Forebrain Electrophysiological Recording in Larval Zebrafish
Summary
A simple method to record extracellular field potentials in the larval zebrafish forebrain is described. The method provides a robust in vivo read-out of seizure-like activity. This technique can be used with genetically modified zebrafish larvae carrying epilepsy-related genes or seizures evoked by administration of convulsant drugs.
Abstract
Epilepsy affects nearly 3 million people in the United States and up to 50 million people worldwide. Defined as the occurrence of spontaneous unprovoked seizures, epilepsy can be acquired as a result of an insult to the brain or a genetic mutation. Efforts to model seizures in animals have primarily utilized acquired insults (convulsant drugs, stimulation or brain injury) and genetic manipulations (antisense knockdown, homologous recombination or transgenesis) in rodents. Zebrafish are a vertebrate model system1-3 that could provide a valuable alternative to rodent-based epilepsy research. Zebrafish are used extensively in the study of vertebrate genetics or development, exhibit a high degree of genetic similarity to mammals and express homologs for ~85% of known human single-gene epilepsy mutations. Because of their small size (4-6 mm in length), zebrafish larvae can be maintained in fluid volumes as low as 100 μl during early development and arrayed in multi-well plates. Reagents can be added directly to the solution in which embryos develop, simplifying drug administration and enabling rapid in vivo screening of test compounds4. Synthetic oligonucleotides (morpholinos), mutagenesis, zinc finger nuclease and transgenic approaches can be used to rapidly generate gene knockdown or mutation in zebrafish5-7. These properties afford zebrafish studies an unprecedented statistical power analysis advantage over rodents in the study of neurological disorders such as epilepsy. Because the “gold standard” for epilepsy research is to monitor and analyze the abnormal electrical discharges that originate in a central brain structure (i.e., seizures), a method to efficiently record brain activity in larval zebrafish is described here. This method is an adaptation of conventional extracellular recording techniques and allows for stable long-term monitoring of brain activity in intact zebrafish larvae. Sample recordings are shown for acute seizures induced by bath application of convulsant drugs and spontaneous seizures recorded in a genetically modified fish.
Protocol
1. Egg Production and Collection
- Zebrafish husbandry follows standard procedures described previously8. Briefly, adult zebrafish are set up in breeding tanks with dividers in place. When the lights in the room come on the following morning, dividers are removed from breeding tanks and fish are allowed approximately 20 to 60 min of undisturbed mating time.
- Eggs from breeding tanks are collected in a strainer and rinsed with egg water. Eggs are then transferred to a Petri dish with egg water. Unfertilized eggs and debris are removed using a transfer pipette.
- Place Petri dish containing the collected eggs in an incubator (28-32 °C). After eggs hatch, around two days post-fertilization (dpf), remove chorion and any other debris with a transfer pipette.
- On desired day post-fertilization (3 to 8 dpf) remove Petri dish containing freely swimming larvae and place on lab bench at room temperature.
2. Microelectrode Pulling and Preparation
- Using a micropipette puller, pull a 1.2 mm OD borosilicate glass capillary into two needles and store in a 150 mm Petri dish or empty pipette box by laying over silly putty ramps. Needles can be pulled in advance. Different OD glass capillaries can be used depending on the type of amplifier headstage available.
- Backload the microelectrode with extracellular recording solution (2 M NaCl) using a 1 ml syringe with a Nalgene syringe filter (4-mm) and Microfil filament (Warner Precision Instruments) attached. Shake or tap the bolus toward the needle tip until there are few or no bubbles remaining.
3. Immobilization in Agar
- Prepare a fresh solution of 1.2% low melting point agar in egg water. Place agar solution in a water bath set at ~37 °C.
- Prepare a coverslip with recording chamber and place in freezer (5-10 min). The recording chamber should match that used on the electrophysiology rig. We use a low-profile open diamond bath imaging chamber from Warner Instruments (Model RC-26GLP).
- Using a Pasteur or transfer pipette, place one larval zebrafish in a small droplet of egg water in a clean Petri dish plate. To this droplet, add a droplet of solution containing an anesthetic (0.02% tricaine) and paralyzing agent (1 mg/ml α-bungarotoxin).
- Monitor zebrafish for loss of movement (5 to 10 min).
- Remove the coverslip/recording chamber from freezer. Place chamber on the stage of a stereomicroscope. Using a transfer pipette, mix several ml of 1.2% low melting point agarose with droplet and transfer solution (with fish) to the coverslip/recording chamber.
- Using a pipette tip or fine blunt needle, position larvae under the stereomicroscope so that the dorsal aspect of the fish is exposed to the agarose gel surface. Allow agarose to harden (5-10 min), then using a flat spatula transfer the agar block to a recording chamber set up on an electrophysiology rig.
3. Extracellular Field Recording
- Add 2-5 ml of zebrafish recording media to the recording chamber. If drug changes are necessary, perfuse chamber with recording solution at a rate of approximately 1 ml/min. No oxygenation of the recording solution is necessary.
- Insert a microelectrode (approximately 1 μm tip diameter, 2-7 MΩ) into the amplifier headstage mounted on a three-dimensional micromanipulator. Check that the micromanipulator is in a proper position to allow for a range of movement and adjustment. Bring the microelectrode tip into the plane of view of the microscope, high off the stage, and using coarse adjustment lower to a point just above and slightly in front of the head of the zebrafish.
- With the amplifier in “current-clamp” mode zero the electrode.
- Using coarse adjustment lower the electrode tip so that it just touches the surface of the zebrafish, slightly in front of the forebrain.
- Using fine adjustment steps advance the electrode tip until it punctures the skin of the zebrafish. Allow to settle and then advance the electrode several microns into forebrain.
- Record electrical activity in current-clamp mode using Axoscope software. Data are acquired at a sampling rate of 10 kHz.
Representative Results
Examples of electrographic seizure-like discharge recorded in the forebrain of an agar-embedded zebrafish larvae are shown in Figure 1. Large-amplitude multi-spike burst discharge in these samples was evoked by bath application of a convulsant drug, 40 mM pilocarpine (in A; 6 dpf) or 1 mM picrotoxin (in B; 8 dpf). In these recordings, immobilized and agar-embedded zebrafish are continuously monitored for up to 90 min. Fish remain viable under these recording conditions for up to 24 hr. Drugs are added to the bathing medium and normally diffuse into the agar to elicit activity in the larval zebrafish forebrain within 30 to 45 min. This method can be used with relatively small volumes of drug solution (2-5 ml) as larvae do not require continuous perfusion and can be recorded in a static bath configuration if necessary. In panel C, a spontaneous burst discharge is shown for a genetically modified zebrafish at 3 dpf; recording was made in zebrafish recording media. Morpholino oligonucleotide injection at the 1-2 cell stage was used to knockdown expression of a gene for Tuberous Sclerosis Complex (tsc1a), a pediatric form of epilepsy associated with seizures and autism. Extracellular recordings can also be obtained from the optic tectum. For examples, see Baraban et al. (2005)9 or Baraban et al. (2007)10. As shown here, the electrical events can vary in waveform and duration depending on the mechanism of action used to elicit seizure activity.
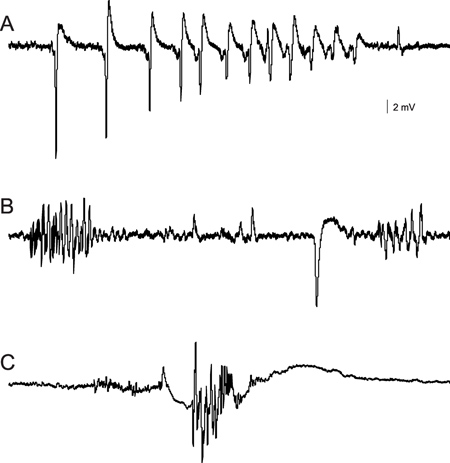
Figure 1. Extracellular field recordings of abnormal burst discharge activity recorded in the forebrain of immobilized and agar-embedded zebrafish larvae. Recording electrodes are positioned under visual observation on an Olympus BX50 upright microscope. Recordings in (A) and (B) were initiated approximately 40 to 45 min after drug application. (A) Multi-spike discharge recorded in pilocarpine, a muscarinic acetylcholine receptor antagonist. (B) Burst discharges recorded in picrotoxin, a GABA-A receptor antagonist. (C) A single multi-spike burst discharge recorded from a 3 dpf zebrafish larvae injected with a morpholino against tsc1a.
Discussion
The extracellular recording method presented here enables a very sensitive and rapid analysis of brain activity. These recordings are analogous to electroencephalographic (EEG) monitoring commonly used to evaluate the presence of abnormal electrical discharge (i.e., seizure) in rodent models of epilepsy11 and patients12. Extracellular recordings can be combined with pharmacological manipulations, as shown here. These types of recordings can also be used to evaluate potential epileptic phenotypes in genetically modified zebrafish. Mutant zebrafish are commonly available for many gene mutations identified in ENU mutagenesis screens (see Zebrafish International Resource Center, http://zebrafish.org/zirc/home/guide.php) or through emerging Tol2 transgenic7 and zinc finger nucleotide13 techniques. As demonstrated here, rapid gene knockdown using morpholino oligonucleotides followed by electrophysiological monitoring as early as 3 dpf provides an additional method to assess seizure-inducing gene defects. The recording electrode can easily be placed in any zebrafish structure including the optic tectum or cerebellum, and multiple recording electrodes can be used if needed. A limitation of these recordings is that it is difficult to assess the precise location of the recording electrode tip relative to specific brain nuclei. The availability of zebrafish carrying fluorescent reporters in specific nuclei or cell types will mitigate this limitation.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The author would like to thank Peter Castro and Matthew Dinday for their early efforts to establish zebrafish in the laboratory. This work was funded by the National Institutes of Health EUREKA grant (#R01NS079214-01).
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| Agarose low melting | Fisher-Scientific | BP1360-100 | Dissolve in embryo media at 1.2% |
| Recording media | Fisher-Scientific | BP3581, P330-3, BP410-1, BP214-500, D16-1, C77-500 | 1 mM NaCl, 2.9 mM KCl, 10 mM HEPES, 1.2 mM MgCl2, 10 mM Dextrose, 2.1 mM CaCl2 pH to approximately 7.3 with 1 N NaOH |
| Tricaine | Argent Labs | MS-222 | 0.02% |
| α-bungarotoxin | Tocris Bioscience | 2133 | 1 mg/ml |
| Capillary glass tubing | Warner Instruments | G120TF-3 | Pull to a resistance of 2 -7 MΩ |
| Patch clamp amplifier | Warner Instruments | PC-505B | We use a Warner amplifier in current-clamp mode; Gain set at 2 mV/pA and Bessel filter set at 2K. Comparable models can be used according to manufacturer’s instructions. |
| Filter/amplifier | Cygnus Technology | FLA-01 | We use a Cygnus pre-amplifier; Gain set at 10-20; Cut-off frequency set at 1-2K; Notch filter IN. Comparable models can be used according to manufacturer’s instructions. |
| Axon A/D board and Axoscope software | Molecular Devices | Axon Digidata 1320A; Axoscope 8.2 | Data is collected in Axoscope using gap-free acquisition mode; sampling at 10 kHz. Comparable models and programs can be used according to manufacturer’s instructions. |
| Egg water | Instant Ocean | 3 g Instant Ocean sea salt, 2 ml 0.1% methylene blue in 10 ml deionized water |
References
- Clark, K. J., et al. Stressing zebrafish for behavioral genetics. Reviews in Neuroscience. 22 (1), 49 (2011).
- Rinkwitz, S., et al. Zebrafish: an integrative system for neurogenomics and neurosciences. Progress in Neurobiology. 93 (2), 231 (2011).
- Penberthy, W. T., et al. The zebrafish as a model for human disease. Frontiers in Bioscience. 7, d1439 (2002).
- Letamendia, A., et al. Development and validation of an automated high-throughput system for zebrafish in vivo screenings. PLoS One. 7, e36690 (2012).
- Nasevicius, A., Ekker, S. C. Effective targeted gene ‘knockdown’ in zebrafish. Nature Genetics. 26 (2), 216 (2000).
- Haffter, P., et al. Mutations affecting development of the zebrafish inner ear and lateral line. Development. 123, 1 (1996).
- Suster, M. L., et al. Transgenesis in zebrafish with the tol2 transposon system. Methods Molecular Biology. 561, 41 (2009).
- Rosen, J. N., Sweeney, M. F., Mably, J. D. Microinjection of Zebrafish Embryos to Analyze Gene Function. J. Vis. Exp. (25), e1115 (2009).
- Baraban, S. C., et al. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 131 (3), 759 (2005).
- Baraban, S. C., et al. A large-scale mutagenesis screen to identify seizure-resistant zebrafish. Epilepsia. 48 (6), 1151 (2007).
- Williams, P., et al. The use of radiotelemetry to evaluate electrographic seizures in rats with kainate-induced epilepsy. Journal of Neuroscience Methods. 155 (1), 39 (2006).
- Marsh, E. D., et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 51 (4), 592 (2010).
- Zhu, C., et al. Evaluation and application of modularly assembled zinc-finger nucleases in zebrafish. Development. 138 (20), 4555 (2011).

