | |
| multiwell 24 well | Fisher Scientific | 087721H | |
| plastic pipette (disposable) | Sarstedt | 861,176 | |
| 1000 mL pipette | Rainin | 17014382 | |
| 2 ml pipette | Eppendorf | 3123000012 | |
| Vortex Genius 3 | IKA | 3340001 | |
| Menzel microscope slides | Fisher Scientific | 10144633CF | |
| Stereoscope | Leica | ||
| LSM800 | Zeiss | Confocal microscope | |
| freezing microtome | Leica | ||
| PBS (10X) | Roche | 11666789001 | |
| PFA | Sigma | P6148-1kg | |
| NaCl | BioFroxx | 1394KG001 | |
| sucrose | neoFroxx | 1104KG001 | |
| Tissue Tek | Sakura | 4583 | OCT |
| Na2HPO4 | BioFroxx | 5155KG001 | |
| NaH2PO4 | Merck | 1,063,460,500 | |
| normal goat serum | Merck Millipore | S26-100ML | |
| normal donkey serum | Merck | S30-100ML | |
| Triton X-100 | Merck | 1,086,031,000 | |
| rabbit anti-Mover | Synaptic Systems | RRID: AB_10804285 | |
| guinea pig anti-Synaptophysin | Synaptic Systems | RRID: AB_1210382 | |
| donkey anti-rabbit AF647 | Jackson ImmunoResearch | RRID: AB_2492288 | |
| goat anti-mouse AF488 | Jackson ImmunoResearch | RRID: AB_2337438 | |
| Mowiol4-88 | Calbiochem | 475904 | |
| ZEN2 blue software | Zeiss | Microscopy software | |
| FIJI | ImageJ | Analysis software | |
| Microsoft Excel | Microsoft | ||
Lab Prep
The presence, absence, or levels of specific synaptic proteins can severely influence synaptic transmission. In addition to elucidating the function of a protein, it is vital to also determine its distribution. Here, we describe a protocol employing immunofluorescence, confocal microscopy, and computer-based analysis to determine the distribution of the synaptic protein Mover (also called TPRGL or SVAP30). We compare the distribution of Mover to that of the synaptic vesicle protein synaptophysin, thereby determining the distribution of Mover in a quantitative manner relative to the abundance of synaptic vesicles. Notably, this method could potentially be implemented to allow for comparison of the distribution of proteins using different antibodies or microscopes or across different studies. Our method circumvents the inherent variability of immunofluorescent stainings by yielding a ratio rather than absolute fluorescence levels. Additionally, the method we describe enables the researcher to analyze the distribution of a protein on different levels: from whole brain slices to brain regions to different subregions in one brain area, such as the different layers of the hippocampus or sensory cortices. Mover is a vertebrate-specific protein that is associated with synaptic vesicles. With this method, we show that Mover is heterogeneously distributed across brain areas, with high levels in the ventral pallidum, the septal nuclei, and the amygdala, and also within single brain areas, such as the different layers of the hippocampus.
The presence, absence, or levels of specific synaptic proteins can severely influence synaptic transmission. In addition to elucidating the function of a protein, it is vital to also determine its distribution. Here, we describe a protocol employing immunofluorescence, confocal microscopy, and computer-based analysis to determine the distribution of the synaptic protein Mover (also called TPRGL or SVAP30). We compare the distribution of Mover to that of the synaptic vesicle protein synaptophysin, thereby determining the distribution of Mover in a quantitative manner relative to the abundance of synaptic vesicles. Notably, this method could potentially be implemented to allow for comparison of the distribution of proteins using different antibodies or microscopes or across different studies. Our method circumvents the inherent variability of immunofluorescent stainings by yielding a ratio rather than absolute fluorescence levels. Additionally, the method we describe enables the researcher to analyze the distribution of a protein on different levels: from whole brain slices to brain regions to different subregions in one brain area, such as the different layers of the hippocampus or sensory cortices. Mover is a vertebrate-specific protein that is associated with synaptic vesicles. With this method, we show that Mover is heterogeneously distributed across brain areas, with high levels in the ventral pallidum, the septal nuclei, and the amygdala, and also within single brain areas, such as the different layers of the hippocampus.
Procedure
The presence, absence, or levels of specific synaptic proteins can severely influence synaptic transmission. In addition to elucidating the function of a protein, it is vital to also determine its distribution. Here, we describe a protocol employing immunofluorescence, confocal microscopy, and computer-based analysis to determine the distribution of the synaptic protein Mover (also called TPRGL or SVAP30). We compare the distribution of Mover to that of the synaptic vesicle protein synaptophysin, thereby determining the distribution of Mover in a quantitative manner relative to the abundance of synaptic vesicles. Notably, this method could potentially be implemented to allow for comparison of the distribution of proteins using different antibodies or microscopes or across different studies. Our method circumvents the inherent variability of immunofluorescent stainings by yielding a ratio rather than absolute fluorescence levels. Additionally, the method we describe enables the researcher to analyze the distribution of a protein on different levels: from whole brain slices to brain regions to different subregions in one brain area, such as the different layers of the hippocampus or sensory cortices. Mover is a vertebrate-specific protein that is associated with synaptic vesicles. With this method, we show that Mover is heterogeneously distributed across brain areas, with high levels in the ventral pallidum, the septal nuclei, and the amygdala, and also within single brain areas, such as the different layers of the hippocampus.
