Sign in to register to receive emails with product information.
Summary
In vitro mammalian cell culture has served as an invaluable tool in cell biology for several decades. Classically, monolayer cultures of adherent cells were grown on flat and rigid two-dimensional (2D) substrates, such as polystyrene or glass. However, many cells, when isolated from tissues and placed onto stiff planar 2D cell culture surfaces, such as tissue culture plastic, become progressively flatter, divide aberrantly, and lose their differentiated phenotype1,2. While these two-dimensional cell culture studies have played a pivotal role in furthering our understanding of many biological processes, they do not emulate in vivo conditions.
Abstract
In vitro mammalian cell culture has served as an invaluable tool in cell biology for several decades. Classically, monolayer cultures of adherent cells were grown on flat and rigid two-dimensional (2D) substrates, such as polystyrene or glass. However, many cells, when isolated from tissues and placed onto stiff planar 2D cell culture surfaces, such as tissue culture plastic, become progressively flatter, divide aberrantly, and lose their differentiated phenotype1,2. While these two-dimensional cell culture studies have played a pivotal role in furthering our understanding of many biological processes, they do not emulate in vivo conditions.
Introduction
In vitro mammalian cell culture has served as an invaluable tool in cell biology for several decades. Classically, monolayer cultures of adherent cells were grown on flat and rigid two-dimensional (2D) substrates, such as polystyrene or glass. However, many cells, when isolated from tissues and placed onto stiff planar 2D cell culture surfaces, such as tissue culture plastic, become progressively flatter, divide aberrantly, and lose their differentiated phenotype1,2. While these two-dimensional cell culture studies have played a pivotal role in furthering our understanding of many biological processes, they do not emulate in vivo conditions.
In the body, cells reside in a complex extracellular matrix (ECM) consisting of a three-dimensional (3D) architecture that allows direct interactions with neighboring cells and matrix molecules through biochemical and mechanical cues. Cell–cell and cell–ECM interactions establish a 3D communication network that maintains the specificity and homeostasis of the tissue3. Therefore it is not surprising that fundamental differences in the microenvironment of 2D and 3D cell culture systems influence various cellular behaviors including the way in which cells attach, spread and grow, their morphology and polarity, gene and protein expression, viability, proliferation, motility, differentiation, response to stimuli, cellular metabolism, and overall function1,2,4-16.
The 3D ECM surrounding a cell performs several critical functions. To begin with, it provides a complex, nanoscale architecture of structural proteins such as collagen, laminin, fibronectin and glycosaminoglycans to establish the mechanical properties inherent in the cellular microenvironment17,18. Cells sense these mechanics through their cell surface integrins and bind to specific adhesion motifs present on the ECM proteins and glycosaminoglycans. Furthermore, the ECM is vital for sequestering soluble biomolecules and growth factors, and releasing these signaling molecules with spatial-temporal control to guide processes such as cell migration, matrix degradation and deposition17,18. Thus, to truly mimic the native ECM, it is necessary to develop 3D culture models that exhibit the mechanical and chemical properties of the ECM, not only at the initial stage of seeding the cells, but rather, in a dynamic and tunable manner as the cells grow and develop. To address the requirements of different cell types and applications in the body, a vast array of materials and fabrication techniques have been employed to develop 3D microenvironments with different physical, biological, and mechanical characteristics. Each model comes with its own set of advantages and limitations, and one distinct model is not suitable for all applications.
Protocol
3D Culture systems
A. 3D Cultures using Hydrogels and Scaffolds
Hydrogels are comprised of networks of cross-linked polymer chains or complex protein or polysaccharide-like molecules of natural or synthetic origin. Due to their significant water content and range of tunable mechanical properties, hydrogels possess biophysical characteristics similar to natural tissue, and serve as highly effective matrices for 3D cell culture. Hydrogels can be used as stand-alone 3D matrices, as a coating, or as a method of encapsulating cells to be combined with other technologies, such as solid scaffolds, permeable supports, cellular microarrays, 3D printing or microfluidics devices. The morphology, growth and functionality of cells within the hydrogel depend on the presentation of biophysical and biochemical cues, as well as physical properties such as permeability and matrix stiffness.
Natural Hydrogels and Extracellular Matrices (ECMs)
Naturally derived hydrogels for cell culture are typically formed of proteins, polysaccharides and ECM components such as collagen, laminin, fibrin, hyaluronic acid, chitosan or Matrigelä (a reconstituted basement membrane extract from Engelbreth–Holm–Swarm mouse tumors containing a mixture of laminin, collagen IV, heparin sulfate proteoglycan, and nidogen/entactin19, Figure 1). Gels derived from natural sources are inherently biocompatible and bioactive20. They also promote many cellular functions due to the presence of various endogenous factors, which can be advantageous for supporting viability, proliferation, function and development of many cell types21.
However, natural hydrogels do present some disadvantages, including their isolation from animal-derived sources, and inherent batch-to-batch variability in composition. Also, they contain endogenous bioactive components such as growth factors that can be advantageous for creating some 3D models, but in other instances, can confound the specific cell behavior or response that is under investigation. For instance Matrigel activates pathways that control angiogenesis22,23, cancer cell motility24, and drug sensitivity25 while collagen type 1 alters the production of matrix metalloproteinases (MMPs), enzymes that degrade ECM components and allow tumor cell invasion26, cell sensitivity to anti-cancer drugs27, cell proliferation, and migration28,29.
To circumvent some of the issues associated with animal-derived biomaterials, matrices have been developed in organisms that are animal-free, or derived from recombinant nucleic acid technology. Hyaluronic acid (hyaluronan or HA) is an increasingly popular polysaccharide-based biologically derived matrix30-32. Most commercial grade HA is of bacterial origin, and characterized by high purity and homogeneous quality. These gels may be modified by the addition of ECM components for improving cell attachment and growth properties.
Synthetic Hydrogels and Extracellular Matrices
Synthetic hydrogels are an option for 3D cell culture applications when naturally derived biological matrices are unsuitable. Synthetic hydrogels are formed of purely non-natural molecules33 such as poly(ethylene glycol) (PEG)34, poly(vinyl alcohol)35, and poly(2-hydroxy ethyl methacrylate)36. They are biologically inert, but provide structural support for various cell types. They are also highly reproducible, allow for facile tuning of the mechanical properties, and are simple to process and manufacture.
Synthetic hydrogels can maintain the viability of encapsulated cells while allowing for ECM deposition as the hydrogel degrades37, thereby demonstrating that synthetic gels can function as 3D cell culture platforms in the absence of integrin-binding ligands. As an alternative approach inert synthetic hydrogels can be modified with appropriate biological components. An example of a synthetic hydrogel that is tunable is Corning PuraMatrix™ Peptide Hydrogel, a self-assembling, synthetic oligopeptide that exhibits nanometer scale fibers (Figure 2). Synthetic hydrogels can be customized with specific peptide sequences to improve cell attachment, cell homing, and other behaviors38 or the hydrogels can be supplemented with bioactive molecules (e.g., growth factors or ECM proteins). Furthermore, devices such as permeable supports (e.g., Transwell® inserts) can be incorporated into 3D culture models with hydrogel substrates to study the interaction between different cell types, soluble factors, and the culture environment.
Solid Scaffolds
Solid scaffolds for 3D cell culture are fabricated with a broad range of materials including metal, ceramics, glass, and polymers. In particular, polymers are a common choice for generating solid scaffolds of diverse size, varying structure, stiffness, porosity, and permeability39. A multitude of fabrication techniques are being utilized to generate solid scaffolds for 3D cell culture, including soft-lithography, electro-spinning, microarray, bio-printing, and many others. The major drawbacks of using solid scaffolds are limited scope for cell imaging and difficulties that are encountered when recovering cells from the matrix.
An important consideration for designing scaffolds for 3D cell culture is the scale and topography of the internal structures within the scaffold. In the body, the ECM provides an intricate nanoscale infrastructure to support cells, and presents an instructive background that governs their behavior40-44. Cells binding to scaffolds that exhibit microscale architectures usually flatten and spread out as they would if cultured on flat surfaces45. Even minute nanoscale level alterations in topography of the cell’s environment can elicit diverse effects on cell behavior46. Apart from scale and structure, the material used for constructing the scaffold, the surface chemical properties, matrix stiffness, permeability, and mechanical forces can significantly impact cell adhesion, growth, and behavior47.
B. Spheroids
Cellular spheroids are simple 3D models that can be generated from a wide range of cell types, which form spheroids naturally due to the tendency of adherent cells to aggregate. Common examples of spheroids include embryoid bodies, mammospheres, tumor spheroids, hepatospheres, and neurospheres. In particular, adherent cells tend to aggregate under circumstances that impede adhesion to cell culture substrates. Common matrix-free methods employed for generating spheroids include the use of ultra-low attachment surfaces for cell culture, or by maintaining the cells as suspension cultures in media (e.g. hanging drop technology, rotary cultures, and bioreactors). Several cell types also form spheroids in 3D hydrogels, and to a limited extent, in some solid scaffolds depending on the structural and mechanical properties of the material. The overall size of the spheroids is limited to a few hundred micrometers, beyond which, necrosis ensues within the core of the spheroids48.
As cells spontaneously aggregate into spheroids they naturally mimic various aspects of solid tissues and are equipped with inherent gradients for efficient diffusion of oxygen and nutrients as well as the removal of metabolic wastes. These cellular aggregates can emulate avascular, solid tumor behavior more effectively than standard 2D environments because spheroids, much like tumors, usually contain a heterogeneous population of surface-exposed and deeply buried cells, proliferating and non-proliferating cells, and well-oxygenated and hypoxic cells49. Additionally, differentiation of pluripotent stem cells typically involves the formation of spherical structures called embryoid bodies, an important step for subsequent cell differentiation studies in vitro. Spheroids thus represent an especially good physiological 3D model for studying solid tumorigenesis and stem cell differentiation. In addition, spheroids can be readily analyzed by imaging using light, fluorescence, and confocal microscopies, which is an advantage over more complex 3D cell culture models. Furthermore, it is relatively simple to mass-produce uniformly sized 3D spheroids making them highly amenable for many in vitro high-throughput (HTP) and toxicity screening applications.
C. Organoids
Another major advancement in stem cell differentiation is the formation of self-organizing 3D mini “organs” known as organoids. Organoids can recapitulate histological details and provide functional representation of multiple cell types that are present within the native organs. Organoids representative of intestinal50,51, retinal52, pancreatic53, mammary54, colonic55, and cerebral tissues56 have been developed recently using 3D models comprised of Matrigel Matrix. Figure 3 illustrates a schematic of 3D culture conditions that enabled efficient expansion of dissociated mouse embryonic pancreatic progenitors53. Another example is shown in Figure 4, where Lancaster et al. generated cerebral organoids with structural similarity to brain tissue57 . While, in vitro culture of organoids is a major step toward elucidating the principles of organ development and the mechanisms responsible for genetic diseases, the consistency and predictability of obtaining the same structures varies from sample to sample57. Similar to spheroids, they are also limited in size by the development of necrotic cores, limiting their usefulness for regenerative approaches.
D. Perfusion bioreactors
One of the major challenges of creating 3D cultures in vitro is the limited mass transfer distances required for nutritional supply and removal. Bioreactors address this challenge by controlling the physiological environment surrounding 3D constructs (i.e. nutrient supply, oxygen tension, waste removal, mechanical inputs, pH, and temperature). Bioreactors therefore are essential when larger tissue constructs are required and also to sustain tissues in vitro for extended periods of time, as increased transport through perfusion helps to avoid necrotic centers. In particular, bioreactors can be used to sustain long term cultures where dynamic changes can be monitored58. Additionally bioreactors control the size and shape of 3D cultures, and promote uniform cell distributions59-64. Bioreactors come in many different formats including: (direct) perfusion bioreactors65, (indirect) hollow fiber perfusion bioreactors66, perfusion bioreactors with mechanical stimulation67, rotating wall vessel bioreactors68, spinner flasks69, and orbital shakers70.
E. Other ways to address limited mass transfer
Another physiologically relevant solution to address limited mass transfer in large tissue constructs is to recreate the tissue vasculature (which provides and removes nutrients in vivo) with interconnected, vascularizable channels 71. As an artificial substitute to support high-density cell growth, ‘pseudovascularization’ through hollow fiber membrane bioreactors has also shown promising results66,72-74. The hollow fibers act as the blood vessels transferring culture medium throughout the tissue engineered constructs while the bulk can be seeded with extracellular matrix or other scaffold materials and cells.
Recent improvements in microscale engineering techniques, such as fabrication of microfluidic devices and microarrays have the potential to address nutrient supply issues by creating micro scale models. Microfluidic devices manipulate small volumes (10-9 to 10-6 L) to generate and precisely control dynamic fluid flow and spatio-temporal gradients, as well as deliver nutrients and other chemical cues to cells in a controlled manner75. Microfluidic networks have been incorporated directly within cell embedded hydrogels, as well as several other matrices, to enable efficient convective transport of nutrients and other soluble cues throughout the 3D scaffolds76. Microarrays consist of a solid support wherein small volumes of different biomolecules and cells can be positioned in defined locations, allowing multiplexed investigation of living cells and their responses to stimuli. Use of these technologies can improve cell viability and functions. Moreover, microfluidic technologies can increase throughput and significantly reduce the cost of culture due to low reagent volume requirements.
F. Cell considerations
Different types of cells can be incorporated in 3D models resulting in altered outcomes. There are three main classifications of cells that can be used: stem cells, primary cells, and immortalized cell lines. Stem cells are undifferentiated cells that can be stimulated to differentiate down different lineages. Stem cells are an attractive option since they self-renew in culture and can be used to create patient specific models. There are many types of stem cells which are defined by their stage of development. The different types include: adult stem cells (often from bone marrow or adipose tissue), fetal stem cells, cord blood stems, embryonic stem cells, or induced pluripotent stem cells. Primary cells on the other hand are obtained directly from a specific tissue and therefore are already committed to a specific lineage. They have a limited lifetime in culture and thus are more limited in numbers, and they eventually undergo senescence and stop proliferating. Immortalized cells in contrast have been altered to proliferate and evade cellular senescence. Gene delivery systems are also an option for modulating a specific phenotype in tissue engineered models.
In the body, communication between different cell types is critical to maintaining tissue homeostasis and specificity. Enhanced differentiation and survival is accomplished by co-culturing appropriate cell types77-81. Furthermore, co-cultures lead to increased ECM deposition82,83, improved functional outcome measurements84, proper morphology83,84, and vascular structures81 not achieved in monocultures. Besides mimicking normal physiology better, 3D co-culture models have been useful for studying complex interactions between cancer cells and other cell types by elucidating their contribution to tumor growth, vascularization and metastasis.
Unique cellular considerations for organs that interface with air have resulted in cultures of multiple cell types at an air-liquid interface, including lung85, epidermis86, cornea87, and inner cheek models88.
Culturing cells at an air-liquid interface using permeable supports has enabled the development ofin vitro3D models for drug discovery, including modeling of the lung epithelium. Using cells from diseased donors, these cellular models are used for studying a variety of pulmonary disorders.
Representative Results
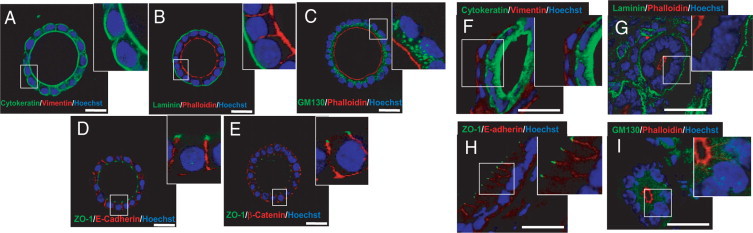
Figure 1. In vitro developed endometrial glands display epithelial apicobasal polarity. Double immunostaining of either in vitro developed glands cultured for 8 days in BIE medium (A–E) or cryostat sections obtained from mouse endometrium (F–I). A and F: Cytokeratin (green) and vimentin (red). B and G: Laminin (green) and phalloidin (red). C and I: GM130 (green) and phalloidin (red). D and H: ZO-1 (green) and E-cadherin (red). E: ZO-1 (green) and b-catenin (red). White scale bar = 20 mm. BIE medium = basal medium supplemented with 5ng/mL EGF and 1:100 dilution of ITS supplement and 3% fresh Matrigel™ Matrix; EGF = epidermal growth factor; ITS = insulin transferrin selenium (Eritja et al., 2010)89.
Figure 1 description - Glandular epithelial cell organization, signaling, and secretion more closely resemble the properties observed in vivo when cultured in a 3D environment, in contrast to the behavior that occurs on 2D surfaces 10,11. A 3D in vitro model for endometrial glands is shown in Figure 1. In this model, endometrial epithelial cells were cultured in medium containing Matrigel™ Matrix for 7-8 days followed by immunofluorescence staining of various polarity markers. Marker expression analysis confirmed that the glandular structures were composed exclusively of cells of epithelial origin (1A and 1F). Furthermore, spheroids representing endometrial glands displayed correct apical-basolateral polarity (1B and 1G), positioning of Golgi apparatus (1C and 1I), adherent junctions (ID and IH), and tight junctions (IE).
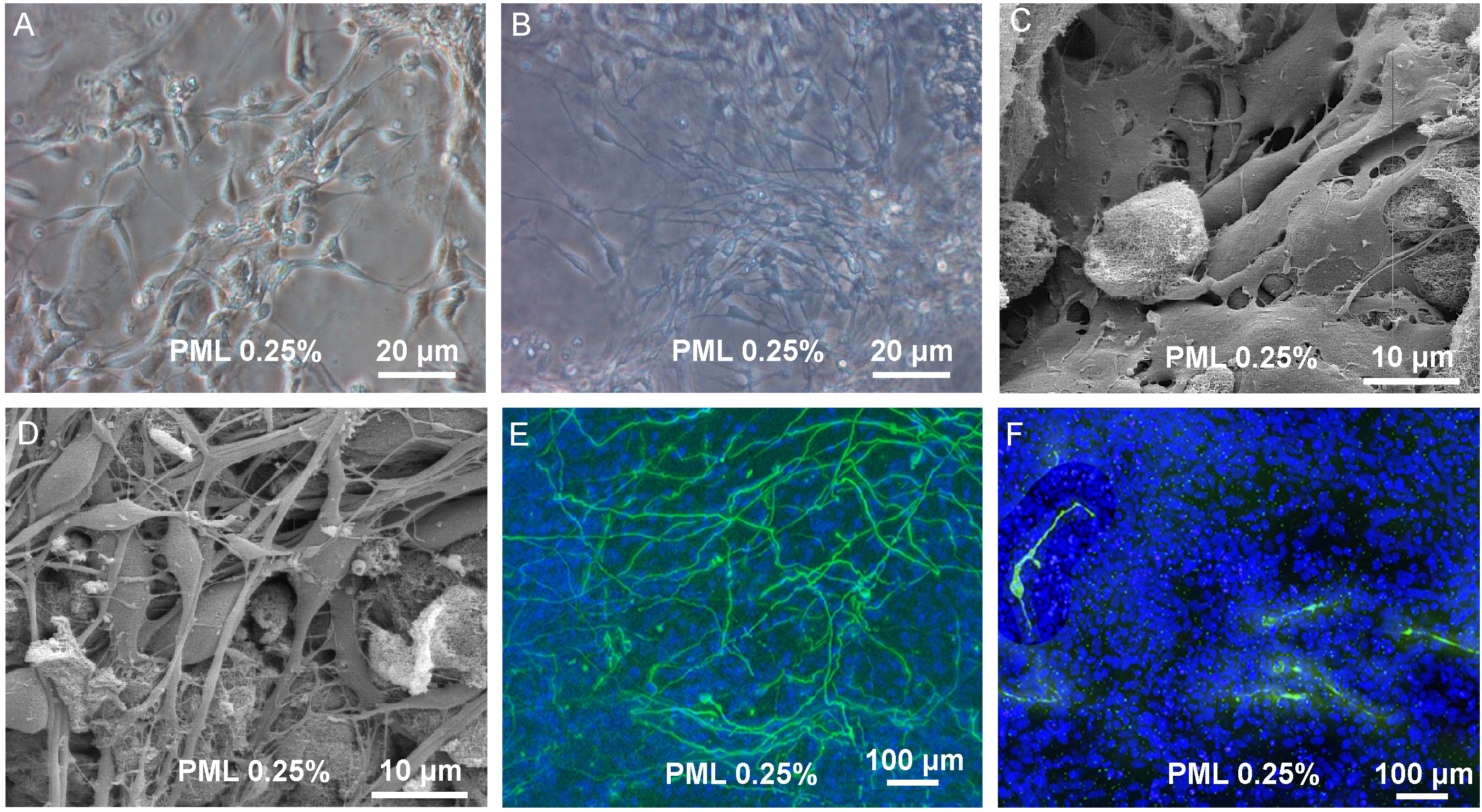
Figure 2. Proliferation and differentiation in 3D scaffold. (A + B) Transmission light picture of proliferating cells in PuraMatrix with 0.25 % laminin (PML) and differentiating cells in PML 0.25% (C + D). Scanning electron microscope picture of proliferating cells and differentiated cells in PML 0.25%. (B + D) Upon induction of differentiation one observes the development of a dense 3 dimensional network of processes. (E) Immunocytochemistry for bIII-tubulin and TH of uninduced cells in PML 0.25% and (F) Cells after 7 days of differentiation revealed a dense network of bIII-tubulin positive cells. TH+ cells were found to possess processes, but without building up a dense network (Ortinau et al. 2010)90.
Figure 2 description – In this study, Ortinau and colleagues used a synthetic hydrogel, PuraMatrix™ supplemented with laminin (PML 0.25%) for neuronal differentiation of human neural progenitor cells90. Using transmission light microscopy (FIGURE 2A + B) and scanning electron microscopy (FIGURE 2C + D) the researchers showed that neural progenitor cells can develop into a dense network of neuronal processes (FIGURE 2B + D). Furthermore, immunocytochemistry revealed that after 7 days of differentiation, the neuronal precursor cells began to express neuronal markers (bIII-tubulin and tyrosine hydroxylase (TH)) (FIGURE 2E + F). These observations demonstrate how functionalization with appropriate biological factors can convert inert synthetic matrices into useful in vitro models for supporting differentiation of stem and progenitor cells.

Fig. 3. Organoids recapitulate progenitor expansion and organized differentiation.
(A-E) Immunohistochemistry on sections of 7-day organoids showing that (A) all cells (DRAQ5, red nuclei) are epithelial (E-cadherin) and many proliferate [phospho-histone H3 (pHH3)] and (B, C) retain pancreatic markers PDX1, SOX9 and HNF1B. (C) Cells polarize and form tubes lined by mucin 1. (D) Exocrine differentiation (amylase) is seen at the periphery. (E) Endocrine differentiation (insulin) is detected in the center. The section in B is close to that in A, and the section in D is close to that in C. (F) Experimental scheme to test endocrine differentiation after back- transplantation of cells grown in organoid in a pancreatic niche. WT, wild type. (G-J) The cells that were first grown in vitro integrate into the host epithelium (white in G and green in H- J). Some remain progenitors (G; HNF1B), some become acinar (H; amylase) or ductal (I; DBA) and others become endocrine (glucagon or insulin). Insets are magnifications of the dashed boxes. Scale bars: 50 μm (Greggio et al., 2013)53.
Figure 3 description – Figure 3 illustrates a schematic of 3D culture conditions that enabled efficient expansion of dissociated mouse embryonic pancreatic progenitors53. By manipulating the medium composition, researchers generated either hollow spheres, which are mainly composed of pancreatic progenitors, or complex organoids that spontaneously undergo pancreatic morphogenesis and differentiation.
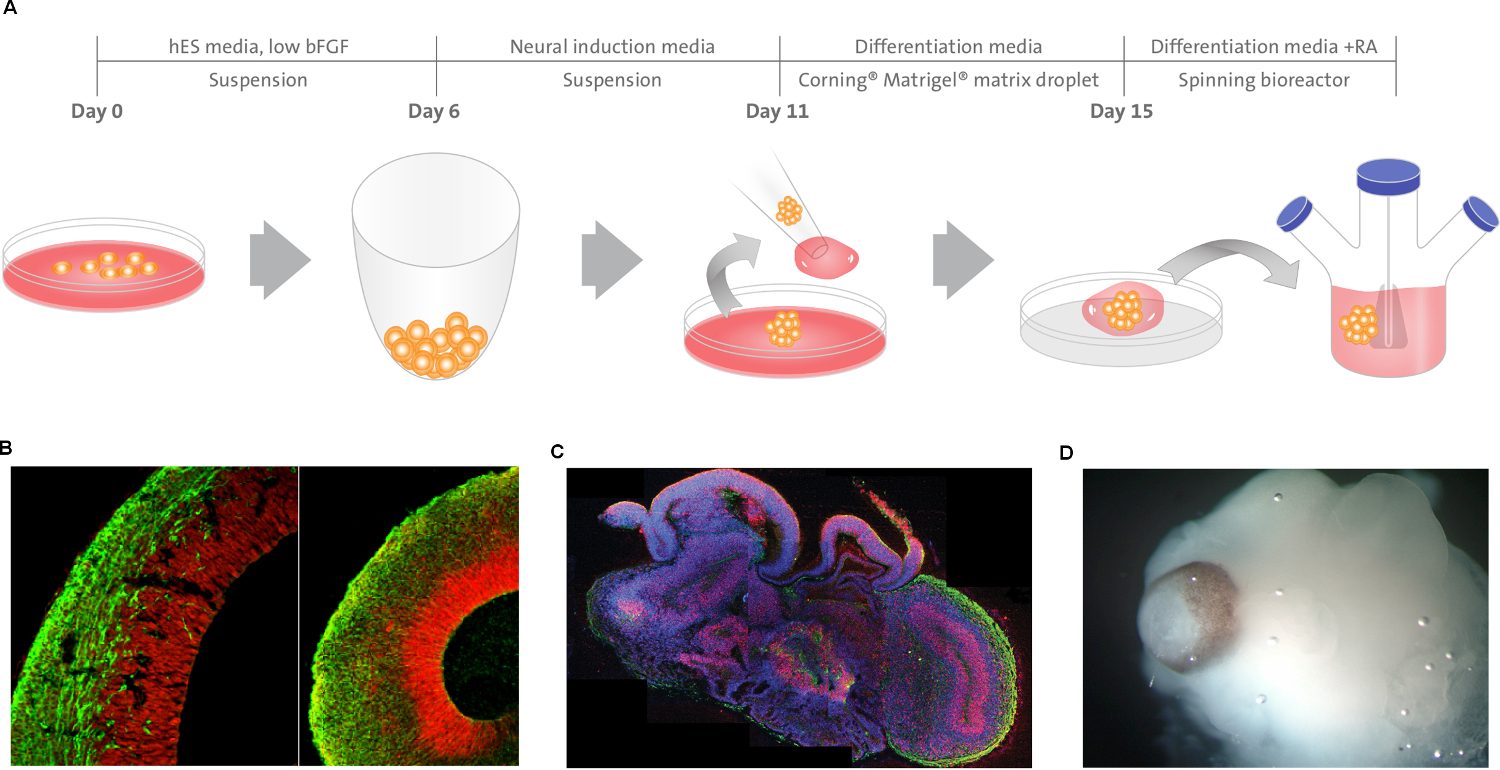
Figure 4 | Description of cerebral organoid culture system. A, Schematic
of the culture system used to generate cerebral organoids. Example images of each
stage are shown. bFGF, basic fibroblast growth factor; hES, human embryonic
stem cell; hPSCs, human pluripotent stem cells; RA, retinoic acid.
B, Neuroepithelial tissues generated using this approach (left) exhibited large fluid-filled cavities and typical apical localization of the neural N-cadherin
(arrow). These tissues were larger and more continuous than tissues grown in stationary suspension without Matrigel (right). C, Sectioning and immunohistochemistry revealed complex morphology with heterogeneous
regions containing neural progenitors (SOX2, red) and neurons (TUJ1, green) (arrow). D, Low-magnification bright-field images revealing fluid-filled cavities reminiscent of ventricles (white arrow) and retina tissue, as indicated by retinal pigmented epithelium (black arrow). Scale bars, 200 mm (Lancaster et al., 2013)57.
Figure 4 description – Lancaster et al. generated cerebral organoids with remarkable structural similarity to brain tissue57. These cerebral organoids grew larger than typical cellular spheroids and survived for weeks to months.
Discussion
Construction of physiologically relevant 3D tissues is a multifaceted research initiative where the resulting tissues can be used for many applications. Since 3D cell culture systems provide a more accurate environment for cell growth, tissue morphogenesis, and stem cell differentiation they have potential to be used for modeling tissue development, disease progression and for implantation in situ for large tissue defects. 3D cell culture models are also suited to bridge the gap between conventional 2D preclinical models for drug and toxicity screening and in vivo clinical studies in humans. Improved 3D preclinical models will better recapitulate pathobiological processes underlying diseases of specific tissues and organs in humans, and also more accurately predict physiological responses to therapeutic compounds.
Great strides have already been achieved over the past few decades in this field, yet there remain many practical limitations before 3D cell culture models can be widely implemented in many applications. To begin with, many of the 3D models are novel, and data generated using these methods need to be validated against established in vivo responses for calibration. Additionally, the outcome of 3D assays can be highly variable owing to a lack of standardized protocols and the use of heterogeneous, and sometimes multiple, cell populations. Nondestructive data analysis techniques (secreted factors, morphological changes, and/or non-invasive functional markers) are therefore essential for tracking the same 3D construct over culture. Also, some 3D matrices have limited permeability or poor diffusion dynamics and therefore must incorporate perfusion systems to maintain viability and function. Furthermore, cell recovery and visualization can be restricted in certain 3D scaffolds. Another major limitation is that many of the 3D culture models cannot be easily automated or scaled for high throughput screening.
These limitations however, are being overcome with emerging technologies and newer 3D in vitro assays. In vitro 3D cell culture models have progressed in supporting cell growth, tissue morphogenesis, stem cell differentiation, disease modeling, drug discovery, and toxicity testing. It is also evident that different 3D models with varying characteristics are required to meet the needs of specific cell types or applications. Combining newer technologies such as microarrays on a chip, microfluidics, or bio-printing with biologically relevant matrices could help to scale-up and drive some of these complex 3D cell culture models to highly predictive and relevant drug screening assays and toxicity tests, as well as provide novel systems for regenerative research.
Disclosures
No conflicts of interest declared.
References
- von der Mark, K., Gauss, V., von der Mark, H., & Muller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 267, 531-532 (1977).
- Petersen, O. W., Ronnov-Jessen, L., Howlett, A. R., & Bissell, M. J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 89, 9064-9068 (1992).
- Kleinman, H. K., Philp, D., & Hoffman, M. P. Role of the extracellular matrix in morphogenesis. Current opinion in biotechnology. 14, 526-532 (2003).
- McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 6, 483-495 (2004).
- Baker, B. M., & Chen, C. S. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. Journal of cell science. 125, 3015-3024, doi:10.1242/jcs.079509 (2012).
- Khetan, S., & Burdick, J. A. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 31, 8228-8234, doi:10.1016/j.biomaterials.2010.07.035 (2010).
- Brock, A. et al. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir : the ACS journal of surfaces and colloids. 19, 1611-1617 (2003).
- Thery, M. et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proceedings of the National Academy of Sciences of the United States of America. 103, 19771-19776, doi:10.1073/pnas.0609267103 (2006).
- Thery, M., Jimenez-Dalmaroni, A., Racine, V., Bornens, M., & Julicher, F. Experimental and theoretical study of mitotic spindle orientation. Nature. 447, 493-496, doi:10.1038/nature05786 (2007).
- Debnath, J., & Brugge, J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nature reviews. Cancer. 5, 675-688, doi:10.1038/nrc1695 (2005).
- Nelson, C. M., & Bissell, M. J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annual review of cell and developmental biology. 22, 287-309, doi:10.1146/annurev.cellbio.22.010305.104315 (2006).
- Singhvi, R. et al. Engineering cell shape and function. Science. 264, 696-698 (1994).
- Fuchs, E., Tumbar, T., & Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell. 116, 769-778 (2004).
- Gomez-Lechon, M. J. et al. Long-term expression of differentiated functions in hepatocytes cultured in three-dimensional collagen matrix. Journal of cellular physiology. 177, 553-562, doi:10.1002/(SICI)1097-4652(199812)177:4<553::AID-JCP6>3.0.CO;2-F (1998).
- Berthiaume, F., Moghe, P. V., Toner, M., & Yarmush, M. L. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 10, 1471-1484 (1996).
- Semino, C. E., Merok, J. R., Crane, G. G., Panagiotakos, G., & Zhang, S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation; research in biological diversity. 71, 262-270, doi:10.1046/j.1432-0436.2003.7104503.x (2003).
- Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science. 326, 1216-1219, doi:10.1126/science.1176009 (2009).
- Daley, W. P., Peters, S. B., & Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. Journal of cell science. 121, 255-264, doi:10.1242/jcs.006064 (2008).
- Kleinman, H. K., & Martin, G. R. Matrigel: basement membrane matrix with biological activity. Seminars in cancer biology. 15, 378-386, doi:10.1016/j.semcancer.2005.05.004 (2005).
- Dawson, E., Mapili, G., Erickson, K., Taqvi, S., & Roy, K. Biomaterials for stem cell differentiation. Advanced drug delivery reviews. 60, 215-228, doi:10.1016/j.addr.2007.08.037 (2008).
- Tibbitt, M. W., & Anseth, K. S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and bioengineering. 103, 655-663, doi:10.1002/bit.22361 (2009).
- Languino, L. R. et al. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. The Journal of cell biology. 109, 2455-2462 (1989).
- Zhou, Z. et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer research. 64, 4699-4702, doi:10.1158/0008-5472.CAN-04-0810 (2004).
- Carpenter, P. M. et al. Motility induction in breast carcinoma by mammary epithelial laminin 332 (laminin 5). Molecular cancer research : MCR. 7, 462-475, doi:10.1158/1541-7786.MCR-08-0148 (2009).
- Miyamoto, H. et al. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 28, 38-44 (2004).
- Ellerbroek, S. M., Wu, Y. I., Overall, C. M., & Stack, M. S. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. The Journal of biological chemistry. 276, 24833-24842, doi:10.1074/jbc.M005631200 (2001).
- Kim, Y. J., Bae, H. I., Kwon, O. K., & Choi, M. S. Three-dimensional gastric cancer cell culture using nanofiber scaffold for chemosensitivity test. International journal of biological macromolecules. 45, 65-71, doi:10.1016/j.ijbiomac.2009.04.003 (2009).
- Menke, A. et al. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer research. 61, 3508-3517 (2001).
- Hall, C. L. et al. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 10, 797-803 (2008).
- Gurski, L. A., Jha, A. K., Zhang, C., Jia, X., & Farach-Carson, M. C. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 30, 6076-6085, doi:10.1016/j.biomaterials.2009.07.054 (2009).
- David, L. et al. Hyaluronan hydrogel: an appropriate three-dimensional model for evaluation of anticancer drug sensitivity. Acta biomaterialia. 4, 256-263, doi:10.1016/j.actbio.2007.08.012 (2008).
- Chen, C. et al. Activation of CD44 facilitates DNA repair in T-cell lymphoma but has differential effects on apoptosis induced by chemotherapeutic agents and ionizing radiation. Leukemi., & lymphoma. 46, 1785-1795, doi:10.1080/10428190500232501 (2005).
- Shapiro, L., & Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 18, 583-590 (1997).
- Sawhney, A. S., Pathak, C. P., & Hubbell, J. A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(.alpha.-hydroxy acid) diacrylate macromers. Macromolecules. 26, 581-587, doi:10.1021/ma00056a005 (1993).
- Martens, P., & Anseth, K. S. Characterization of hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 41, 7715-7722, doi: http://dx.doi.org/10.1016/S0032-3861(00)00123-3 (2000).
- Chirila, T. V. et al. Poly(2-hydroxyethyl methacrylate) sponges as implant materials: in vivo and in vitro evaluation of cellular invasion. Biomaterials. 14, 26-38 (1993).
- Bryant, S. J., & Anseth, K. S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. Journal of biomedical materials research. 59, 63-72 (2002).
- Zhang, S. Beyond the Petri dish. Nature biotechnology. 22, 151-152, doi:10.1038/nbt0204-151 (2004).
- Lee, J., Cuddihy, M. J., & Kotov, N. A. Three-dimensional cell culture matrices: state of the art. Tissue engineering. Part B, Reviews. 14, 61-86, doi:10.1089/teb.2007.0150 (2008).
- Zagris, N. Extracellular matrix in development of the early embryo. Micron. 32, 427-438 (2001).
- Gullberg, D., & Ekblom, P. Extracellular matrix and its receptors during development. The International journal of developmental biology. 39, 845-854 (1995).
- Aumailley, M., & Gayraud, B. Structure and biological activity of the extracellular matrix. Journal of molecular medicine. 76, 253-265 (1998).
- Scott, J. E. Extracellular matrix, supramolecular organisation and shape. Journal of anatomy. 187 ( Pt 2), 259-269 (1995).
- Wallner, E. I., Yang, Q., Peterson, D. R., Wada, J., & Kanwar, Y. S. Relevance of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. The American journal of physiology. 275, F467-477 (1998).
- Stevens, M. M., & George, J. H. Exploring and engineering the cell surface interface. Science. 310, 1135-1138, doi:10.1126/science.1106587 (2005).
- Curtis, A., & Wilkinson, C. New depths in cell behaviour: reactions of cells to nanotopography. Biochemical Society symposium. 65, 15-26 (1999).
- Haycock, J. W. 3D cell culture: a review of current approaches and techniques. Methods in molecular biology. 695, 1-15, doi:10.1007/978-1-60761-984-0_1 (2011).
- Mueller-Klieser, W., Freyer, J. P., & Sutherland, R. M. Influence of glucose and oxygen supply conditions on the oxygenation of multicellular spheroids. British journal of cancer. 53, 345-353 (1986).
- Frieboes, H. B. et al. An integrated computational/experimental model of tumor invasion. Cancer research. 66, 1597-1604, doi:10.1158/0008-5472.CAN-05-3166 (2006).
- Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 470, 105-109, doi:10.1038/nature09691 (2011).
- McCracken, K. W., Howell, J. C., Wells, J. M., & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nature protocols. 6, 1920-1928, doi:10.1038/nprot.2011.410 (2011).
- Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 472, 51-56, doi:10.1038/nature09941 (2011).
- Greggio, C. et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 140, 4452-4462, doi:10.1242/dev.096628 (2013).
- Gray, R. S., Cheung, K. J., & Ewald, A. J. Cellular mechanisms regulating epithelial morphogenesis and cancer invasion. Current opinion in cell biology. 22, 640-650, doi:10.1016/j.ceb.2010.08.019 (2010).
- Wang, Y. et al. Capture and 3D culture of colonic crypts and colonoids in a microarray platform. Lab on a chip. 13, 4625-4634, doi:10.1039/c3lc50813g (2013).
- Bershteyn, M., & Kriegstein, A. R. Cerebral organoids in a dish: progress and prospects. Cell. 155, 19-20, doi:10.1016/j.cell.2013.09.010 (2013).
- Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature. 501, 373-379, doi:10.1038/nature12517 (2013).
- Bellas, E. et al. Sustained volume retention in vivo with adipocyte and lipoaspirate seeded silk scaffolds. Biomaterials. 34, 2960-2968, doi:10.1016/j.biomaterials.2013.01.058 (2013).
- Gaspar, D. A., Gomide, V., & Monteiro, F. J. The role of perfusion bioreactors in bone tissue engineering. Biomatter. 2, 167-175, doi:10.4161/biom.22170 (2012).
- Mabvuure, N., Hindocha, S., & Khan, W. S. The role of bioreactors in cartilage tissue engineering. Current stem cell researc., & therapy. 7, 287-292 (2012).
- Salter, E. et al. Bone tissue engineering bioreactors: a role in the clinic? Tissue engineering. Part B, Reviews. 18, 62-75, doi:10.1089/ten.TEB.2011.0209 (2012).
- Oragui, E., Nannaparaju, M., & Khan, W. S. The role of bioreactors in tissue engineering for musculoskeletal applications. The open orthopaedics journal. 5 Suppl 2, 267-270, doi:10.2174/1874325001105010267 (2011).
- Martin, I., Wendt, D., & Heberer, M. The role of bioreactors in tissue engineering. Trends in biotechnology. 22, 80-86, doi:10.1016/j.tibtech.2003.12.001 (2004).
- Sistino, J. J. Bioreactors for tissue engineering--a new role for perfusionists? The Journal of extra-corporeal technology. 35, 200-202 (2003).
- Flaibani, M., Luni, C., Sbalchiero, E., & Elvassore, N. Flow cytometric cell cycle analysis of muscle precursor cells cultured within 3D scaffolds in a perfusion bioreactor. Biotechnology progress. 25, 286-295, doi:10.1002/btpr.40 (2009).
- Mueller, D. et al. In-depth physiological characterization of primary human hepatocytes in a 3D hollow-fiber bioreactor. Journal of tissue engineering and regenerative medicine. 5, e207-218, doi:10.1002/term.418 (2011).
- Seidel, J. O. et al. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 41, 445-458 (2004).
- Lin, H. J., O'Shaughnessy, T. J., Kelly, J., & Ma, W. Neural stem cell differentiation in a cell-collagen-bioreactor culture system. Brain research. Developmental brain research. 153, 163-173, doi:10.1016/j.devbrainres.2004.08.010 (2004).
- Bellas, E., Marra, K., & Kaplan, D. L. P. Sustainable 3D tissue model of human adipose tissue. Tissue engineering. Part C, Methods., doi:10.1089/ten.TEC.2012.0620 (2013).
- Farrell, M. J. et al. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. Journal of biomechanics, doi:10.1016/j.jbiomech.2013.10.030 (2013).
- Tiruvannamalai-Annamalai, R., Armant, D. R., & Matthew, H. W. A glycosaminoglycan based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues. PloS one. 9, e84287, doi:10.1371/journal.pone.0084287 (2014).
- Chesnick, I. E. et al. Evaluation of bioreactor-cultivated bone by magnetic resonance microscopy and FTIR microspectroscopy. Bone. 40, 904-912, doi:10.1016/j.bone.2006.10.020 (2007).
- Bettahalli, N. M. et al. Integration of hollow fiber membranes improves nutrient supply in three-dimensional tissue constructs. Acta biomaterialia. 7, 3312-3324, doi:10.1016/j.actbio.2011.06.012 (2011).
- Ellis, M. J., & Chaudhuri, J. B. Poly(lactic-co-glycolic acid) hollow fibre membranes for use as a tissue engineering scaffold. Biotechnology and bioengineering. 96, 177-187, doi:10.1002/bit.21093 (2007).
- Huh, D., Hamilton, G. A., & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends in cell biology. 21, 745-754, doi:10.1016/j.tcb.2011.09.005 (2011).
- Whitesides, G. M. The origins and the future of microfluidics. Nature. 442, 368-373, doi:10.1038/nature05058 (2006).
- Ikuta, S., Sekino, N., Hara, T., Saito, Y., & Chida, K. Mouse epidermal keratinocytes in three-dimensional organotypic coculture with dermal fibroblasts form a stratified sheet resembling skin. Bioscience, biotechnology, and biochemistry. 70, 2669-2675 (2006).
- Futai, N., Gu, W., Song, J. W., & Takayama, S. Handheld recirculation system and customized media for microfluidic cell culture. Lab on a chip. 6, 149-154, doi:10.1039/b510901a (2006).
- Majumdar, D., Gao, Y., Li, D., & Webb, D. J. Co-culture of neurons and glia in a novel microfluidic platform. Journal of neuroscience methods. 196, 38-44, doi:10.1016/j.jneumeth.2010.12.024 (2011).
- Gingras, M., Beaulieu, M. M., Gagnon, V., Durham, H. D., & Berthod, F. In vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia. 56, 354-364, doi:10.1002/glia.20617 (2008).
- Hofmann, A. et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 29, 4217-4226, doi:10.1016/j.biomaterials.2008.07.024 (2008).
- Hayden, R. S., Quinn, K. P., Alonzo, C. A., Georgakoudi, I., & Kaplan, D. L. Quantitative characterization of mineralized silk film remodeling during long-term osteoblast-osteoclast co-culture. Biomaterials., doi:10.1016/j.biomaterials.2014.01.034 (2014).
- Wang, P. C., & Takezawa, T. Reconstruction of renal glomerular tissue using collagen vitrigel scaffold. Journal of bioscience and bioengineering. 99, 529-540, doi:10.1263/jbb.99.529 (2005).
- Hussain, A., Collins, G., Yip, D., & Cho, C. H. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnology and bioengineering. 110, 637-647, doi:10.1002/bit.24727 (2013).
- Choe, M. M., Tomei, A. A., & Swartz, M. A. Physiological 3D tissue model of the airway wall and mucosa. Nature protocols. 1, 357-362, doi:10.1038/nprot.2006.54 (2006).
- Margulis, A., Zhang, W., & Garlick, J. A. In vitro fabrication of engineered human skin. Methods in molecular biology. 289, 61-70 (2005).
- Liu, X. Y. et al. In vitro tissue engineering of lamellar cornea using human amniotic epithelial cells and rabbit cornea stroma. International journal of ophthalmology. 6, 425-429, doi:10.3980/j.issn.2222-3959.2013.04.03 (2013).
- Bhargava, S., Chapple, C. R., Bullock, A. J., Layton, C., & MacNeil, S. Tissue-engineered buccal mucosa for substitution urethroplasty. BJU international. 93, 807-811, doi:10.1111/j.1464-410X.2003.04723.x (2004).
- Eritja, N. et al. A novel three-dimensional culture system of polarized epithelial cells to study endometrial carcinogenesis. The American journal of pathology. 176, 2722-2731, doi:10.2353/ajpath.2010.090974 (2010).
- Ortinau, S. et al. Effect of 3D-scaffold formation on differentiation and survival in human neural progenitor cells. Biomedical engineering online. 9, 70, doi:10.1186/1475-925X-9-70 (2010).



