Dual-Task Stroop Paradigm for Detecting Cognitive Deficits in High-Functioning Stroke Patients
Instructor Prep
concepts
Student Protocol
This project was approved by the Medical Ethics Association of the Fifth Affiliated Hospital of Guangzhou Medical University (No. KY01-2020-08-06) and has been registered at the China Clinical Trial Registration Center (No. ChiCTR2000036514). Informed consent was obtained from patients for using their data in this study.
1. Recruitment
- Recruit stroke patients with stable conditions as confirmed by imaging examination-diagnosis conforms to the diagnostic criteria of cerebrovascular disease of the Chinese Medical Association Neurology Branch (2005). Choose patients with stroke at Brunnstrom stage IV21.
- Ensure the patients can complete basic daily activities independently. Ensure the patients are without obvious cognitive impairment and meet the following requirements: MoCA in the normal range; no unilateral neglect (Albert's Test, number of omissions ≤2)22; no other neurological diseases, such as language defects; and can cooperate with relevant instructions to complete this study.
- Ensure the subjects participate in the test voluntarily and sign an informed consent form.
2. Clinical evaluation
- Record the subject's information, including name, gender, date of birth, education level, body mass index, medical history, and medication history.
- Perform cognitive function assessment.
- Perform MoCA23 on stroke patients by asking 11 questions addressing subjects' attention and concentration, executive function, memory, language, visual structure skills, abstract thinking, computing, and orientation.
- The total score of the MoCA is 30, which is related to education level. If the subject took less than 12 years of education, add one point to the total score of MoCA. Consider a score of 26 and higher as normal23.
- Perform a CDR24 on the stroke patients. Collect information during structured interviews with stroke patients and their families and assess the subjects' abilities in six aspects: memory, orientation, judgment and problem solving, work and social interaction, family life and personal hobby, and independent living.
- The highest possible score is 3. Assess the scores obtained as follows: total score = 0 indicates no dementia; total score = 0.5 indicates suspected dementia; total score = 1 indicates mild cognitive impairment; total score = 2 indicates moderate cognitive impairment; and total score = 3 indicates severe cognitive impairment24.
- Perform the Albert's test to detect the presence of unilateral spatial neglect (USN) in patients with stroke. Ask the subject to cross out all the lines that are placed in random orientations on a piece of paper.
- Present the subject with a series of 40 black lines, each about 2 cm long, randomly oriented on a sheet of white 11 in x 8.6 in size paper in six rows. Assess the presence or absence of USN, based on the number of lines left uncrossed on each side of the test sheet. If any lines are left uncrossed and more than 70% of these uncrossed lines are on the same side as the motor deficit, this indicates unilateral spatial neglect.
- Perform motor function assessment.
- Perform the Fugl-Meyer Assessment (FMA) on the stroke patients to assess motor function, sensation, balance, joint range of motion, and joint pain in patients with post-stroke hemiplegia. The motor domain includes items assessing movement, coordination, and the reflex actions of the shoulder, elbow, forearm, wrist, hand, hip, knee, and ankle.
- The motor function score ranges from 0 (hemiplegia) to 100 points (normal motor performance), divided into 66 points for the upper extremities and 34 points for the lower extremities. Assess the score as follows: 0-49 points indicate severe motor impairment; 50-84 points indicate marked motor impairment; 85-95 points indicate moderate motor impairment; and 96-99 points indicate slight motor impairment.
- Perform balance function assessment.
- Perform the Berg balance scale (BBS)27 on the stroke patient, with a total of 14 items from easy to difficult, including sitting balance, standing balance, body transfer, turning, and single-leg standing.
- Asses the scores as follows: the highest score on the scale is 56; a total score of <40 points suggests a risk of falling; 0-20 points scored indicates poor balance function and that a wheelchair is required; 21-40 points scored suggests that the subject has a certain balance function and needs to walk with assistance; 41-56 points scored suggests good balance function and that the subject can walk independently28.
- Perform risk of falling assessment.
- Perform the timed up and go test (TUGT)29 on stroke patients. Ask the subject to stand up from the chair, walk for 3 m, turn their body, then return and sit in the chair at a comfortable speed to ensure safety. Concurrently, ask the evaluator to time the whole process from issuing the departure order to sitting in the chair.
- Assess the result obtained as follows: if the total time for the subject to complete TUGT ≥14 s, it indicates that the subject has the risk of falling29.
3. Stroop task evaluations
- Perform the Stroop single task evaluation (Stroop task only; Figure 1).
- Ask the patient to sit in a stable chair.
- Run the commercial stimulus presentation software and select the congruence test trials. Make a new profile for the patient. Select the congruence test trials of the Stroop task and repeat three trials.
- Carry out the following experimental paradigm. Design the experiment with a patient rest time of 10 s and then ask the patient to perform one cognitive test with a frequency of 6 s for a total of three trials, with each trial having a 60 s stimulus + 60 s rest.
- Set the total duration of the experiment to 370 s (the specific process is shown in Figure 1). In the resting stage, ask the patient to relax. When the experiment is in the stimulation stage, ask the patient to perform the attention-related test, complete the task in 6 s, and complete it 10x in 60 s.
- Ask the patients to follow the instructions for the two test trials as described below.
- Select the congruence test trials. Click the arrow button to the left (←) as soon as possible when
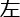 is shown on the left of the square. Click the arrow button to the right (→) as soon as possible when
is shown on the left of the square. Click the arrow button to the right (→) as soon as possible when 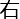 is shown on the right of the square.
is shown on the right of the square. - Select the incongruence test trials, which share the same step as the congruence test trials. Click the arrow button to the left (←) as soon as possible when
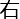 is shown on the left of the square, ignoring the meaning of the character and focusing on its position.
is shown on the left of the square, ignoring the meaning of the character and focusing on its position. - Click the arrow button to the right (→) as soon as possible, when
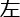 is shown on the right of the square, ignoring the meaning of the character and focusing on its position. Finish the task, save the data, and export the data to a self-built database.
is shown on the right of the square, ignoring the meaning of the character and focusing on its position. Finish the task, save the data, and export the data to a self-built database.
- Select the congruence test trials. Click the arrow button to the left (←) as soon as possible when
- Perform the Stroop dual-task evaluation (Stroop task + balance control).
- Ask the patient to sit on a balance ball with the therapist being responsible for the patient's protection. Let the patient complete the Stroop experimental paradigm with the steps mentioned above (steps 3.1.1.-3.1.5.).
- When the experiment is in a resting stage, ask the patient to keep balance and relax on the balance ball as much as possible. When the experiment is in the state of stimulation, ask the patient to carry out the attention-related test while maintaining balance on the balance ball as much as possible.
- Ask the patient to sit on a balance ball with the therapist being responsible for the patient's protection. Let the patient complete the Stroop experimental paradigm with the steps mentioned above (steps 3.1.1.-3.1.5.).
4. fNIRS evaluation
- Place 10 light sources and 12 receivers on the fNIRS test cap to correspond to this study's four regions of interest (ROIs), which include the left prefrontal cortex (LPFC), right prefrontal cortex (RPFC), left promoter cortex (LPMC), and right promoter cortex (RPMC).
- Preparation of the subject
- Inform the subjects of the experimental purpose and observe the patients.
- Ensure the Cz site at the top of the test cap, the fourth point from the forehead to the occipital lobe on the midline of the full cap. Ensure the midpoint of the connection lies between the nasal root to the lower edge of the occipital protuberance, the intersection point of the connection from the nasal root to the occipital protuberance, or the connection between the superior auricular fossa of both ears (cymba conchae).
- Place the cap on the subject's head and adjust the position of the cap so that the Cz point on the subject's head coincides with the Cz point on the cap. Tighten the tie on both sides of the cap and allow the subject's ears to pierce through the gap; the front of the cap is naturally attached to the forehead, and the back is naturally attached to the occiput.
- Acquisition and pre-acquisition
- Open the software, select the experimental subject, and input the patients' basic information. Set the sampling frequency to 11 Hz.
- Click the Pre-acquisition button to start pre-acquisition and calibrate the test signal. According to the signal intensity of each point displayed by the functional near-infrared spectroscopy, adjust the weak signal points by moving the cap or further exposing the scalp. When the signal intensity of each point collected by the cap tends to be stable, stop pre-collection, and click the automatic gain button. Click the Start button to collect the signal.
NOTE: Ensure the signal quality in acquisition and pre-acquisition as follows. The original light intensity signal curve should be stable, accompanied by a 1-2 Hz heartbeat signal fluctuation, and the value should meet the reasonable threshold set by the equipment. The intensity of the signal can be indicated by color, where a gray display signal intensity is low, yellow is good, green is excellent, and red is too strong.
- Perform Stroop single task evaluation synchronized with fNIRS. Then, perform Stroop dual-task evaluation synchronized with fNIRS.
5. Data processing and analysis
- Process general information and clinical evaluation data of the patients.
- Analyze the near-infrared data using the NirSpark software package in MATLAB.
- Perform data pre-procession.
- Click the Exclude button to eliminate the time interval unrelated to the experiment. Click the Motion button to eliminate motion artifacts caused by physiological activities like breathing, heartbeat, pulse, etc., and involuntary activities like blinking, swallowing, etc., and convert the light intensity signal to an optical density signal.
- Click the Filter button to select the bandpass filter (0.01-0.2 Hz) to remove physiological and instrumental noise. Click the Hemo button to calculate the relative changes of oxyhemoglobin (HbO2) and deoxyhemoglobin (HbR) according to the modified Beer-Lambert Law and convert the optical density signal into the blood oxygen concentration signal.
NOTE: HbO2 is more sensitive to changes between conditions than HbR, so subsequent analysis uses only HbO2 data in this study protocol.
- General linear model (GLM) building
- Choose HbO2 in Hemo Type as the analysis data. Click the Specification button to take seconds as the time unit and select the standard HRF type as the basis function. Then, eliminate the rest stage to establish the GLM design matrix and select the stimulus stage in the task according to the experimental design.
- Click the Estimation button to fit the established design matrix with the collected data. Click the View button to check out the calculated β value.
NOTE: GLM is a linear combination of observed hemodynamic signals (dependent variable) as interesting regressions (task variable), redundant covariates (such as surface noise measured in short-range channels), and error terms.
- Calculate the β value as follows. Calculate experimental data in the ROIs by using the established linear correlation model. Obtain the GLM parameters of the required channel and derive the β value of brain activation under each experimental condition (that is, the weight coefficient in the linear model) for analysis.
- Perform data pre-procession.
- Run the commercial stimulus presentation software to export the performance data of cognitive tasks in the Stroop task and obtain the accuracy (ACC) and reaction time (RT) for final data analysis.
Dual-Task Stroop Paradigm for Detecting Cognitive Deficits in High-Functioning Stroke Patients
Learning Objectives
This study presents results from a high-functioning stroke patient, who was a 71-year-old male who suffered from ischemic stroke with left hemiplegia 2 years ago. The magnetic resonance imaging (MRI) presented bilateral chronic infarction from the basal ganglia to the radiating crown. He was able to walk and live independently in the community but was not satisfied with his cognitive recovery. However, the functional assessments were all within the normal range: FMA = 100, BBS = 56/56, TUGT = 6, MoCA = 26/30, CDR = 0.5, Albert's Test = 0. Moreover, we also recruited one young female healthy subject as the control. The subjects' information is shown in Table 1.
The single/dual-task assessment results based on the Stroop paradigm showed that, in high-functioning stroke patient performing the single-task Stroop test, the RT of the congruence test trials was shorter than that of the incongruence test trials, and the ACC was comparable to the incongruence test trials (RTCongruence = 547.62 ms, RTIncongruence = 565.07 ms; ACCCongruence = ACCIncongruence = 100%). When performing dual-task congruence test trials, the RT of high-functioning stroke patients was higher than that of healthy young subjects, and their ACC was also relatively lower (RTstroke = 587.03 ms, RThealth = 363.07 ms; ACCstroke = 93.33%, ACChealth = 100%), and the difference in the incongruence test trials was greater than that in the congruence test trials (RTstroke = 613.03 ms, RThealth= 384.67 ms; ACCstroke = 90%, ACChealth = 100%; Table 2).
The results for brain function showed that the β value of ROIs in the stroke patient was lower than that in the healthy young subject during the process of performing dual tasks (RDLPFC: βstroke = −0.006, βhealth = 0.1366; LDPFC: βstroke = −0.0196, βhealth = 0.0976). The rest of the brain regions are shown in Figure 2 and Figure 3.
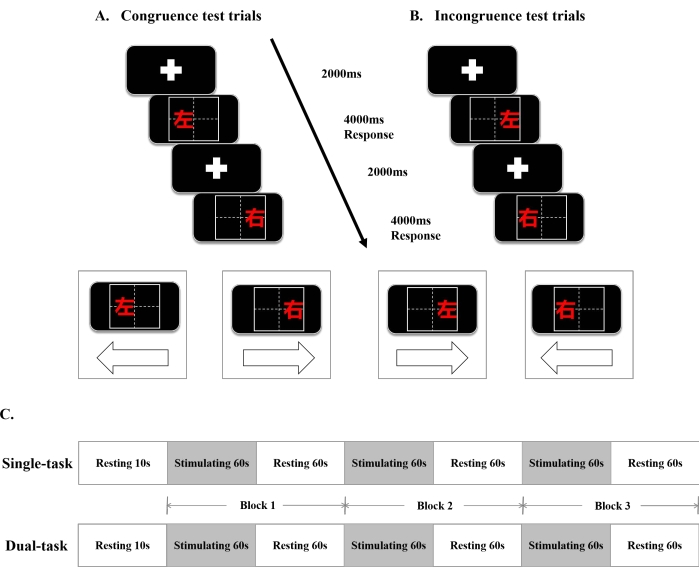
Figure 1: The single/dual-task Stroop paradigm and the fNIRS design. (A) Congruence test trials. (B) Incongruence test trials. (C) The timeline diagram of the single/dual-task Stroop paradigm. Abbreviations:ms = millisecond; s = second; 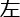 = left;
= left; 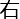 = right. Please click here to view a larger version of this figure.
= right. Please click here to view a larger version of this figure.
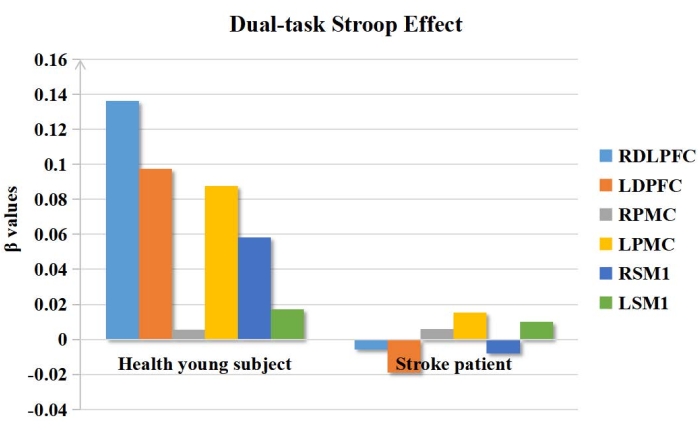
Figure 2: The β values in ROIs of the dual-task Stroop effect. The β values of ROIs in the stroke patient was lower than that of the healthy young subject during the dual-task Stroop. Abbreviations: ROIs = regions of interest; RDLPFC = right dorsolateral prefrontal cortex; LDPFC = left dorsolateral prefrontal cortex; RPMC = right promoter cortex; LPMC = left promotor cortex; RSM1 = right primary sensory-motor cortex 1; RPMC = right primary sensory-motor cortex. Please click here to view a larger version of this figure.
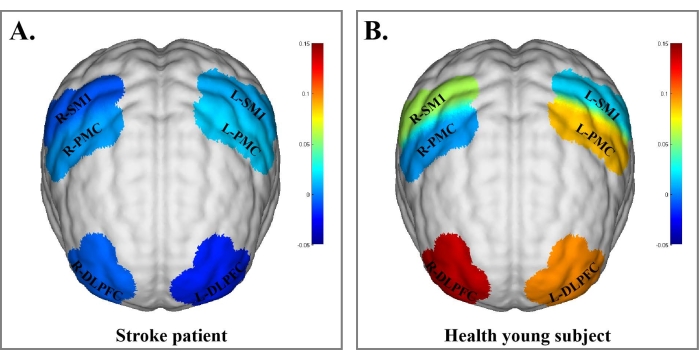
Figure 3: Blood oxygen concentration in brain regions of the stroke patient and healthy young subject under the dual-task Stroop effect. (A) Blood oxygen concentration in brain regions of the stroke patient under the dual-task Stroop effect. (B) Blood oxygen concentration in brain regions of the healthy young subject under the dual-task Stroop effect. The β values are indicated by color bars. The results of brain function showed that the β value of ROIs in the stroke patient was lower than that in the healthy young subject during dual-task performance. Abbreviations: R-DLPFC = right dorsolateral prefrontal cortex; L-DLPFC = left dorsolateral prefrontal cortex; R-PMC = right promoter cortex; L-PMC = left promotor cortex; R-SMI = right primary sensory-motor cortex; R-PMC = right primary sensory-motor cortex. Please click here to view a larger version of this figure.
| Characteristics | Healthy young subject | Stroke patient |
| Age (year) | 21 | 71 |
| Gender | female | male |
| BMI (kg/m2) | 22.27 | 23.81 |
| Cognitive assessment | ||
| Montreal Cognitive Assessment (MoCA) | 30/30 | 26/30 |
| Clinical Dementia Rating (CDR) | 0 | 0.5 |
| Albert’s Test | 0 | 0 |
| Motor and balance assessment | ||
| Brunnstrom stage | NT | V stage |
| Fugl-Meyer Assessment (FMA) | 100 | 100 |
| Berg Balance Scale (BBS) | 56/56 | 52/56 |
| Timed Up and Go Test (TUGT) (s) | 6 | 11 |
| Abbreviations: BMI, Body Mass Index; kg/m2, kilogram per square meter; NT, Not testable; s, second. | ||
Table 1: The baseline information and characteristics of the healthy young subject and the stroke patient.
| Congruence test trials | Incongruence test trials | |||
| ACC | RT(ms) | ACC | RT(ms) | |
| the stroke patient | 93.33% | 587.03 | 90% | 613.03 |
| the healthy young subject | 100% | 363.07 | 100% | 384.67 |
| Abbreviations: ACC, accuracy; RT, reaction time; ms, millisecond. | ||||
Table 2: The ACC and RT of the healthy young subject and the stroke patient in the dual task. Abbreviations: ACC = accuracy; RT = reaction time; ms = millisecond.
List of Materials
| Balance Ball | Shanghai Fanglian Industrial Co, China | PVC-KXZ-EVA01-2015 | NA |
| E-Prime 3.0 | Psychology softwares Tools | commercial stimulus presentation software | |
| fNIRS | Hui Chuang, China | NirSmart-500 | NA |
Lab Prep
General clinical cognitive assessment scales are not sensitive enough to cognitive impairment in high-functioning stroke patients. The dual-task assessment has advantages for identifying cognitive deficits in high-functioning stroke patients and has been gradually applied in clinical assessment and cognitive training. Moreover, the Stroop paradigm has higher sensitivity and specificity for attentional assessment than conventional clinical cognitive assessment scales. Therefore, this study presents the dual-task assessment based on the Stroop paradigm to identify cognitive deficits in high-functioning stroke patients. This study demonstrates a single- and dual-task evaluation based on the Stroop paradigm and confirms its feasibility through case experiments and synchronized functional near-infrared spectroscopy evaluation. The Stroop reaction time and correct rate are used as the main indicators to evaluate the cognitive level of the subjects. This study protocol aims to provide new ideas to figure out the ceiling effect in general clinical assessment failure for high-functioning stroke patients.
General clinical cognitive assessment scales are not sensitive enough to cognitive impairment in high-functioning stroke patients. The dual-task assessment has advantages for identifying cognitive deficits in high-functioning stroke patients and has been gradually applied in clinical assessment and cognitive training. Moreover, the Stroop paradigm has higher sensitivity and specificity for attentional assessment than conventional clinical cognitive assessment scales. Therefore, this study presents the dual-task assessment based on the Stroop paradigm to identify cognitive deficits in high-functioning stroke patients. This study demonstrates a single- and dual-task evaluation based on the Stroop paradigm and confirms its feasibility through case experiments and synchronized functional near-infrared spectroscopy evaluation. The Stroop reaction time and correct rate are used as the main indicators to evaluate the cognitive level of the subjects. This study protocol aims to provide new ideas to figure out the ceiling effect in general clinical assessment failure for high-functioning stroke patients.
Procedure
General clinical cognitive assessment scales are not sensitive enough to cognitive impairment in high-functioning stroke patients. The dual-task assessment has advantages for identifying cognitive deficits in high-functioning stroke patients and has been gradually applied in clinical assessment and cognitive training. Moreover, the Stroop paradigm has higher sensitivity and specificity for attentional assessment than conventional clinical cognitive assessment scales. Therefore, this study presents the dual-task assessment based on the Stroop paradigm to identify cognitive deficits in high-functioning stroke patients. This study demonstrates a single- and dual-task evaluation based on the Stroop paradigm and confirms its feasibility through case experiments and synchronized functional near-infrared spectroscopy evaluation. The Stroop reaction time and correct rate are used as the main indicators to evaluate the cognitive level of the subjects. This study protocol aims to provide new ideas to figure out the ceiling effect in general clinical assessment failure for high-functioning stroke patients.
