Use of Magnetic Resonance Imaging and Biopsy Data to Guide Sampling Procedures for Prostate Cancer Biobanking
Instructor Prep
concepts
Student Protocol
The protocol adheres to local guidelines and is approved by the UCL/UCLC Biobank Research Ethics Committee (Reference 15/YH/0311).
NOTE: As this method involves the sampling of human tissue, all local procedures regarding ethics and consent must be observed in advance of beginning the protocol. Radical prostatectomy cases may be included if both MRI and biopsy data are available in advance of surgery, with tumor diameter ≥5 mm. Cases should be excluded if the index lesion is not well defined, i.e., only diffuse changes are visible by MRI.
1. Prostate Slicing Apparatus
- Purchase the prostate slicing apparatus (Table of Materials). Alternatively, print a blade handle using a 3D printer as previously published10.
NOTE: The device and disposable blades used here were purchased under material transfer agreement from the Institute of Cancer Research, London, UK.
2. Tumor Targeting
- Review clinical notes to identify the index lesion as indicated by diagnostic biopsy, e.g., left posterior.
- Review MRI images to measure location of the above tumor.
- Find the sequence where the tumor is most visible in the axial plane, e.g., T2-weighted.
- Scroll through axial images to find the image where the tumor is largest and print image for reference.
- In the corresponding coronal image, measure the distance from the base of the prostate to the selected axial position, and the full length of the prostate from apex to base (mm), and print for reference.
3. Collection of pProstate
- Check patient notes to ensure appropriate informed consent has been obtained for this procedure and any downstream research applications.
- Following radical prostatectomy, collect the prostate in a dry pot. Ensure no formalin or other fixative has been added to the prostate.
- Transfer to a suitable sterile location for sampling, e.g., a laminar flow hood in a pathology laboratory.
- Proceed to sampling as soon as possible if fresh tissue is required.
NOTE: For certain applications (e.g., assessment of DNA which should not degrade as quickly as RNA), it may be appropriate to refrigerate the specimen and take samples the next day.
4. Specimen Preparation
- Prepare laminar flow hood and prostate slicing apparatus according to local decontamination procedures, using sterile technique. Here, spray 70% ethanol and wipe across all surfaces. Use sterile single-use needles and scalpels. Use slicer blades up to three times; wash after each use in hot soapy water, then spray and wipe with 70% ethanol.
- Weigh the prostate (g) using a standard scale.
- Ink the prostate. Paint the left side with blue ink and right side with black ink. Cover the full capsule and seminal vesicles with ink to later denote the surgical margins.
NOTE: Inking procedures may vary locally and can be modified accordingly.
5. Prostate Slicing
- Assemble the slicing apparatus by inserting the walls perpendicularly into the base of the stand (Figure 1A).
- Place prostate so that the base and apex are facing opposite walls, with the posterior side down and anterior up. Place gold pins around prostate. Push prostate inwards slightly if necessary to get a snug fit, which will support the prostate during slicing.
- Measure prostate length from base to apex, using a ruler, and compare with prostate length as measured by MRI. If the prostate has shrunk, apply an ad hoc correction to the anticipated distance from base to target transverse slice. For example, if the full length of the prostate in the MRI image is 50 mm, but when measured with a ruler at this point it has shrunk to 45 mm, reduce the anticipated slicing position by 10%.
- Measure from the base to the desired transverse slice. Choose the pin that sits closest to this measurement to slice around.
- Wearing chainmail gloves to prevent injury, hold slicing device (Figure 1B), place blades either side of the identified pin and use the spacer to keep blades 5 mm apart. Take slice by slowly and firmly moving the blades downwards, forwards and backwards with long strokes (Figure 1C). Ensure a full slice has been separated before disassembling apparatus.
- Remove walls and pins and carefully take the slice out onto a sterile sheet of cork board using gloves.
6. Tissue Sampling
- Visually inspect the transverse slice and compare with the axial MRI image. In some cases, the tumor area may appear paler than surrounding tissue.
- Palpate the transverse slice gently. In some cases, the tumor may feel firmer than the surrounding tissue.
- Using the axial MRI image as a guide, select one or more areas for sampling.
- Take biopsy punches of desired area of tissue.
- Using a 6 mm punch, push down on the desired area of tissue.
- Twist the tissue punch on the spot and down against the cork to ensure full separation and use a sharp scalpel to separate if necessary.
- Remove the punch and place into tubes/molds as necessary by ejecting using the plunger.
- Repeat for further tumor and benign samples as required, with separate sterile biopsy punches. Ink the holes where punches were taken in red.
- Note the location of each punch along with the weight of the prostate and any observations on tissue color/firmness.
7. Submission of Prostate for Local Diagnostics
- Pin the prostate to cork with sterile single-use needles prior to fixation in order to prevent tissue shrinkage and warping, which could alter the appearance of the surgical margins.
- Following pinning to cork, submit the prostate to the histopathology department for standard clinical diagnostics.
8. Decontamination of Apparatus
- Discard all disposable equipment in biomedical waste streams and/or sharps containers as designated locally.
- Decontaminate the laminar flow hood and prostate slicing apparatus in accordance with local risk assessments appropriate to human tissue (e.g., by spraying with 70% EtOH and wiping).
Use of Magnetic Resonance Imaging and Biopsy Data to Guide Sampling Procedures for Prostate Cancer Biobanking
Learning Objectives
Fresh prostate tissue sampled using the PEOPLE method can be used for a variety of downstream techniques, including genomic sequencing and ex vivo culture. The first 59 cases sampled using this method have been previously published in comparison with an earlier version of the method, along with initial downstream data8. The time from first slicing the prostate to freezing/fixing the punch biopsies here was approximately 1 min, which was kept to a minimum to avoid degradation of RNA. Time from removal of the prostate to prostate slicing should also be kept to a minimum, though here this took approximately 20 min due to our theatre and pathology labs being in different locations.
Depending on the downstream application, typically at least two samples are taken: one from an area of anticipated tumor tissue and one from an area of anticipated benign tissue. The key measure of success for the sampling method itself is to assess the tumor content in a given sample.
For entry into the 100,000 genomes project, an H&E stained tissue section must be assessed by a uropathologist, and the sample must contain at least 40% tumor cells. Samples that contain less than 40% tumor may still be included in the project if they are successfully macrodissected. Of the first 92 cases sampled in this manner, 64% contained at least 40% tumor and were submitted to the 100,000 Genomes Project without macrodissection. DNA was extracted and was of sufficient yield and quality in all cases (Table 1). An initial subset of 59 of these samples was previously published in comparison with an earlier method8.
For ex vivo culture, matched tumor and benign tissue must be of sufficient quality to withstand 72 h culture without significant degradation. Multiple tissue samples from a total of three patients were cultured successfully8.
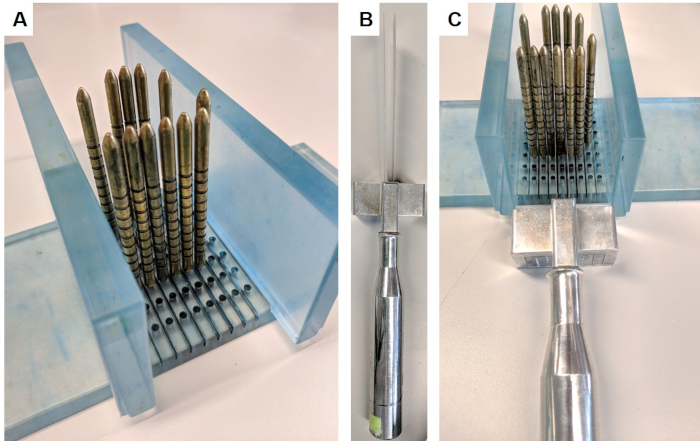
Figure 1: Prostate slicing apparatus. This apparatus was obtained under material transfer agreement from the Institute of Cancer Research. (A) The walls are inserted perpendicular to the base, and gold pins are inserted into the base surrounding the prostate (prostate not pictured). (B) The replaceable parallel blades are inserted into the blade handle. (C) The blades pass between the gold pins in order to slice a 5 mm section of the prostate. Please click here to view a larger version of this figure.
| n (%) | |
| Hit (>40% tumor) | 59 (64%) |
| Partial hit (5-30% tumor) | 6 (7%) |
| Miss (0% tumor) | 27 (29%) |
| Total | 92 (100%) |
Table 1: Tumor hit rate. Tumor hit rate was determined by a consultant pathologist specializing in prostate cancer, following review of H&E stained tissue. Tumor cell content of >40% was determined to be suitable for inclusion in the 100,000 Genomes Project, as per Genomics England guidelines.
List of Materials
| 6 mm biopsy punch | Fisher Scientific | 13404607 | Disposable biopsy punches for removing 6 mm tissue samples |
| Black Ink | Leica Biosystems | 3801753 | Tissue marking & margin dye |
| Blue Ink | Leica Biosystems | 3801751 | Tissue marking & margin dye |
| Chainmail hand glove | Arco | 1456803 | Chainmail gloves to protect hand during slicing |
| Cork board | Fisher Scientific | 12396447 | Cork board for pinning prostate to following sampling procedure |
| Needles | SLS (Scientific Laboratory supplies) | SYR6112 | Sterile needles to use to pin tissue to cork board following sampling |
| Prostate slicing aparatus | Insitute of Cancer Research, London | NA – must be obtained under MTA | A kit containing the slicer handle, blades, spacer, base, walls and pins |
Lab Prep
Previous methods for biobanking prostate tissue, following radical prostatectomy, generally involved random sampling. In order to increase efficiency, and enable a greater range of downstream applications, a more targeted method of sampling prostate tissue was developed. Here we use both magnetic resonance imaging (MRI) and biopsy data to target specific areas of the organ for sampling. The method involves use of a previously published prostate slicing device which removes a 5 mm transverse slice from a predetermined region of the prostate, followed by the removal of 6 mm punch biopsies from predetermined areas of this slice. These samples can be stored frozen or fixed for biobanking purposes, or used fresh immediately with 70% confidence of tumor content, as compared with 10% confidence from the random sampling approach. This enables the use of all standard downstream techniques such as genomics, proteomics or histological work, but also work that requires fresh tissue such as live tissue imaging or ex vivo culture.
Previous methods for biobanking prostate tissue, following radical prostatectomy, generally involved random sampling. In order to increase efficiency, and enable a greater range of downstream applications, a more targeted method of sampling prostate tissue was developed. Here we use both magnetic resonance imaging (MRI) and biopsy data to target specific areas of the organ for sampling. The method involves use of a previously published prostate slicing device which removes a 5 mm transverse slice from a predetermined region of the prostate, followed by the removal of 6 mm punch biopsies from predetermined areas of this slice. These samples can be stored frozen or fixed for biobanking purposes, or used fresh immediately with 70% confidence of tumor content, as compared with 10% confidence from the random sampling approach. This enables the use of all standard downstream techniques such as genomics, proteomics or histological work, but also work that requires fresh tissue such as live tissue imaging or ex vivo culture.
Procedure
Previous methods for biobanking prostate tissue, following radical prostatectomy, generally involved random sampling. In order to increase efficiency, and enable a greater range of downstream applications, a more targeted method of sampling prostate tissue was developed. Here we use both magnetic resonance imaging (MRI) and biopsy data to target specific areas of the organ for sampling. The method involves use of a previously published prostate slicing device which removes a 5 mm transverse slice from a predetermined region of the prostate, followed by the removal of 6 mm punch biopsies from predetermined areas of this slice. These samples can be stored frozen or fixed for biobanking purposes, or used fresh immediately with 70% confidence of tumor content, as compared with 10% confidence from the random sampling approach. This enables the use of all standard downstream techniques such as genomics, proteomics or histological work, but also work that requires fresh tissue such as live tissue imaging or ex vivo culture.
