Evaluating Toxicity of Chemicals using a Zebrafish Vibration Startle Response Screening System
Instructor Prep
concepts
Student Protocol
All zebrafish husbandry and handling were performed in compliance with the German animal protection standards and approved by the Government of Baden-Württemberg, Regierungspräsidium Karlsruhe, Germany (Aktenzeichen 35-9185.64/BH KIT).
1. Preparing stock solutions of chemicals to be tested
- Label the glass vial (or chemical aliquot) with the compound name/abbreviation, stock concentration, and date of preparation. For example, CdCl2_2.5 g·L-1_210813.
- Centrifuge the chemical aliquot at 2000 x g for 1 min at room temperature (RT).
- Under a fume hood, weigh the compound (if necessary) on a scale sensitive to 0.001 g and transfer it to the labeled vial. If the compound is a liquid, add it to the vial using a pipette and plastic pipette tip.
NOTE: For example, for the tricaine methanesulfonate stock used in the result example shown in Figure 2, 400 mg was weighed into the labeled vial. - Add solvent (e.g., sterile pure water or dimethyl sulfoxide (DMSO), depending on the physicochemical properties of the compounds) using a pipette and plastic pipette tip. If possible, water is the preferred solvent.
NOTE: For example, for the tricaine stock, a 15.3 mM solution in 100 mL water was prepared. - Seal the vial, shake gently, and check for precipitation.
- Prepare dilute stock solutions (if necessary) in glass vials using pipettes and plastic pipette tips.
NOTE: For example, no further dilution was necessary for the tricaine stock. - Store the stock solution(s) at -20 °C until use.
- Store the remaining chemical aliquot in the same conditions as before.
- Record stock aliquot information in the lab notebook.
2. Collecting and raising zebrafish embryos
- Collect embryos in cleavage stages (2-8 cell stage) from natural spawning of group matings in 10 cm tissue culture dishes.
- Select a batch of appropriate quality: less than 10% unfertilized/dead eggs.
- Clean the dishes (remove unfertilized eggs, debris, scales, etc.).
- Place 60 embryos per 10 cm tissue culture dish in 15 mL of E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4)25.
- Place dishes into a humidified chamber prepared by laying out paper towels soaked with water.
- Raise embryos until 72 hpf in an incubator at 28.5 °C.
3. Preparing working dilution of chemicals to be tested
- Remove the stock solution from the -20 °C freezer and let it thaw.
- If compound solubility is high enough, prepare serial dilutions in E3 medium in glass bottles, 1 mL per replicate per concentration. Ensure that the the concentration is 10 times higher than the desired exposure concentration. This avoids having to change the entire medium of the embryos at the beginning of the exposure. In case of low solubility, prepare the serial dilutions directly at the desired exposure concentrations, 10 mL per replicate per concentration.
- Check for precipitation (if necessary). If the solution has precipitated, record this, then further dilute to achieve the next highest concentration. Check again for precipitation. Repeat until there is no precipitation.
- Check the pH of the exposure solution. If outside the range of pH 7.0-8.5, record this, and prepare the solutions in E3 containing 5 mM HEPES/pH 7.4, adjusting the pH with HCl or NaOH.
- Dispose of unused exposure media according to local regulations.
4. Exposing embryos to the chemicals to be tested
- Check the dishes with the 72 hpf old embryos for dead/unhatched embryos and remove them. If a batch of embryos contains more than 5% of unhatched eggs, remove the batch.
- Place 10 embryos per 6 cm tissue culture dish in 9 mL of E3 medium (exposure plate).
- Label exposure plates with the compound name, exposure concentration, and replicate number. For example, "CdCl2 1 mg·L-1 01". Include sufficient E3-only plates and a solvent control plate, if needed (see section 5).
- Add 1 mL of exposure solution to each plate, starting with the lowest concentration, and swirl the plate. For compounds with low solubility, replace the entire 10 mL of the plate with the exposure solution (see step 3.2.)
- Record the temporal order in which compound solutions were added to the embryos.
- Incubate the plates in the humidified chamber in an incubator at 28.5 °C for 48 h until they reach 120 hpf.
5. Performing the vibration startle assay
NOTE: Analyse the plates in the same order as recorded in step 4.5. Each run should include an E3 control plate.
- Switch on the computer and the vibration device (blue LED light should be on).
- Prepare the configuration file in a spreadsheet, as seen in Figure 1D and attached as Supplementary File 1. Record exposure information for each of the 5 plate positions (compound, concentration, replicate).
- Open the general user interface (GUI) program (available at https://git.scc.kit.edu/xk4962/vibration-startle-assay-kit , project ID 43215.)
- Check the camera movement by selecting the different positions in the GUI program and observing camera movement.
- Take out the sample plates to be measured from the incubator. Place the sample plates on the 5 positions (see Figure 1A, "loudspeakers") and let the embryos settle for several minutes.
- Click on Record; a window will open to select the configuration file.
- Select the appropriate configuration file prepared in step 5.2 for this run.
- Check that the sample description corresponds to the samples on each position (1-5).
- Measuring will be conducted automatically (10 s / position). When the sound pulse is applied by the program, an LED is turned on. Recording for 10 s/position allows acquiring enough data to estimate swimming speed and distance traveled both before and after the stimulus is applied and prevents habituation to subsequent stimuli.
- When the recording is completed, the camera goes back to position 1 and the software starts to compress the files. During this time, replace the samples with the next set that needs to be measured.
- Go to step 5.2. to record the next run.
- When all plates have been measured, collect the exposure solutions. Use a sieve to retain the embryos.
- Euthanize the embryos by rapid chill in an ice/isopropanol (5%) bath.
- Dispose of the exposure solutions and dead embryos according to the local regulations.
6. Data analysis
- Open the video data with VirtualDub (1.10.4).
- Score visually the number of embryos responding to the sound pulse (when the control LED is on).
- Enter data into a spreadsheet. Record the compound name, the replicate, the concentration of the compound, and the percentage of immotile embryos according to the template provided in Supplementary File 2, which includes the example dataset shown in Figure 2C.
NOTE: The template has a flexible structure and principally allows application for data of other organisms and endpoints. It describes the responses for each concentration and also provides endpoint descriptions as well as definitions of the parameters generated in the subsequent concentration-response modeling. - Conduct the benchmark concentration (BMC) analysis using a KNIME workflow (KNIME analytics 4.626) with embedded R scripts (R version 3.6., R-packages plotrix, drc and bmd27,28).
NOTE: In principle, the assessment could also be conducted directly in R. However, for convenience and to allow assessment as a web-based service, the analysis has been organized in a KNIME server-compliant workflow. The output of the KNIME workflow is provided in Supplementary File 3. For details on the statistical parameters generated by the KNIME workflow, refer to this template. The statistical parameters to estimate the goodness of fit and thresholds to accept determined BMC values are shown in Table 1. The KNIME workflow itself is available via GitHub (https://github.com/precisiontox/range-finding-drc). The concentration-response is modeled using a 4-parameter log-logistic model. Two of the curve fitting parameters can be fixed. Typically, one would fix the maximum to 100 in the case of percentage data. In case no background effects are observed, the minimum can be fixed to 0%.
Evaluating Toxicity of Chemicals using a Zebrafish Vibration Startle Response Screening System
Learning Objectives
Figure 2A shows the percentage of immotile embryos in 48 clutches of untreated wild-type embryos (AB2O2 strain). On average, 14.33% of untreated wild-type embryos do not react to the vibration stimulus. In 4 clutches, the percentage of immotile larvae reached 50%, but 75% of the clutches had a percentage of immotile larvae below 20%.
Figure 2B,C show an example of a typical calculation of a benchmark concentration/dose (BMC/BMD29,30) for compound effects on motility with the vibration startle assay workflow, as currently performed within the PrecisionTox consortium24. BMC10, BMC25, and BMC50 values correspond to the concentrations at which 10%, 25%, and 50% of embryos show immotility levels higher than the background, respectively. Only embryos that are completely immotile are included in this calculation, not those that still show partial responses, such as only a C-bend without subsequent escape swimming or only tail movements (Figure 2B). The embryos were exposed to 8 concentrations of the sodium channel inhibitor tricaine methanesulfonate, which is frequently used for fish anesthesia31. The data indicate a background level of around 25% immotility in response to the vibration stimulus. Starting at 1% tricaine, motility is reduced and then ceases above 2.5%. The KNIME workflow calculates the BMC50 as 164.9 µM, which corresponds to 1.07% tricaine and an immotility level of 75% (Figure 2C). The small 95% confidence intervals (indicated by the grey shades in the curve) indicate robust reproducibility of the motility values in this assay.
Figure 2D shows an example of a suboptimal assay run, the data of which should not be used for BMC calculations. Five E3 treated control groups with different embryos derived from the same clutch are shown (AB2O2 [wild-type strain] replicate 1-5). Only the first group shows a near normal response, showing around 25% immotility that is consistent with literature values32 and those obtained in the assay described here, as shown in Figure 2A, while all other groups show reduced and/or incomplete behavioral responses (e.g., showing only a C-bend not followed by swimming activity, or motility without a clear C-bend at the beginning). Such a response may occur when embryos do not develop properly and are in an immature state due to developmental delay, which impacts the robustness of the startle response14,33.
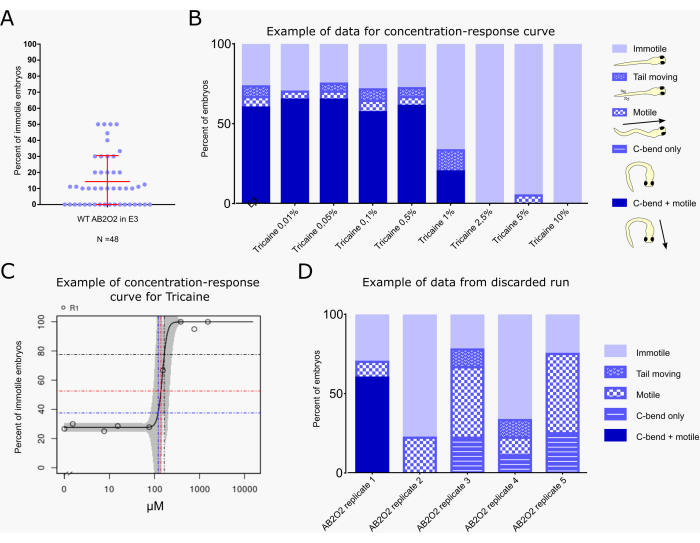
Figure 2: Example of a typical result, including benchmark dose calculation. (A) Percentage of non-responsive embryos after the sound pulse for wild-type untreated larvae for 48 clutches (n = 10 per clutch). The mean (14.33%) and standard deviation (±16.19%) are indicated in red. (B) Evaluation of startle response behavior of embryos (n=20 per condition) treated with the indicated concentration of tricaine in E3 medium or with E3 alone as a control. Behavior is classified according to the color scheme and cartoons indicated to the right of the graph, with each embryo assigned to only one of the following classes: "immotile": embryo does not show any movement; "tail moving": embryo shows tail movement, but neither C-bend nor swimming behavior; "motile": embryo shows swimming movement, but no C-bend in response to the vibrational stimulus; "C-bend only": embryo shows C-bend, but not escape swimming; "C-bend + motile": embryo shows typical C-bend behavior followed by escape swimming (the typical full startle response). The different behaviors are shown as a percentage of the total number of embryos for each treatment. (C) BMC calculation graph generated by the KNIME workflow, indicating the percentage of "immotile" embryos for each treatment concentration. Blue, red, and black lines indicate the BMC10, BMC25, and BMC50 values, i.e., the concentrations at which 10%, 25%, and 50% of embryos show immotility levels higher than the background, respectively. (D) Example of a discarded assay run. Five E3-treated control runs with different AB2O2 wild-type embryos derived from the same clutch are shown (replicate 1-5). Only replicate 1 shows a nearly normal response, while embryos of the remaining runs do not show the typical C-bend + escape swimming response. Please click here to view a larger version of this figure.
Table 1: Statistical parameters to estimate the goodness of fit and thresholds to accept determined BMC values. Please click here to download this Table.
Table 2: Properties of a selection of vibrational startle response assay systems. Please click here to download this Table.
Supplementary File 1: Excel template for configuration file. Please click here to download this File.
Supplementary File 2: KNIME input template with an example data set. Please click here to download this File.
Supplementary File 3: KNIME output file example. Please click here to download this File.
List of Materials
| Fine test sieves, Brass frame, pore size 250 μm | Sigma-Aldrich | Z289744-1EA | Or comparable material |
| High-speed camera | XIMEA | MQ013MG-ON USB 3 | |
| Laboratory Bottles, Narrow Neck, with Screw Cap | VWR | 215-3261 | Reference number for 50 mL, available up to 20 L. Or comparable material |
| Pipette tip, working volume: 10 µL | SARSTEDT | 70.3010.210 | Or comparable material |
| Pipette tip, working volume: 1000 µL | SARSTEDT | 70.3050.100 | Or comparable material |
| Pipette tip, working volume: 20 µL | SARSTEDT | 70.3020.210 | Or comparable material |
| Pipette tip, working volume: 200 µL | SARSTEDT | 70.3030.100 | Or comparable material |
| Serological pipette 10 mL | SARSTEDT | 86.1254.001 | Or comparable material |
| Serological pipette 25 mL | SARSTEDT | 86.1685.001 | Or comparable material |
| Serological pipette 5 mL | SARSTEDT | 86.1253.001 | Or comparable material |
| Tissue culture dish 60,0 mm/15,0 mm vented (Polystyrene) | Greiner bio-one | 628102 | Or comparable material |
| Tissue culture dish 100, suspension (Polystyrene) | SARSTEDT | 83.3902.500 | Or comparable material |
| Transfer pipette 6 mL | SARSTEDT | 86.1175 | Or comparable material |
| Tube 15 mL 120 mm x 17 mm PP | SARSTEDT | 62.554.502 | Or comparable material |
| Tube 50 mL 114mm x 28 mm PP | SARSTEDT | 62.5472.54 | Or comparable material |
Lab Prep
We developed a simple screening system for the evaluation of neuromuscular and general toxicity in zebrafish embryos. The modular system consists of electrodynamic transducers above which tissue culture dishes with embryos can be placed. Multiple such loudspeaker-tissue culture dish pairs can be combined. Vibrational stimuli generated by the electrodynamic transducers induce a characteristic startle and escape response in the embryos. A belt-driven linear drive sequentially positions a camera above each loudspeaker to record the movement of the embryos. In this way, alterations to the startle response due to lethality or neuromuscular toxicity of chemical compounds can be visualized and quantified. We present an example of the workflow for chemical compound screening using this system, including the preparation of embryos and treatment solutions, operation of the recording system, and data analysis to calculate benchmark concentration values of compounds active in the assay. The modular assembly based on commercially available simple components makes this system both economical and flexibly adaptable to the needs of particular laboratory setups and screening purposes.
We developed a simple screening system for the evaluation of neuromuscular and general toxicity in zebrafish embryos. The modular system consists of electrodynamic transducers above which tissue culture dishes with embryos can be placed. Multiple such loudspeaker-tissue culture dish pairs can be combined. Vibrational stimuli generated by the electrodynamic transducers induce a characteristic startle and escape response in the embryos. A belt-driven linear drive sequentially positions a camera above each loudspeaker to record the movement of the embryos. In this way, alterations to the startle response due to lethality or neuromuscular toxicity of chemical compounds can be visualized and quantified. We present an example of the workflow for chemical compound screening using this system, including the preparation of embryos and treatment solutions, operation of the recording system, and data analysis to calculate benchmark concentration values of compounds active in the assay. The modular assembly based on commercially available simple components makes this system both economical and flexibly adaptable to the needs of particular laboratory setups and screening purposes.
Procedure
We developed a simple screening system for the evaluation of neuromuscular and general toxicity in zebrafish embryos. The modular system consists of electrodynamic transducers above which tissue culture dishes with embryos can be placed. Multiple such loudspeaker-tissue culture dish pairs can be combined. Vibrational stimuli generated by the electrodynamic transducers induce a characteristic startle and escape response in the embryos. A belt-driven linear drive sequentially positions a camera above each loudspeaker to record the movement of the embryos. In this way, alterations to the startle response due to lethality or neuromuscular toxicity of chemical compounds can be visualized and quantified. We present an example of the workflow for chemical compound screening using this system, including the preparation of embryos and treatment solutions, operation of the recording system, and data analysis to calculate benchmark concentration values of compounds active in the assay. The modular assembly based on commercially available simple components makes this system both economical and flexibly adaptable to the needs of particular laboratory setups and screening purposes.
