Clinical Indications for Rapid Sequence MRI in Pediatric Neurosurgical Patients and the Limitations and Barriers to Implementation
Instructor Prep
concepts
Student Protocol
This protocol follows the guidelines of the institutional human research ethics committee of the University of North Carolina, as it was created secondary to a literature review and did not require real human subjects. Required permissions from volunteers and for filming have been obtained. The representative RS-MRI images used in this study have been deidentified.
NOTE: A review of the literature was conducted using keywords like "rapid MRI" and "fast brain". A total of 15 articles were reviewed, and the imaging protocols were retrieved and combined to create the protocol below.
1. Patient positioning
- Before patient positioning, ensure that a thorough review of contraindications to the use of MRI has been completed. Tell the patient that contraindications for RS-MRI currently include various types of metallic implants such as vascular clips, foreign bodies, prosthetic heart valves, and other types of metal devices. Scan the patient with a metal detector to ensure no loose metal objects.
- Patients who have anxiety or claustrophobia may need special attention to patient positioning to reduce exacerbations of these conditions. Give patients an alarm bell with an explanation for its use.
- Consult members of the Child Life Specialist team. Request that they review videos with patients to prepare them for what to expect.
- Some RS-MRI coils have mirrors. Fix them so the patient may see out of the MRI scanner. Ensure accurate patient positioning is practiced in pediatric patients and proper coils are chosen to optimize the RS-MRI images.
- Cranial imaging
- For a brain MRI, position the patient supine and centered on the brain coil with the chin tilted upwards3. Use landmarking, touch sensors, or laser marking with the patient's eyes closed.
- Provide earplugs for patient comfort and safety and immobilization pads to decrease motion and noise.
- Spine imaging
- Cervical spine
- Place the patient in the supine position, with the larynx aligned to the center of the brain coil3. Use the same patient safety measures applied above.
- Thoracic spine
- Place the patient in the supine position. Utilize a spine coil and the center of the spine coil to align with the sternum3.
- Lumbar spine
- Position the patient in the supine position. Use the spine coil and align it centrally about 5 cm superior to the iliac bones3. Use an upright MRI if there is difficulty obtaining the image4.
- Cervical spine
- Comforting techniques
- Use comforting techniques to reduce motion artifacts during RS-MRI. Attempt comforting techniques, including feeding, swaddling, and standard restraints5.
- Request guardian involvement to assist with soothing techniques. If a guardian is unavailable, involve experienced staff members like Child Life Specialists to attempt soothing techniques.
- Always attempt conservative soothing methods before the escalation of care. If standard restraints are required, do a thorough skin check after removal to assess for bruising.
- Sedation
- If the patient continues to remain inconsolable despite soothing techniques, consult anesthesiology for sedation recommendations and dosing. Obtain guardian consent with an escalation in care.
2. Spine evaluation
- The following RS-MRI protocol recommendations capture sequences for the routine detection and evaluation of spinal pathologies. Perform these sequences using a 1.5 Tesla (T) or 3T scanner6.
- Review representative parameters such as matrix size, field of view (FoV), repetition time (TR), and echo time (TE). Follow institution parameters or the parameters listed below.
- Adjust the full spine series field of view to single or separate (cervical-upper thoracic, lower thoracic-lumbar/sacral). Calculate adjustments based on the patient's body habitus.
- Syrinx evaluation
- Using the NUMARIS/4 software, select the Patient tab in the top left corner. From the drop-down menu, select Patient Browser.
- A separate screen will display a list of options. From this list, select Scheduler. Click once on the patient's name, followed by the register button in the lower half of the screen.
- A separate screen will display the patient's Name, Date of Birth, Height, and Weight. Review these parameters to ensure they are correct.
- Under patient positioning, select Head First- Supine. On the same screen, under Study, select SYRINX/TETHERED CORD NON-SEDATION EVALUATION protocol.
- At the start of the imaging study, ensure the localizer sequence is running. This sequence determines the orientation of the study. Run this sequence 2-3 times in spine cases.
- Next, run the selected T2 weighted half-Fourier acquisition single-shot turbo spin echo (HASTE) axial and sagittal sequences.
- Follow the imaging protocol listed here: slice thickness 3.0 mm, FoV 240 mm, TE 82 ms, TR 1500 ms.
- After the study, repeat step 2.3.1. From the separate screen display, select Local Database.
- Select the patient's name and the study completed. Click Transfer at the top left corner, followed by Transfer to PACS.
- Notify the support team that the study is concluded and transfer the patient out of the MRI scanner room. Once the patient is safely removed, reunite the patient with a guardian.
- Other spinal pathologies
- If a clinical indication or suspicion of a cord pathology, Add a T2 Short-Ti Inversion Recovery (STIR) sequence. Include this sequence in the protocol above by repeating step 2.3.1.
- Select ______- SPINE WO sequence. Select the sequence relevant to the portion of the spine that is being imaged (i.e., C-SPINE WO).
- From the list of additional sequences that populate in the right column, select the STIR sequence. Follow these protocol parameters: slick thickness 3.0 mm, FoV 280 mm, TE 58.0 ms, TR 4000 ms.
- Of note, STIR nullifies fat tissue, which helps with tissue distinction. STIR has better sensitivity for cord pathologies than HASTE, which is more useful for CSF and cord differentiation.
- Repeat steps 2.3.7-2.3.8 to transfer the additional images for interpretation by the radiologist.
3. Traumatic brain injury evaluation
- Perform the recommended protocol with a 1.5 T or 3 T scanner. Select scanners from the list available in Table 1.
- Ensure that traumatic brain injury (TBI) sequences include but are not limited to axial fluid-attenuated inversion recovery (FLAIR), axial gradient echo sequences (GRE), axial diffusion-weighted imaging (DWI)- single-shot turbo spin echo, and axial and coronal HASTE.
- Be aware that insignificant variations may exist in TE, TR, matrix size, and field of view. Follow institutional imaging protocols or the parameters listed below.
- Of note: T2 GRE and T2 HASTE sequences most likely identify traumatic pathology.
- Hemorrhage
- Follow steps 2.3.1-2.3.3 to select the patient for the study. After selecting patient positioning as Head First Supine, under Study, select NEURO BRAIN.
- An additional list of protocols will populate, and from that list, select PEDS TRAUMA. Review this list to ensure it contains the sequences listed above in step 3.2.
- For suspected hemorrhage, ensure that a radiologist interprets the GRE images. Use these parameters for best GRE imaging quality: slice thickness 4.0 mm, FoV 230 mm, TE 2.46 ms, TR 240 ms.
NOTE: This sequence is notable for increased detection of extra-axial hemorrhage when compared to CT imaging. - Repeat steps 2.3.7-2.3.8 to transfer the additional images for interpretation by the radiologist.
- Diffuse axonal injury
- In addition to the GRE sequence, add an additional axial susceptibility weighted image (SWI) to the evaluation for diffuse axonal injury.
NOTE: SWI images are more sensitive than GRE in terms of volume and number of detected hemorrhagic lesions. - Repeat steps 3.4.1-3.4.2. Use these parameters for best SWI imaging quality: slice thickness 3.0 mm, FoV 220 mm, TE 20 ms, TR 27 ms.
- SWI imaging may result in longer acquisition times when compared with GRE and, therefore, is more likely to be degraded by motion artifacts. Review the soothing techniques above to assist in reducing motion artifacts.
- In addition to the GRE sequence, add an additional axial susceptibility weighted image (SWI) to the evaluation for diffuse axonal injury.
- Skull fractures
- For suspected skull fractures, the above sequences have little sensitivity. Add a black bone MRI sequence to the protocol above.
- Select the black bone sequence by returning to the Patient Browser tab. From this tab, select the Neuro Brain protocol.
- From the list of additional protocols displayed on the left, select PEDS Trauma followed by the Black Bone sequence.
- The black bone sequence is a GRE sequence with shorter TE and TR and an optimal flip angle to differentiate soft tissue and bone. Select these imaging protocols1,7: TE 4.20 ms, TR 8.60 ms, and flip angle of 5° under the Routine tab of the study properties screen.
- Head CT is the gold standard for evaluating skull fractures, as seen in Figure 1. Discuss risks and benefits with guardians and determine the most appropriate course. If the patient has completed a skeletal survey in TBI workup, examine the skull radiograph before initiating Head CT.
4. Hydrocephalus and shunt evaluation
- Perform the protocol on 1.5 T or 3 T. Review sequences with standard commercially available hardware and software.
- Hydrocephalus evaluation
- Follow steps 2.3.1-2.3.3 to select the patient for the study. After selecting patient positioning as Head First Supine, under Study, select Neuro Brain.
- An additional list of protocols will populate. From that list, select Rapid Sequence.
- Begin the study with a localizer sequence named AAHScout. Ensure that this localizer sequence automatically begins at the start of the study.
- For evaluation of hydrocephalus, include a TurboFLASH T1-weighted sequence and a HASTE T2 weighted sequence. The TurboFLASH sequence is a modified GRE sequence with shorter TE, TR, and flip angles.
- For HASTE T2 performed on a 1.5 T, use the following recommended parameters8: Repetition time (TR) 744 ms, echo time 104 ms, flip angle 150°, field of view 230 mm, matrix 256 × 156, number of acquisitions 1, slice thickness 4 mm with a skip of 1 mm, and I-PAT factor of 2.
- For HASTE T2 performed on a 3 T, use the following recommended parameters8: 3-Tesla: TR 358 ms, echo time 90 ms, flip angle 150°, field of view 220 mm, matrix 256 × 156, number of acquisitions 1, slice thickness 4 mm with a skip of 1 mm and I-PAT factor of 2.
NOTE: HASTE T2 weight images provide the best imaging quality for ventricular assessment. If a catheter is placed, the TurboFLASH T1-weighted images are better suited for catheter visualization.
- Use these imaging protocols for TurboFLASH T1-weighted sequence: slice thickness 4.0 mm, FoV 230 mm, TE 2.46 ms, TR 240 ms. Viewing the Exam tab on the left, ensure both sequences are in three planes- axial, sagittal, and coronal. Multiplanar imaging provides better visualization of the catheter when compared with uniplanar imaging.
- Transfer images using steps 2.3.7-2.3.8.
- Shunt evaluation
- Follow the protocol above for hydrocephalus evaluation. Repeat the imaging sequence until a clear visualization of the shunt catheter is obtained.
NOTE: A summary of recommended sequences can be found below in Table 1. Only high-yield sequences are included.
- Follow the protocol above for hydrocephalus evaluation. Repeat the imaging sequence until a clear visualization of the shunt catheter is obtained.
Clinical Indications for Rapid Sequence MRI in Pediatric Neurosurgical Patients and the Limitations and Barriers to Implementation
Learning Objectives
Spine evaluation
Ryan et al. conducted a prospective study to determine the feasibility of rapid spine MRI in the evaluation of syrinx in pediatric patients. Patients with known or suspected syrinx or Chiari malformations underwent rapid spinal MRI (HASTE) and standard non-contrast MR. Images were blindly reviewed by pediatric neuroradiologists who measured the following outcomes: Presence or absence of syrinx, syrinx measurement, clonus position, cerebellar tonsillar ectopia and degree, and filum detection. They identified syrinx (sensitivity 87.8%, specificity 94.7%) if greater than 2.3 mm in size and if the patient was older than 1 year old. There was no clinically significant difference between spinal rapid MRI and standard spinal imaging9. An example of a lumbosacral syrinx identified by RS-MRI can be seen in Figure 2.
Gewirtz et al. conducted a retrospective chart review of patients who underwent spinal rapid MRI. Patients' (n = 45) scans were reviewed and compared with radiographic reports and clinical notes, and 47 scans were included in the analysis. Clinical indications for scan included syrinx (n = 30) and spinal dysraphism (n = 22) evaluation. All 47 scans were interpretable and usable (n = 8 moderate motion artifacts). Subsequent standard MRI follow-up scans (n =7) were completed within 1 year, and no new abnormalities were detected10.
Traumatic brain injury evaluation
Lindberg et al. conducted a prospective cohort study where they attempted RS-MRI in children <6 years old who had a prior head CT. The primary outcomes were feasibility and accuracy. Feasibility was measured by RS-MRI completion rate and imaging time. Accuracy was measured against CT and was assessed by the ability of the RS-MRI to identify skull fracture, intracranial hemorrhage, and parenchymal injury. A total of 223 RS-MRIs were conducted with a median imaging time of 365 s. Of the 111 patients identified with TBI on CT, RS-MRI detected 103 of these (sensitivity 92.8%, 95% confidence interval 86.3-96.3). RS-MRI failed to detect 6 isolated skull fractures and 2 subarachnoid hemorrhages. These findings concluded that RS-MRI is feasible and accurate relative to CT in clinically stable patients5.
Kessler et al. conducted a systematic review of the use of RS-MRI in the setting of pediatric head trauma. A total of 13 articles were identified and reviewed. In addition to the Lindberg article listed above, they reviewed Kralik et al., Missios et al., and Sheridan et al., which were a combination of retrospective and prospective studies of multisequence MRI. These four studies concluded that RS-MRI can be used without concurrent imaging modalities. Additional studies that utilized RS-MRI with tri-planar T2-weighted imaging alone were reviewed and compared to concurrent HCT or standard brain MRI. The sensitivity and specificity of RS-MRI were 100% and 97% for the detection of IPH, 86% and 96% for extra-axial hemorrhage, 10% and 100% for SAH, 50% and 100% for IVH, and 47% and 97% for skull fractures respectively. Further, Ryan et al. discussed the decreased sensitivity of RS-MRI to skull fractures, noting that only 11 of the 41 fractures were detected. The articles reviewing the utilization of the T2 sequence only concluded that across all pathologies, the sensitivity to TBI pathology was increased when used concurrently with HCT. To address the poor detection of skull fractures by RS-MRI, an article by Dremmen et al. was reviewed, which included the novel black bone sequence to T1-weighted imaging and determined that RS-MRI had a sensitivity and specificity of 66.7% for detected skull fracture. Of those skull fractures, there were 2 false negatives and 2 false positives. The false positives were later noted to be sutures and the patient population most affected by these findings were children under 2 years old. Lastly, a conglomerate of articles was reviewed where RS-MRI alone was compared to matched cohorts who received standard imaging (HCT/standard brain MRI). Cohen et al. found that more radiographic abnormalities were found in the HCT group, and those patients were, on average, triaged at higher levels of care. From this systematic review, Kessler et al. concluded that RS-MRI is a promising option when compared with HCT and standard MRI but may be less sensitive to traumatic pathology and that appropriate imaging modalities should be selected in the clinical and institutional context1.
Ryan et al. examined the ability of RS-MRI T2 sequences to detect intracranial hemorrhage. Patients who presented to the pediatric hospital with acute intracranial hemorrhage on CT had a follow-up RS-MRI 48 h later, and the two imaging modalities were compared. RS-MRI had modest sensitivity to detect subdural and epidural hemorrhages in the absence of prior CT; sensitivity ranged from 61%-74% but increased to 80%-86% with the review of the prior CT. The addition of GRE sequences to standard T2 sequences increased the sensitivity of detecting subarachnoid hemorrhage from 10%-25% to 71%-93%. Ryan et al. included that RS-MRI with GRE is most sensitive for detecting intracranial hemorrhages when prior CT is available and is not adequate to replace CT in the initial evaluation6. A T2 Haste from RS-MRI showcasing right extra-axial hemorrhage along right cerebral convexity is shown in Figure 3.
Hydrocephalus and shunt evaluation
A retrospective chart review conducted by O'Neill et al. included patients who underwent RS-MRI and compared that to prior HCT. The median age was 1.3 years. Patients underwent an average of 2.38 RS-MRI and 10.1 HCTs. All RS-MRIs were reviewed by a radiologist and blinded neurosurgeon, and image quality and catheter visualization were graded. Secondary outcomes were the amount of motion artifact and ventricular size. The radiologist rated 51.2% of RS-MRIs as having excellent imaging quality compared to the 76.5% rated by the neurosurgeon. Further, there were differences in catheter visualization by radiologists (24.4%) when compared to neurosurgeons (42.9%), and visualization was most problematic in the setting of small ventricles. It was concluded that axial RS-MRI provides good visualization of ventricular anatomy with the risk of potential valve failure11. An example of this can be seen in Figure 4, which demonstrates a sagittal view of a shunt catheter (Figure 4A) and an axial view showcasing ventriculomegaly (Figure 4B).
Yue et al. conducted a two-site retrospective chart review to compare RS MRI vs non-contrast CT in the evaluation of shunt malfunction of pediatric patients who presented to the ED. Shunt malfunction was defined as necessary neurosurgical shunt revision within 30 days of initial imaging. There were 997 scans used in the analysis (RS-MRI= 724, CT=273). There was a total of 235 shunt revisions (RS-MRI= 188, CT= 47). The sensitivity to detect shunt malfunction in the RS-MRI group was 58.5% (95% CI 51.1%-65.6%), and the specificity was 93.3% (95% CI 90.8%-95.3%). In the CT group, the sensitivity to detect shunt malfunction was 53.2% (95% CI 38.1%-67.9%), and specificity was 95.6% (95% CI 92%-97.9%). It was found that there was no statistically significant difference between sensitivity (p=0.51) or specificity (p=0.23)12.
Boyle et al. conducted a single-center retrospective chart review of pediatric patients who presented to Boston's Children's ED with concerns about shunt malfunction to determine the diagnostic accuracy between rapid cranial MRI and CT to diagnose ventricular shunt malfunction. Shunt malfunction was defined as the need for surgical intervention due to mechanical shunt flow alterations within 72 h of initial ED evaluation. The noninferiority test of the accuracy of rapid cranial to CT for diagnosing shunt malfunction, with a noninferiority margin of 10%, was used as the primary analysis. A total of 698 ED visits were included in the analysis (between 286 patients), of which patients received rapid cranial MRI scans (n = 362) and CT scans (n = 336). ED visits (n = 140) resulted in shunt revision. The accuracy of RS-MRI was similar to that of CT scans for diagnosing ventricular shunt malfunction (81.8% MRI vs. 82.4% CT), with an increase in RS-MRI use over the study period. Neurosurgical attending and neuroimaging modality were positively correlated (χ2 = 93.9, P < .001)13.
Boyle and Nigrovic reviewed the literature to compare the different neuroimaging modalities used to diagnose shunt malfunction in pediatric patients in the emergent setting. A review of the literature concludes rapid cranial MRI is an alternative non-inferior modality compared to CT when diagnosing shunt revision in children14. Table 2 showcases a summary of the representative results and their conclusions1,5,6,9,10,11,12,13,14.
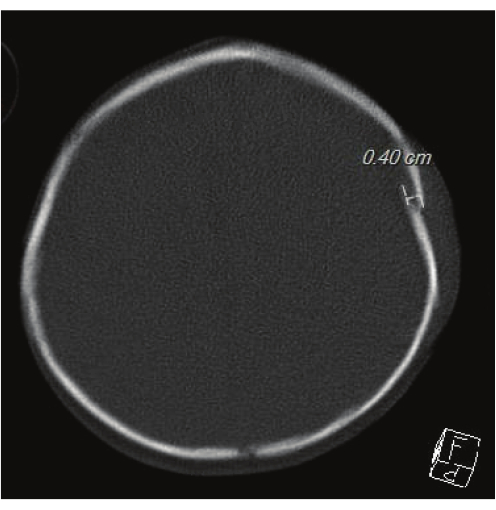
Figure 1: Head CT for evaluating skull fractures. This image showcases the gold standard Head CT. A 0.40 cm left parietal fracture can be seen. Please click here to view a larger version of this figure.
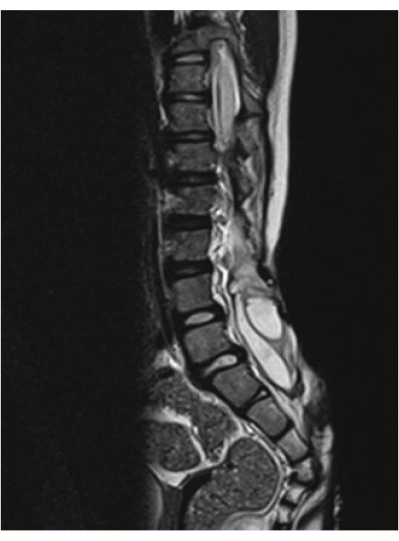
Figure 2: T2 spinal imaging showcasing syrinx. The image showcases a lumbosacral syrinx that is identified by RS-MRI. Please click here to view a larger version of this figure.
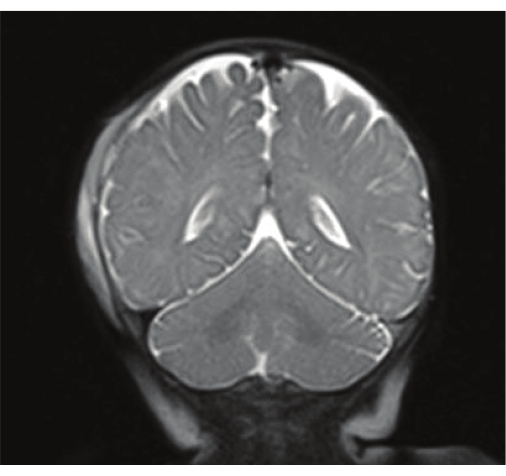
Figure 3: TBI findings from RS-MRI. The image is a T2 Haste from RS-MRI showcasing right extra-axial hemorrhage along right cerebral convexity. Please click here to view a larger version of this figure.
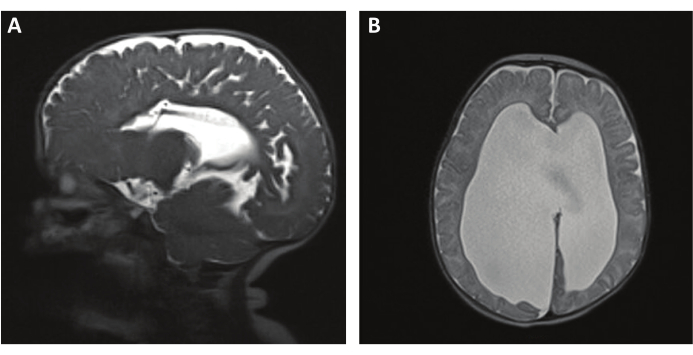
Figure 4: RS-MRI T2 HASTE for shunt evaluation and ventriculomegaly. (A) A T2 HASTE sequence showing a sagittal view of a shunt catheter. (B) An axial T2 HASTE image showing ventriculomegaly. Please click here to view a larger version of this figure.
Table 1: Summary of RS-MRI sequences for CNS pathology. The table provides a summary of recommended MRI sequences from the combined protocols above. BB= black bone Please click here to download this Table.
Table 2: Summary of representative results. The table provides a summary of the representative results showcasing the study type, comparative sequences, sensitivity and specificity, and conclusions. Please click here to download this Table.
List of Materials
| Alarm bell | Siemens | https://www.siemens.com/global/en/products/buildings/fire-safety/evacuation/notification-ul.html | |
| Brain and spine coils | Siemens | https://www.siemens-healthineers.com/magnetic-resonance-imaging | |
| Consent form to be filled out by parents or guardian | Local Health System | N/A | |
| Ear plugs | 3M Classic Ear Plugs | https://www.3m.com/3M/en_US/p/?Ntt=classic+ear+plugs | |
| Ferroguard Metal Detector | Metrasens | https://www.metrasens.com/solution/ferroguard-assure/ | |
| Immobilization restraints | Siemens | https://www.siemens-healthineers.com/magnetic-resonance-imaging | |
| Landmarkers, laser markers, or touch sensors | Siemens | https://www.siemens-healthineers.com/magnetic-resonance-imaging | |
| MR power cut-off | Siemens | https://www.siemens-healthineers.com/magnetic-resonance-imaging | |
| MR quench button | Siemens | https://www.siemens-healthineers.com/magnetic-resonance-imaging | |
| MRI scanner | Magnetom Avanto | https://www.siemens-healthineers.com/en-us/magnetic-resonance-imaging/0-35-to-1-5t-mri-scanner/magnetom-avanto | Other brands: Discovery 750, HDXT Signa scanners, GE Healthcare, , Aera and Skyra, Siemens, Erlangen, and Germany |
| Radiologic technologist | Local Health System | N/A | |
| Radiologist | Local Health System | N/A | |
| Standard MRI hardware and software | NUMARIS | Version 4 | |
| Support pads and pillows | Medline | www.medline.com | Alternative: Quality electrodynamics |
Lab Prep
Rapid and fast magnetic resonance imaging (MRI) protocols have become increasingly popular for pediatric neurosurgical patients as they are a great way to reduce ionizing radiation and sedation. While their popularity has increased, there are hurdles to overcome when transitioning to using them clinically, such as cost, staffing training, and motion artifact. Through this paper, we developed a protocol for clinical applications where rapid MRI can be a substitute or adjuvant in diagnostic workup. Further, we outline the relevant literature for the use of RS-MRI for the spine, TBI, and hydrocephalus pathologies while expanding upon the limitations and logistical barriers when transitioning to their use, a few of which are discussed above. Through this, we conclude that RS-MRI can be used diagnostically for spinal pathologies such as syrinx and hydrocephalus. Further, its lack of sensitivity for TBI findings makes rapid sequence magnetic resonance imaging (RS-MRI) a strong adjuvant with other advanced imaging or computed tomography (CT) for traumatic brain injury (TBI) pathologies.
Rapid and fast magnetic resonance imaging (MRI) protocols have become increasingly popular for pediatric neurosurgical patients as they are a great way to reduce ionizing radiation and sedation. While their popularity has increased, there are hurdles to overcome when transitioning to using them clinically, such as cost, staffing training, and motion artifact. Through this paper, we developed a protocol for clinical applications where rapid MRI can be a substitute or adjuvant in diagnostic workup. Further, we outline the relevant literature for the use of RS-MRI for the spine, TBI, and hydrocephalus pathologies while expanding upon the limitations and logistical barriers when transitioning to their use, a few of which are discussed above. Through this, we conclude that RS-MRI can be used diagnostically for spinal pathologies such as syrinx and hydrocephalus. Further, its lack of sensitivity for TBI findings makes rapid sequence magnetic resonance imaging (RS-MRI) a strong adjuvant with other advanced imaging or computed tomography (CT) for traumatic brain injury (TBI) pathologies.
Procedure
Rapid and fast magnetic resonance imaging (MRI) protocols have become increasingly popular for pediatric neurosurgical patients as they are a great way to reduce ionizing radiation and sedation. While their popularity has increased, there are hurdles to overcome when transitioning to using them clinically, such as cost, staffing training, and motion artifact. Through this paper, we developed a protocol for clinical applications where rapid MRI can be a substitute or adjuvant in diagnostic workup. Further, we outline the relevant literature for the use of RS-MRI for the spine, TBI, and hydrocephalus pathologies while expanding upon the limitations and logistical barriers when transitioning to their use, a few of which are discussed above. Through this, we conclude that RS-MRI can be used diagnostically for spinal pathologies such as syrinx and hydrocephalus. Further, its lack of sensitivity for TBI findings makes rapid sequence magnetic resonance imaging (RS-MRI) a strong adjuvant with other advanced imaging or computed tomography (CT) for traumatic brain injury (TBI) pathologies.
