Subconjunctival Administration of Adeno-associated Virus Vectors in Small Animal Models
Instructor Prep
concepts
Student Protocol
All animal procedures were performed in accordance with the regulations of the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. The use of AAV vectors is a Biosafety Level 1 biohazard risk. Wear proper personal protective equipment, including a lab coat, gloves, and goggles when handling AAV. For the experiment described herein, a recombinant AAV vector packaged with the serotype 8 capsid and encoding a generic ubiquitous cytomegalovirus (CMV) promoter controlling the expression of green fluorescence protein (GFP) was utilized.
1. AAV vector handling and storage
- Store the virus in a -80 °C freezer in 100 µL aliquots in siliconized or low-retention microcentrifuge tubes.
- Thaw all vector stock solutions on ice before use.
NOTE: Dyes such as sodium fluorescein solution (at a final concentration of 0.1-2%) are often mixed with the AAV vectors to visualize the injected solution. Additionally, visualization of injected solutions helps detect air bubbles and monitor AAV distribution and/or leakage after injection.
2. Subconjunctival (SCJ) injection
- Assemble the injection system.
- To assemble the injection system, place a stereomicroscope and a syringe pump in a biosafety cabinet.
NOTE: An infusion pump is needed to perform injections with high precision. Herein, a Standard Infuse/Withdraw Programmable Syringe Pump is utilized (see the Table of Materials), which includes a tight grip and a secure syringe clamp for syringes ranging in volume from 0.5 µL to 60 mL. This pump also offers enhanced flow performance with high accuracy and smooth flow rates from 1.28 pl/min to 88.28 ml/min. - Cut the polyethylene tubing to a length of approximately 50 cm (see the Table of Materials).
- Insert the hub end of a 36 G needle into one of the ends of the tubing.
NOTE: Slide the needle hub end into the tube for ~3 mm to ensure that no leakage occurs. The 36 G needle is used for the subsequent SCJ injection. Needles ranging between 32 G and 36 G are the most commonly used sizes for SCJ injections. The use of a hemostat to assist this step is highly recommended to avoid the potential risk of sharps injury. - Fill a disposable 3 mL syringe with sterile water; insert this disposable syringe into the side of the tube opposite the needle and flush the water throughout the tubing/needle. Repeat this step with 70% alcohol.
- Repeat step 2.1.4 three more times, alternating flushes with sterile water and 70% alcohol, to disinfect the tubing and ensure no leaks, clogs, or damage are observed throughout the tubing.
- Use the disposable 3 mL syringe to fill the tubing with sterile water and leave the tubing attached to the disposable syringe.
- Place a piece of parafilm on the bench surface and add a pool of sterile water to it (~1 mL). Submerge the portion of the tubing connected to the needle into the pool of sterile water. Pull the disposable syringe out from the tube opening at the opposite end to prevent any air from entering the tubing/needle system upon removal of the syringe. Leave the portion of the tubing connected to the needle submerged in the pool of water.
NOTE: Perform procedures 2.1.4 to 2.1.7 in a laminar hood. - Fill a 10 µL Hamilton syringe/needle with sterile water and avoid air in the syringe. Connect the Hamilton syringe/needle to the remaining open end of the tubing by submerging the tubing and needle tip of the Hamilton syringe into the pool of sterile water on the parafilm.
- Press the fast reverse button on the pump screen to move the pusher block to the approximate length of the syringe. Unscrew the bracket clamping knobs to loosen the retaining brackets on the pusher and the syringe holder blocks. Load the Hamilton syringe onto the syringe holder block and secure the syringe following the manufacturer's instructions.
NOTE: To secure the syringe, the syringe barrel clamp should be tight against the syringe barrel; however, do not overtighten, especially when using glass syringes. The syringe plunger should be secured by the pusher block retaining bracket. - Adjust the parameters in the pump settings screen.
- Press the Force button, and set the force level at 30%. Accept the changes to go back to the settings screen.
- Press the quick start button and select Method | Infuse/withdraw.
- For the Syringe, select Hamilton 1700, glass, 10 µL. Select the infusion and withdraw rate and the injection volume.
NOTE: The Force level is set depending on the syringe type/material/capacity/manufacturers; see factory manufacturer instructions for suggested force for each syringe. The injection speed used in this experiment was 200 nL/s. SCJ injections are relatively safe, and there is less of a concern for the induction of elevated intraocular pressure (IOP) resulting from the injection. A slower injection speed is often desirable for certain applications to avoid reflux into the needle and maintain consistency in injections among animals.
- Eject the water from the Hamilton syringe but leave the tubing and injection needle full of water. Slightly pull back on the Hamilton syringe by pressing the Reverse button to introduce a small air bubble in the tubing/needle.
NOTE: The air bubble will serve as a barrier between the water in the tube and the therapeutic drug (in this case, AAV), ensuring the accuracy of the administered dose. - Withdraw the virus by placing the injection needle into an aliquot of the virus stock. Ensure that a visible air bubble remains between the virus and the water in the tubing.
NOTE: AAV vectors can bind to the plastic tubing and metal needle, leading to a loss of virus and/or inaccurate dosing regimens. Thus, to ensure rigor, reproducibility, and an accurate dose of AAV, precoating the surfaces that subsequently come into contact with the AAV is recommended. To coat the tubing/needle system with the virus, draw the viral vector solution into the tubing/needle and incubate it at room temperature for 10 min to allow for saturation of virus binding to the wall of the needle and/or tubing. Discard the virus.
- To assemble the injection system, place a stereomicroscope and a syringe pump in a biosafety cabinet.
- Virus injection
- Anesthetize the mouse with inhaled anesthesia (isoflurane) or intraperitoneal injection of ketamine/xylazine/acepromazine. Confirm the surgical plane of anesthesia by a lack of response to firm toe pinches.
NOTE: Use female and/or male C57BL/6J or BALB/c mice that are at least 6 weeks old. Ketamine/xylazine/acepromazine doses are as follows: ketamine at 70 mg/kg, xylazine at 7 mg/kg, and acepromazine at 1.5 mg/kg. - Apply topical anesthesia to the eye that will receive the injection.
NOTE: Use 0.1% proparacaine hydrochloride and/or tetracaine hydrochloride ophthalmic solution (0.5%) for topical anesthesia. - Apply topical ointment to the other eye that will not receive an injection to prevent dryness and injury.
- Position the mouse on the microscopic stage and expose the mouse eye under the stereomicroscope.
- Place two fingers on the eyelid and slightly pull it away from the mouse's eye to expose the conjunctiva, which is the inner membrane connecting the eyelid to the sclera.
- Grab the conjunctiva with forceps.
- Release the eyelid and hold the needle with the bevel facing upwards using the dominant hand.
- Insert the needle into the conjunctiva. Insert the needle until the bevel is completely covered by the conjunctival membrane. Lay the needle against the globe.
NOTE: As the conjunctiva is a clear membrane, the needle tip/bevel is easily visible. - Start the injection by pressing the Start button using the footswitch.
NOTE: The movement of the air and the Hamilton plunger are synchronized; any delay indicates excess air in the injecting system or possibly a loose connection between the tubing, needle, and/or syringe components. - After the injection is finished, hold the needle in place for 10 s before withdrawing the needle from the conjunctiva to decrease the chances of backflow.
NOTE: It is common for a bleb to appear at the site of the SCJ injection. Such blebs normally resolve completely within a few hours post injection. - Put a drop of the topical lubricant gel on the mouse's eyes to prevent ocular dryness/injury, and then place the mouse on a heating pad to recover.
- Perform ocular examinations such as tear production, IOP, and slit-lamp examination in conjunction with corneal fluorescein staining to assess ocular abnormities following the injection.
NOTE: Tear production is measured by a Phenol Red Thread test, and a tonometer is often used to examine the IOP of the mouse eye. It is reported that some intraocular injections, such as intravitreal injections, might result in a significant increase in IOP; however, IOP changes after SCJ injection are not obvious13,22,23,24.
- Anesthetize the mouse with inhaled anesthesia (isoflurane) or intraperitoneal injection of ketamine/xylazine/acepromazine. Confirm the surgical plane of anesthesia by a lack of response to firm toe pinches.
- AAV biodistribution and transduction efficiency examination following subconjunctival injection
- To investigate the viral genome biodistribution and/or transduction profile of AAV vectors delivered via SCJ, euthanize the mice by AVMA approved method.
NOTE: In this experiment, the mice were sacrificed 8 weeks post injection. - For biodistribution and transgene expression in targeted ocular compartments, dissect the relevant tissue of interest such as the eyelids, cornea, conjunctiva, eye muscle, retina, and optic nerve. Flash-freeze all tissues and store at -80 °C. To examine whole-body AAV biodistribution, collect organs such as the submandibular lymph nodes and liver, and flash-freeze and store them at -80 °C.
- Using a DNA/RNA extraction kit, collect gDNA and RNA from the same sample to examine transgene expression and AAV biodistribution, respectively. If only vector biodistribution is desired, use a DNA extraction kit to extract gDNA.
- Perform standard qPCR and RT-qPCR to determine the AAV vector biodistribution and cDNA abundance using vector transgene-specific primers/probes13,25.
- For histology analysis, fix the eyes, embed them in paraffin, and section them at a thickness of 5 µm. Perform standard immunofluorescence staining to reveal transgene expression26.
- To investigate the viral genome biodistribution and/or transduction profile of AAV vectors delivered via SCJ, euthanize the mice by AVMA approved method.
Subconjunctival Administration of Adeno-associated Virus Vectors in Small Animal Models
Learning Objectives
Solution injected into the subconjunctival space presents as a bleb depending on the injection volume.
In this experiment, 7 µL of AAV (7 × 109 viral genomes (vg)/eye) mixed with fluorescein at a final concentration of 0.1% was injected with a 36 G needle under a stereomicroscope, and the injection speed/pressure was held constant using a programmable syringe pump at 1 µL/s. A bleb can appear upon injection (arrow). A microscopic view of AAV vector administration to the murine SCJ compartment is shown in Figure 1.
Substances injected into the subconjunctival space diffuse around the globe of the eye and are distributed throughout the periocular tissues.
To define the distribution of AAV administered via SCJ injection, 7 µL of diluted India ink was injected into the subconjunctival space of a 10-month-old mouse after anesthetization. No bleeding, leakage, or backflow was detected during or following the SCJ injection. Thirty minutes post injection, the ocular and surrounding tissues were harvested and subsequently stained with Hematoxylin and Eosin (H&E) to visualize the distribution of India ink. The representative sagittal sections depicted in Figure 2 demonstrate that the dispersion of India ink occurred mainly adjacent to the extraocular muscles, in the outer surface of the sclera, and the periocular loose connective tissues (Figure 2).
Self-complementary AAV8 successfully transduces the cornea and periocular muscles following SCJ.
To determine the transduction profile of self-complementary AAV8 at eight weeks post injection, GFP abundance in whole-globe cross-sections was examined via immunofluorescence staining using an anti-GFP antibody at a dilution of 1:500. Images were taken under a fluorescence microscope (Figure 3). These results revealed that AAV vectors administered via SCJ injection efficiently transduce the periocular muscles posterior to the eye and the cornea.
Abundant vector genome and transgene expression in distinct eye compartments following SCJ injection
To quantitatively analyze the vector biodistribution and transgene expression, vector genome copy numbers in distinct eye compartments and organs such as the liver and heart were examined by qPCR (Figure 4A), while the transgene expression was tested by qRT-PCR (Figure 4B). These results suggest that SCJ injection of AAV8 results in transgene expression in the eyelid, conjunctiva, cornea, and optic nerve.
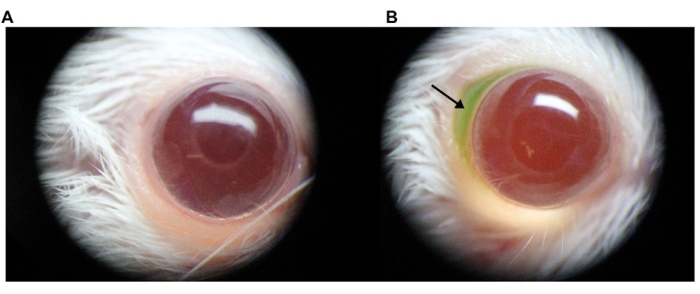
Figure 1: Microscopic view of AAV vector administration into the murine SCJ space. To allow for visualization of the formation of a bleb during the procedure, 1% fluorescein was directly added to the AAV vector preparation. Images were taken using a digital camera attached to a stereomicroscope. (A) Representative image of a noninjected eye; (B) representative image of an injected eye. The arrow indicates the injected AAV solution containing fluorescein in the SCJ space. Abbreviations: AAV = Adeno-associated virus; SCJ = subconjunctival. Please click here to view a larger version of this figure.
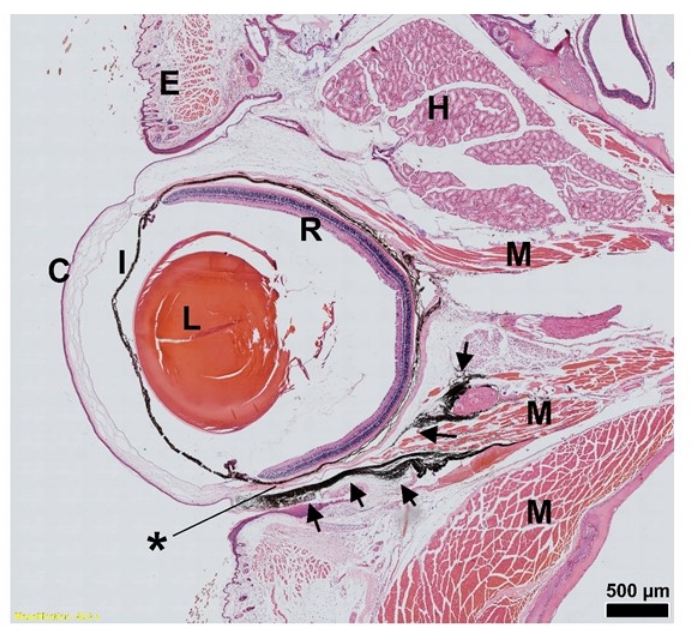
Figure 2: H&E staining of India ink distribution (arrow) after SCJ injection in the mouse eye. Sagittal sections of an eye injected with India ink are presented; 7 µL of India ink was injected at the indicated site. *, Injection site. Scale bar = 500 µm. Abbreviations: SCJ = subconjunctival; H&E = hematoxylin and eosin; C = cornea; I = iris; L = lens; R = Retina; E = eyelid; H = Harderian gland; M = muscle. Please click here to view a larger version of this figure.
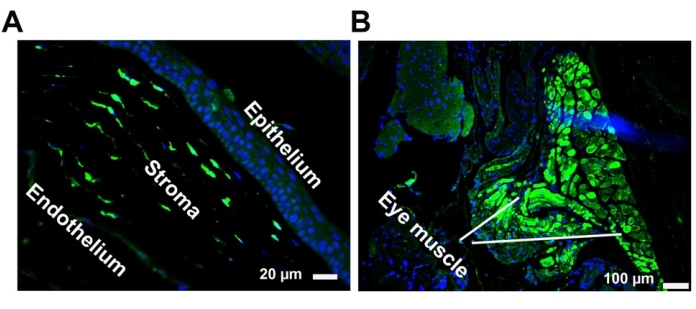
Figure 3: Representative GFP histology images of self-complementary AAV8 after SCJ injection. Transduction of the cornea (A) and eye muscles (B) following SCJ injection. GFP expression (green) was visualized via immunostaining in paraffin-embedded tissue sections with an anti-GFP antibody. Nuclei were stained with DAPI (blue). Scale bar = 100 µm (eye muscle), 20 µm (cornea). Abbreviations: GFP = green fluorescent protein; AAV = adeno-associated virus; SCJ = subconjunctival; DAPI = 4',6-diamidino-2-phenylindole. Please click here to view a larger version of this figure.
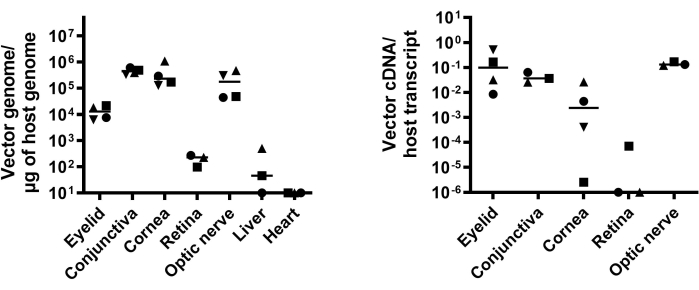
Figure 4: Quantitative analysis of vector biodistribution and transgene expression. (A) Vector biodistribution in eye compartments (eyelid, conjunctiva, cornea, optic nerve, and retina) and other organs (liver and heart) following SCJ is presented as vector genome copy number/µg of host genome DNA. (B) GFP abundance determined by qRT-PCR is presented as vector cDNA copy number/host transcript. This figure is modified from 13. Abbreviations: SCJ = subconjunctival; qRT-PCR = quantitative revere-transcription PCR; GFP =green fluorescent protein. Please click here to view a larger version of this figure.
List of Materials
| 36 G NanoFil Needles | World Precision Instruments | NF36BV-2 | |
| AAV vector | University of North Carolina at Chapel Hill | / | |
| Acepromazine | Henry Schein | NDC 11695-0079-8 | |
| anti-GFP antibody | AVES labs Inc. | ||
| Digital camera | Cannon | Cannon EOS T5i | |
| DNA/RNA extraction kit | Qiagen | 80204 | |
| Forceps | Fine Science Tools | F6521 | |
| Hamilton syringe | Hamilton | 7654-01 | |
| India ink | StatLab | NC9903975 | |
| Ketamine hydrochloride injection solution | Henry Schein | NDC 0409-2051-05 | |
| Moisture-resistant film | Parafilm | 807-6 | |
| Polyethylene tubing | Becton Dickinson and Company | 427401 | |
| Proparacaine 0.1% | Bausch Health US | NDC 24208-730-06 | |
| Rebound tonometer | Tonovet | / | |
| Sodium fluorescein solution | Sigma-Aldich | 46960 | |
| Standard Infuse/Withdraw Pump 11 Pico Plus Elite Programmable Syringe Pump | Harvard Bioscience | 70-4504 | |
| Stereo microscopye | Leica | Mz6 | |
| Tetracaine Hydrochloride Ophthalmic Solution 0.5% | Bausch and Lomb | Rx only | |
| Topical ointment | GenTeal | NDC 0078-0429-47 | |
| Xylazine | Akorn | NDC 59399-110-20 | |
| Zone-Quick Phenol Red Thread Box 100 Threads | ZONE-QUICK | PO6448 |
Lab Prep
Ocular diseases include a wide range of inherited genetic and acquired disorders that are appealing targets for local drug delivery due to their relative ease of accessibility via multiple administration routes. Subconjunctival (SCJ) injections offer advantages over other intraocular administration routes as they are simple, safe, and usually performed in an outpatient setting. SCJ injections in small animals usually require the assistance of an operating microscope due to the size of the eye. Previous work has demonstrated that SCJ injection of specific adeno-associated virus (AAV) serotypes is a valid gene delivery strategy for targeted transduction of the ocular surface, eye muscle, cornea, and optic nerve, providing a potential approach for the treatment of many ocular diseases.
Herein, a detailed protocol is presented for SCJ injections in a mouse model using an injection system consisting of a programmable infusion/withdrawal syringe pump (which allows for consistent and precise injection speed and pressure) and a gastight removable syringe coupled with microinjection needles. The injection system is also adaptable for other intraocular administration routes such as intrastromal, intracameral, intravitreal, and subretinal injections in small animals. Although the delivery of adeno-associated viral vectors for ocular gene therapy studies is described, the protocol herein can also be adapted for a variety of ophthalmic solutions in small animal models. The key practical steps in the administration route, setup for the injection platform, preparation of the injection, and tips from direct experience will be discussed in detail. In addition, common validation techniques for AAV delivery confirmation to the desired tissues will also be briefly discussed.
Ocular diseases include a wide range of inherited genetic and acquired disorders that are appealing targets for local drug delivery due to their relative ease of accessibility via multiple administration routes. Subconjunctival (SCJ) injections offer advantages over other intraocular administration routes as they are simple, safe, and usually performed in an outpatient setting. SCJ injections in small animals usually require the assistance of an operating microscope due to the size of the eye. Previous work has demonstrated that SCJ injection of specific adeno-associated virus (AAV) serotypes is a valid gene delivery strategy for targeted transduction of the ocular surface, eye muscle, cornea, and optic nerve, providing a potential approach for the treatment of many ocular diseases.
Herein, a detailed protocol is presented for SCJ injections in a mouse model using an injection system consisting of a programmable infusion/withdrawal syringe pump (which allows for consistent and precise injection speed and pressure) and a gastight removable syringe coupled with microinjection needles. The injection system is also adaptable for other intraocular administration routes such as intrastromal, intracameral, intravitreal, and subretinal injections in small animals. Although the delivery of adeno-associated viral vectors for ocular gene therapy studies is described, the protocol herein can also be adapted for a variety of ophthalmic solutions in small animal models. The key practical steps in the administration route, setup for the injection platform, preparation of the injection, and tips from direct experience will be discussed in detail. In addition, common validation techniques for AAV delivery confirmation to the desired tissues will also be briefly discussed.
Procedure
Ocular diseases include a wide range of inherited genetic and acquired disorders that are appealing targets for local drug delivery due to their relative ease of accessibility via multiple administration routes. Subconjunctival (SCJ) injections offer advantages over other intraocular administration routes as they are simple, safe, and usually performed in an outpatient setting. SCJ injections in small animals usually require the assistance of an operating microscope due to the size of the eye. Previous work has demonstrated that SCJ injection of specific adeno-associated virus (AAV) serotypes is a valid gene delivery strategy for targeted transduction of the ocular surface, eye muscle, cornea, and optic nerve, providing a potential approach for the treatment of many ocular diseases.
Herein, a detailed protocol is presented for SCJ injections in a mouse model using an injection system consisting of a programmable infusion/withdrawal syringe pump (which allows for consistent and precise injection speed and pressure) and a gastight removable syringe coupled with microinjection needles. The injection system is also adaptable for other intraocular administration routes such as intrastromal, intracameral, intravitreal, and subretinal injections in small animals. Although the delivery of adeno-associated viral vectors for ocular gene therapy studies is described, the protocol herein can also be adapted for a variety of ophthalmic solutions in small animal models. The key practical steps in the administration route, setup for the injection platform, preparation of the injection, and tips from direct experience will be discussed in detail. In addition, common validation techniques for AAV delivery confirmation to the desired tissues will also be briefly discussed.
