A Rat Dry Eye Model with Lacrimal Gland Dysfunction Induced by Scopolamine
Instructor Prep
concepts
Student Protocol
All animal experiments performed following this protocol are performed under the approval of the Institutional Animal Care and Use Committee (IACUC).
1. Animal preparation
- Prepare 12 healthy 6-week-old SPF Wistar female rats weighing 160 g ± 20 g.
- Use a slit lamp and ophthalmoscope to examine the eye conditions of all rats, ensuring that there are no anterior segment or retinal diseases.
- Raise all the rats for 1 week with sufficient food and water sources.
- Randomly divided all rats into normal, scopolamine drug concentration 2.5 mg /mL, scopolamine drug concentration 5 mg/mL, and scopolamine drug concentration 7.5 mg/mL groups, with three animals in each group.
2. Solution preparation
- Prepare scopolamine hydrobromide by dissolving it in 0.9% sodium chloride solution to make a solution with concentrations of 7.5 mg/mL, 5 mg/mL, and 2.5 mg/mL.
- Prepare a 0.9% sodium chloride solution without scopolamine hydrobromide to be used as an injection for the control group of rats.
3. Equipment and material preparation
- Prepare a small animal microscope.
- Prepare materials for the experiment, including 1 mL disposable syringe with needle (26 G); fluorescein sodium ophthalmic strips; Schirmer tear test strip; absolute ethanol; 4% paraformaldehyde; xylene; neutral balsam; hematoxylin, eosin; and periodic acid-Schiff staining kit.
4. Subcutaneous injection
NOTE: This procedure requires assistance from a second person to help secure the rats.
- Hold the rat's body steady and catch and stretch its left (or right) hind legs.
NOTE: An assistant can help in holding the animal. - Clean injection site with alcohol.
- Insert 1 mL disposable syringe with needle (26 G) at the base of skin fold between thumb and finger.
- Aspirate the syringe by pulling back the syringe plunger. Any blood in the syringe indicates improper needle placement; remove and reposition the needle.
- Administer 0.9% sodium chloride solution with or without scopolamine hydrobromide in a steady, fluid motion.
- Inject all rats according to different concentrations, with 0.5 mL injected each time and four times daily (at 9:00, 12:00, 15:00, and 18:00) for a consecutive period of 19 days, alternating between left and right limbs.
NOTE: The groups are named as follows:
Group without scopolamine hydrobromide: 0 group (control)
Group with scopolamine hydrobromide 2.5 mg/mL: 2.5 group
Group with scopolamine hydrobromide 5 mg/mL: 5 group
Group with scopolamine hydrobromide 7.5 mg/mL: 7.5 group - Return the animal to its cage and monitor breathing and behavior for 5-10 min.
5. Tear secretion test (Schirmer tear test, STT)
- Create a modified filter paper strip for rats11. Cut half of the filter paper strip used for humans along the centerline (1 mm × 15 mm), and trim the head of the strip to make it smooth.
NOTE: Before conducting the tear secretion test, manually restrain the rat's body to prevent movement and ensure exposure of the rat's eyes. - Place the filter paper strip on the outer 1/3 of the lower eyelid conjunctival sac of the rat.
- Time the test for 5 min. Control the closure of the rat's eyes throughout the procedure.
- After measuring, use tweezers to clamp the filter paper strip into a microcentrifuge tube and record the tear volume by making a mark on the wall of the tube.
- Measure tear secretion on day 0, day 1, day 3, day 5, day 7, day 11, day 15, and day 19.
6. Corneal fluorescein staining
- Drop 0.5 µL of 0.5% fluorescein sodium solution into the inferior conjunctival sac of each rat.
- Observe the cornea under blue light for 3 min after fluorescein instillation.
- Record the fluorescence staining of each rat's cornea and observe whether there is a corneal defect.
- Perform corneal fluorescein staining on day 0, day 1, day 3, day 5, day 7, day 11, day 15, and day 19.
7. Histological observation of conjunctival tissue
- After completing the model development, anesthetize the rats deeply with an intraperitoneal injection of 0.4 mL/100 g of 10% aqueous chloral hydrate to alleviate the animals' tension. Then, euthanize the rats by cervical dislocation.
- Take the bulbar conjunctiva from the same regions of each rat, with a size of approximately 2 mm x 2 mm.
- Fix the tissues immediately in 4% paraformaldehyde for 24 h and embed in paraffin13.
- Cut 5 µm thickness sections and stain with hematoxylin and eosin (HE)14 and periodic acid-Schiff (PAS) stain (follow manufacturer's instructions).
8. Histological observation of corneal and lacrimal gland tissue
- After completing the model development, euthanize the rat as described in step 7.1.
- Take the cornea on the right side of each rat and fix it immediately in 4% paraformaldehyde solution.
- Cut the cephalic epidermis and subcutaneous tissue along the line connecting the ear and the outer corner of the eye, expand the incision to both sides and further isolate the yellowish extra orbital gland.
- Thoroughly remove the rat's fur and separate the extraorbital gland with 0.9% sodium chloride solution.
- Place the isolated extraorbital glands in 4% paraformaldehyde solution for 24 h and embed in paraffin.
- Cut continuous sections of ~5 µm thickness and stain them with HE for corneal and extraorbital gland tissue specimens.
9. Statistical analysis
- Use appropriate software for statistical analysis of the data.
- Perform one-way analysis of variance (ANOVA) to analyze the data and the least significant difference (LSD) test for comparison between groups. Set the statistical significance level at α = 0.05, with P < 0.05 indicating statistical significance.
NOTE: SPSS 20 software was used for statistical analysis of the experimental data.
- Perform one-way analysis of variance (ANOVA) to analyze the data and the least significant difference (LSD) test for comparison between groups. Set the statistical significance level at α = 0.05, with P < 0.05 indicating statistical significance.
A Rat Dry Eye Model with Lacrimal Gland Dysfunction Induced by Scopolamine
Learning Objectives
Schirmer I test, SIT I
The tear volume of the rats was measured on days 0, 3, 5, 7, 11, 15, and 19 after the start of the experiment. The experimental results showed that the tear secretion of the scopolamine group (2.5 group, 5 group, 7.5 group), compared with the control group (0 group), was significantly decreased, and the difference was statistically significant (P < 0.01). There was no statistical significance between the 2.5 group, 5 group, and 7.5 group (P > 0.05). There was no significant difference observed between the different groups in terms of the number of days (P > 0.05) (Figure 1, Table 1).
Corneal fluorescein staining
Corneal fluorescein staining was performed on days 0, 3, 5, 7, 11, 15, and 19 of the experiment. The results showed that there was no corneal fluorescein staining in any group, indicating that no obvious corneal epithelial defects were formed during the 20-day experiment with different concentrations of scopolamine drugs (Figure 2).
Pathological analysis of corneal epithelium
After the experiment, corneal tissues from each rat were collected for HE staining to observe the morphology of the corneal epithelium and measure the thickness of the corneal epithelial layer. The corneal epithelium of the control group was composed of 4-6 layers of orderly arranged epithelial cells, among which the basal layer consisted of a single layer of columnar epithelial cells arranged neatly and closely. The corneal epithelium of scopolamine groups 2.5, 5, and 7 were significantly thinner than the control group, with flattened and atrophic cell morphology and disordered cell structure. In group 7.5, there was a loose intercellular connection and vacuolar structure in the basal layer (indicated by the red arrow in Figure 3). Compared with the corneal epithelium of scopolamine groups, the corneal epithelium of the normal control group showed statistical differences in the thickness of the corneal epithelial layer (Figure 4).
Pathological analysis of lacrimal gland
The main gland for tear secretion in rats is the extrorbital lacrimal gland15. When observing lacrimal gland slices, changes in the morphology of the lacrimal gland epithelial cells were observed with the increase of scopolamine concentration, accompanied by inflammation and tissue edema. No such changes were observed in the control group. The pathology results suggest that inflammatory changes of the lacrimal gland, cell edema, and atrophy of the glandular epithelial cells can be used as indicators for functional damage of the lacrimal gland16 (Table 2). These indicators can be used to measure the severity of dry eye in relation to the amount of tear secretion (Figure 5).
Analysis of conjunctival staining results
The structure of the conjunctiva in the control group is complete, mainly composed of the surface layer and the lamina propria. The surface layer is laminated columnar epithelial cells, smooth and complete, with microvilli on the cell surface. Scattered goblet cells were present between the epithelial cells, with large cell volume and mucous granules in the cell cytoplasm. The surface layer of the conjunctival epithelium in the three scopolamine drug groups was significantly thinner, the number of microvilli and goblet cells was reduced, the cell arrangement structure was incomplete, accompanied by edema, and a small amount of inflammatory cells as observed in HE staining (Figure 6).
By staining the conjunctiva with PAS, the average number of goblet cells per 40x microscopic field in three independent samples of each mouse was calculated and expressed as mean ± SD (Figure 7).
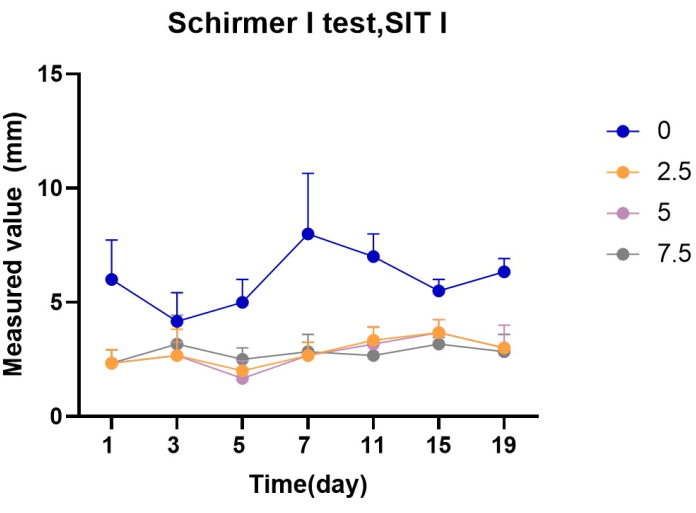
Figure 1: Statistics of Schirmer test value in each group (mm) Please click here to view a larger version of this figure.
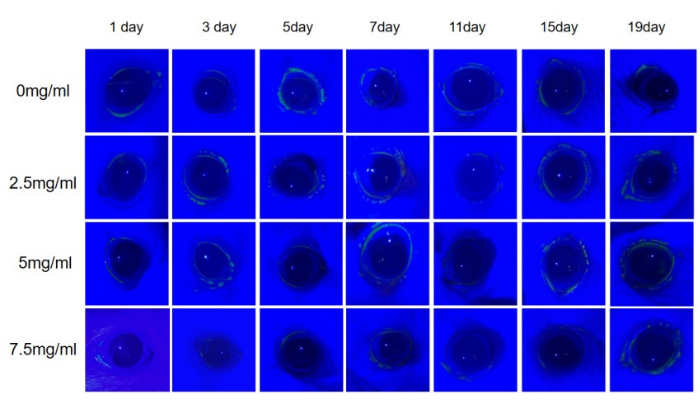
Figure 2: Fluorescein sodium staining of rat cornea. In the 20-day experiment with fluorescein sodium, no positive findings were observed in the corneas of all rats. Please click here to view a larger version of this figure.
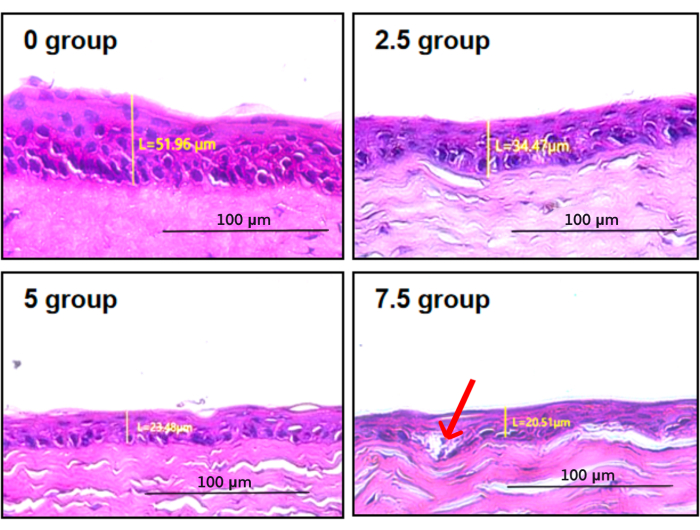
Figure 3: Corneal epithelium staining and thickness measurement. In group 7.5, there was loose intercellular connection and vacuolar structure in the basal layer (indicated by the red arrow) Please click here to view a larger version of this figure.
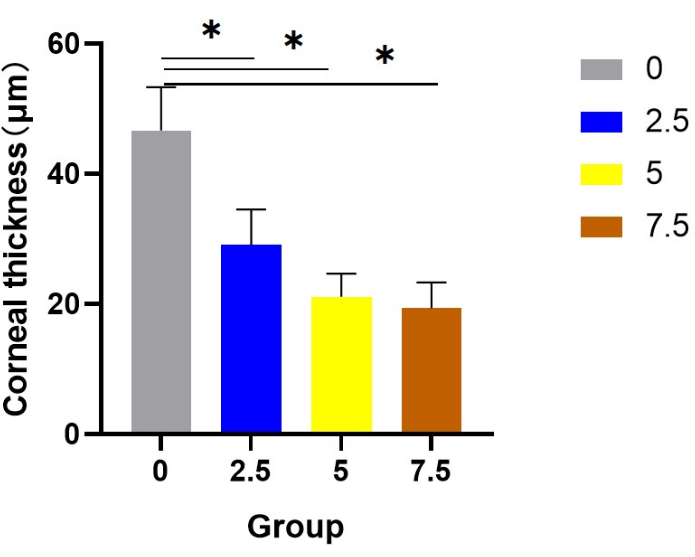
Figure 4: Statistics of corneal epithelial thickness in each group. Compared with the corneal epithelium of scopolamine groups, the corneal epithelium of the normal control group showed statistical differences in the thickness of the corneal epithelial layer. Please click here to view a larger version of this figure.
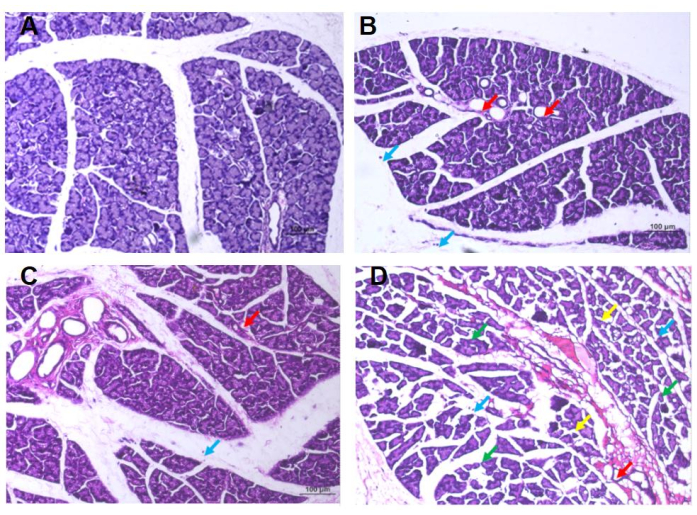
Figure 5: HE staining results of the extrorbital lacrimal gland of rats. (A) Group 0: In the visual field, lacrimal glands showed lobular structure and were composed of ducts and tubular glands, with no obvious abnormalities in the morphology of the ducts, while the tubular glands were composed of cone-shaped glandular cells with abundant mucous substances in the cytoplasm, no obvious edema in connective tissues, no obvious abnormalities in interstitial blood vessels, and no obvious necrosis and inflammatory cell infiltration. (B) Group 2.5: In the visual field, occasional atrophy of the lacrimal gland epithelial cells is observed, with reduced volume, irregularly-shaped dilated glandular cavities, and reduced mucinous substance within the cavity (indicated by the red arrow). There is also occasional infiltration of free lymphocytes in the stroma (indicated by the blue arrow), but no obvious abnormalities in the duct morphology or signs of edema are observed. (C) Group 5: In the visual field, lacrimal epithelial cells were occasionally atrophied and reduced in size, the glandular cavity was enlarged, the mucous substance in the cavity was reduced (red arrow), and free lymphocyte infiltration was occasionally observed in the stroma (blue arrow), and no obvious abnormalities in duct morphology or connective tissue edema between lacrimal gland lobules are observed. (D) Group 7.5: Edema can be seen in the visual field; the spacing between the lacrimal glands is widened, and the arrangement is irregular (green arrow), the epithelial cells of the lacrimal glands are often atrophied, the volume becomes smaller, and the shape is irregular (yellow arrow), occasionally the gland cavity is enlarged, the mucous matter in the cavity is reduced (red arrow), occasionally the free lymphocyte is infiltrated (blue arrow), with no apparent abnormalities in duct morphology. Please click here to view a larger version of this figure.
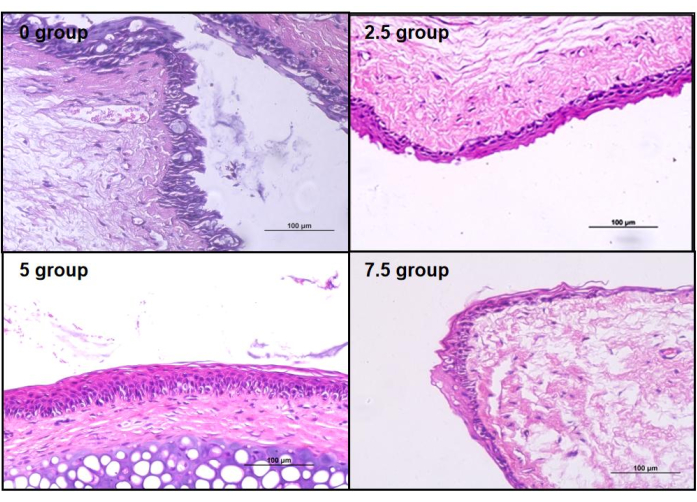
Figure 6: HE staining of rat conjunctiva. Compared to the control group's conjunctival epithelium, all three groups of scopolamine-medicated conjunctival epithelium showed varying degrees of structural damage. Please click here to view a larger version of this figure.
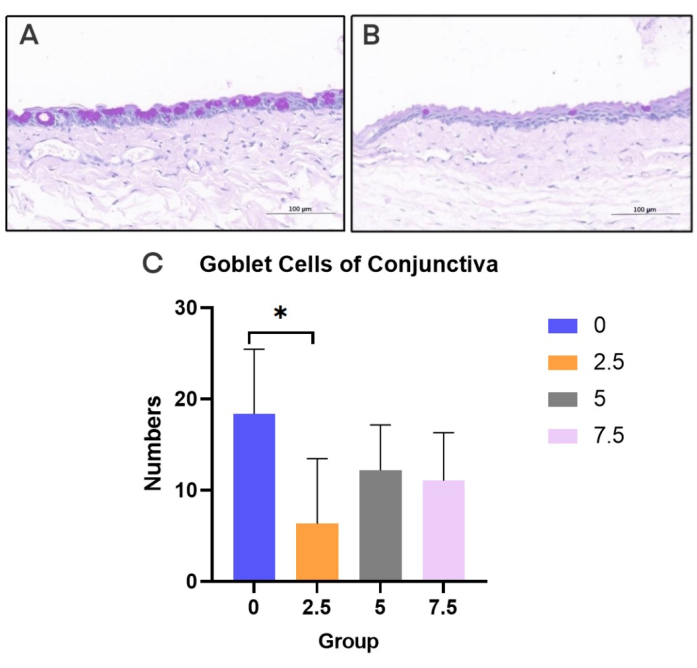
Figure 7: PAS staining of conjunctiva. (A) Normal control rats. (B) scopolamine group rats. (C) Goblet cell density in each group (20x). Black bar = 100 µm. Please click here to view a larger version of this figure.
| Schirmer I test, SIT(Mean value, unit [mm]) | |||||||
| Group | 0 days | 3 days | 5 days | 7 days | 11 days | 15 days | 19 days |
| 0 | 6 | 4.2 | 5 | 8 | 7 | 5.5 | 6.3 |
| 2.5 | 2 | 2.7 | 2 | 2.7 | 3.3 | 3.7 | 3 |
| 5 | 2.3 | 2.7 | 1.7 | 2.3 | 3.2 | 3.7 | 3 |
| 7.5 | 2.3 | 3.2 | 2.5 | 2.8 | 2.7 | 3.2 | 2.8 |
Table 1: Schirmer test of rats in the four groups at different time points (mm). After applying medication, the secretion of tears in rats significantly decreased.
| Number | Necrosis | Inflammation | Edema | Epithelial atrophy |
| 0 Grp-1 | 0 | 0 | 0 | 0 |
| 0 Grp -2 | 0 | 0 | 0 | 0 |
| 0 Grp -3 | 0 | 0 | 0 | 0 |
| 2.5 Grp -1 | 0 | 1 | 0 | 0 |
| 2.5 Grp -2 | 0 | 0 | 0 | 0 |
| 2.5 Grp -3 | 0 | 1 | 0 | 1 |
| 5 Grp -1 | 0 | 1 | 0 | 1 |
| 5 Grp -2 | 0 | 1 | 0 | 1 |
| 5 Grp -3 | 0 | 0 | 0 | 1 |
| 7.5 Grp -1 | 0 | 1 | 2 | 1 |
| 7.5 Grp -2 | 0 | 1 | 0 | 1 |
| 7.5 Grp -3 | 0 | 1 | 2 | 2 |
Table 2: Pathological tissue score of rat lacrimal gland. Scoring criteria: 0: Under normal conditions, considering factors such as animal age, sex, and strain, the tissue is deemed normal;
1: The observed changes have just exceeded the normal range; 2: Lesions can be observed, but they are not yet severe; 3: Lesions are evident and continue to worsen; 4: Lesions are extremely severe and have affected the entire tissue16.
List of Materials
| 0.9% sodium chloride solution | SJZ No.4 Pharmaceutical | H13023201 | |
| 4% paraformaldehyde | Wuhan Servicebio Technology Co., Ltd | G1113 | |
| Absolute ethanol | Sinopharm Chemical Reagent Co., Ltd. | 10009218 | |
| Fluorescein sodium ophthalmic strips | Tianjin Yinuoxinkang Medical Device Tech Co., Ltd | YN-YG-I | |
| Hematoxylin and eosin | Nanjing Jiancheng Bioengineering Institute | D006 | |
| Neutral balsam | Beijing Solarbio Science & Technology Co., Ltd. | G8590 | |
| Paraffin | Beijing Solarbio Science & Technology Co., Ltd. | YA0012 | |
| Periodic Acid-Schiff Staining Kit | Beyotime Biotechnology | C0142S | |
| Schirmer tear test strips | Tianjin Yinuoxinkang Medical Device Tech Co., Ltd | YN-LZ-I | |
| Scopolamine hydrobromide | Shanghai Macklin Biochemical Co., Ltd | S860151 | |
| Small animal microscope | Head Biotechnology Co,. Ltd | ZM191 | |
| Xylene | Sinopharm Chemical Reagent Co., Ltd. | 10023418 |
Lab Prep
Aqueous-deficient dry eye (ADDE) is a type of dry eye disease that can result in the reduction of tear secretion quantity and quality. Prolonged abnormal tear production can lead to a disturbance in the ocular surface environment, including corneal damage and inflammation. In severe cases, ADDE can cause vision loss or even blindness. Currently, dry eye treatment is limited to eye drops or physical therapy, which can only alleviate eye discomfort symptoms and cannot fundamentally cure dry eye syndrome. To restore the function of the lacrimal gland in dry eye, we have created an animal model of lacrimal gland dysfunction in rats induced by scopolamine. Through the comprehensive evaluation of the lacrimal gland, corneas, conjunctivas, and other factors, we aim to provide a full understanding of the pathological changes of ADDE. Compared with the current dry eye mouse model, this ADDE animal model includes a functional evaluation of the lacrimal gland, providing a better platform for studying lacrimal gland dysfunction in ADDE.
Aqueous-deficient dry eye (ADDE) is a type of dry eye disease that can result in the reduction of tear secretion quantity and quality. Prolonged abnormal tear production can lead to a disturbance in the ocular surface environment, including corneal damage and inflammation. In severe cases, ADDE can cause vision loss or even blindness. Currently, dry eye treatment is limited to eye drops or physical therapy, which can only alleviate eye discomfort symptoms and cannot fundamentally cure dry eye syndrome. To restore the function of the lacrimal gland in dry eye, we have created an animal model of lacrimal gland dysfunction in rats induced by scopolamine. Through the comprehensive evaluation of the lacrimal gland, corneas, conjunctivas, and other factors, we aim to provide a full understanding of the pathological changes of ADDE. Compared with the current dry eye mouse model, this ADDE animal model includes a functional evaluation of the lacrimal gland, providing a better platform for studying lacrimal gland dysfunction in ADDE.
Procedure
Aqueous-deficient dry eye (ADDE) is a type of dry eye disease that can result in the reduction of tear secretion quantity and quality. Prolonged abnormal tear production can lead to a disturbance in the ocular surface environment, including corneal damage and inflammation. In severe cases, ADDE can cause vision loss or even blindness. Currently, dry eye treatment is limited to eye drops or physical therapy, which can only alleviate eye discomfort symptoms and cannot fundamentally cure dry eye syndrome. To restore the function of the lacrimal gland in dry eye, we have created an animal model of lacrimal gland dysfunction in rats induced by scopolamine. Through the comprehensive evaluation of the lacrimal gland, corneas, conjunctivas, and other factors, we aim to provide a full understanding of the pathological changes of ADDE. Compared with the current dry eye mouse model, this ADDE animal model includes a functional evaluation of the lacrimal gland, providing a better platform for studying lacrimal gland dysfunction in ADDE.
