Ultrahigh Density Array of Vertically Aligned Small-molecular Organic Nanowires on Arbitrary Substrates
Summary
We report a simple method for fabricating an ultrahigh density array of vertically ordered small-molecular organic nanowires. This method allows for synthesis of complex heterostructured hybrid nanowire geometries, which can be inexpensively grown on arbitrary substrates. These structures have potential applications in organic electronics, optoelectronics, chemical sensing, photovoltaics and spintronics.
Abstract
In recent years π-conjugated organic semiconductors have emerged as the active material in a number of diverse applications including large-area, low-cost displays, photovoltaics, printable and flexible electronics and organic spin valves. Organics allow (a) low-cost, low-temperature processing and (b) molecular-level design of electronic, optical and spin transport characteristics. Such features are not readily available for mainstream inorganic semiconductors, which have enabled organics to carve a niche in the silicon-dominated electronics market. The first generation of organic-based devices has focused on thin film geometries, grown by physical vapor deposition or solution processing. However, it has been realized that organic nanostructures can be used to enhance performance of above-mentioned applications and significant effort has been invested in exploring methods for organic nanostructure fabrication.
A particularly interesting class of organic nanostructures is the one in which vertically oriented organic nanowires, nanorods or nanotubes are organized in a well-regimented, high-density array. Such structures are highly versatile and are ideal morphological architectures for various applications such as chemical sensors, split-dipole nanoantennas, photovoltaic devices with radially heterostructured “core-shell” nanowires, and memory devices with a cross-point geometry. Such architecture is generally realized by a template-directed approach. In the past this method has been used to grow metal and inorganic semiconductor nanowire arrays. More recently π-conjugated polymer nanowires have been grown within nanoporous templates. However, these approaches have had limited success in growing nanowires of technologically important π-conjugated small molecular weight organics, such as tris-8-hydroxyquinoline aluminum (Alq3), rubrene and methanofullerenes, which are commonly used in diverse areas including organic displays, photovoltaics, thin film transistors and spintronics.
Recently we have been able to address the above-mentioned issue by employing a novel “centrifugation-assisted” approach. This method therefore broadens the spectrum of organic materials that can be patterned in a vertically ordered nanowire array. Due to the technological importance of Alq3, rubrene and methanofullerenes, our method can be used to explore how the nanostructuring of these materials affects the performance of aforementioned organic devices. The purpose of this article is to describe the technical details of the above-mentioned protocol, demonstrate how this process can be extended to grow small-molecular organic nanowires on arbitrary substrates and finally, to discuss the critical steps, limitations, possible modifications, trouble-shooting and future applications.
Introduction
A template-assisted method is commonly used for the fabrication of vertically oriented nanowire arrays 1-3. This method allows straightforward fabrication of complex nanowire geometries such as an axially 4-6 or radially 7 heterostructured nanowire superlattice, which are often desirable in various electronic and optical applications. In addition, this is a low-cost, bottom-up nanosynthesis method with high throughput and versatility. As a result, template-directed methods have gained immense popularity among researchers worldwide 2,3.
The basic idea of the “template-directed method” is as follows. First a template is fabricated, which contains an array of vertically oriented cylindrical nanopores. Next, the desired material is deposited within the nanopores until the pores are filled. As a result the desired material adopts the pore morphology and forms a nanowire array hosted within the template. Finally, depending on the target application, the host template may be removed. However, this also destroys the vertical order. The geometry and dimensions of the final nanostructures mimic the pore morphology and hence synthesis of the host template is a critical part of the fabrication process.
Various types of nanoporous templates have been reported in literature 8. The most commonly used templates include (a) polymer track-etched membranes, (b) block copolymers and (c) anodic aluminum oxide (AAO) templates. To create the polymer track etched membranes a polymer foil is irradiated with high-energy ions, which completely penetrate the foil and leave latent ion tracks within the bulk foil 9. The tracks are then selectively etched to create nanosized channels within the polymer foil 9. The nanosized channels can be further widened by a suitable etching step. Key problems with this method are the non-uniformity of the nanochannels, lack of control of location, non-uniform relative distance between the channels, low density (number of channels per unit area ~108/cm2), and poorly ordered porous structure 1. In the block copolymer method a similar cylindrical nanoporous template is first created, followed by the growth of desired material within the pores 8.
In the past, methods (a) and (b) mentioned above have been used to fabricate polymer nanowires 8. However, these methods may not be suitable for synthesizing nanowires of any arbitrary organic material due to the potential absence of selective etching during post-processing steps. Post-processing typically involves removal of the host template, which for the above-mentioned templates would require organic solvents. Such solvents may have deleterious effect on the structural and physical properties of the organic nanowires. However, these templates work as ideal hosts for inorganic nanowires such as cobalt 10, nickel, copper and metallic multilayers 11, which remain unaffected in the etching process that removes the polymer host. Another potential challenge for the above-mentioned methods is the poor thermal stability of the host matrix at higher temperatures. High temperature annealing is often required to improve crystallinity of the organic nanowires, which indicates the necessity of good thermal stability of the host matrix.
Controlled electrochemical oxidation of aluminum (also known as “anodization” of aluminum) is a well-known industrial process and is commonly used in the automobile, cookware, aerospace and other industries to protect aluminum surface from corrosion 12. The nature of the oxidized aluminum (or “anodic alumina”) depends critically on the pH of the electrolyte used for anodization. For corrosion-resistance applications, anodization is generally performed with weak acids (pH ~5-7), which create a compact, non-porous, “barrier-type” alumina film 12. However, if the electrolyte is strongly acidic (pH < 4), the oxide becomes “porous” due to local dissolution of the oxide by the H+ ions. The local electric field across the oxide determines the local concentration of the H+ ions and hence surface pre-patterning prior to anodization offers some control over the final porous structure. The pores are cylindrical, with small diameter (~10-200 nm) and hence such nanoporous anodic alumina films have been used extensively in recent years for synthesizing nanowires of various materials 2,3.
Nanoporous anodic alumina templates offer better thermal stability, high pore density, long-range pore order, and excellent tunability of pore diameter, length, inter-pore separation and pore density via judicious choice of anodization parameters such as pH of the electrolyte and anodization voltage 2,3. Due to these reasons we choose AAO templates as the host matrix for the organic nanowire growth. Further, inorganic oxides such as alumina have high surface energy, thus facilitating uniform spreading of the organic solution (low surface energy) on the alumina surface 13. In addition, our goal is to grow these nanowire arrays directly on a conductive and/or transparent substrate. As a result, the pore is closed at the bottom end, which needs additional consideration as we describe below. Growth of nanowires within a through-pore template and subsequent transfer to the desired substrate is often undesirable due to poor interface quality and this method is not even feasible for short-length nanowires (or thin templates) due to poor mechanical stability of the thin templates.
π-conjugated organic materials can be broadly classified into two categories: (a) long-chain conjugated polymers and (b) small molecular weight organic semiconductors. Many groups have reported synthesis of long chain polymer nanowires within the cylindrical nanopores of an AAO template in the past. Comprehensive review on this topic is available in refs 8,14. However, synthesis of nanowires of commercially important small molecular organics (such as rubrene, tris-8-hydroxyquinoline aluminum (Alq3), and PCBM) in AAO is extremely rare. Physical vapor deposition of rubrene and Alq3 within the nanopores of AAO template has been reported by several groups 4,15-17. However, only a thin layer (~30 nm) of organics can be deposited within the pores (~50 nm diameter) and prolonged deposition tends to block the pore entrance 4,16,17. Complete pore filling can be achieved in this method if the pore diameter is sufficiently large (~200 nm) 15. Thus it is important to find an alternative method that is applicable for pore diameters in the sub 100 nm range.
Another approach that has been used for some other small-molecular organics is a so-called “template wetting” method 8,14. However, in most reports thick commercial templates (~50 μm) with both side open pores and large diameter (~200 nm) have been used. Such method has not produced nanowires in one-side closed pores as mentioned before, presumably due to the presence of trapped air pockets within the pores, which prevents infiltration of the solution within the pores. We have previously reported a novel method that overcomes these challenges and allows growth of small molecular organic nanowire arrays with arbitrary dimensions on any desired substrate. In what follows, we will describe the detailed protocol, potential limitations and future modifications.
Protocol
As mentioned above, the two key steps in the AAO-based fabrication process are (a) synthesis of the empty AAO template on arbitrary (primarily conductive and/or transparent) substrates (schematic description in Figure 1) and (b) growth of small molecular organic nanowires within the nanopores of the AAO template (Figure 2). In this section we provide a detailed description of these processes.
1. Growth of Anodic Aluminum Oxide (AAO) Templates on Conductive Aluminum Substrates
- Create nanoporous alumina templates by first polishing aluminum foils and then anodizing (or electrochemically oxidizing) them. Begin by cutting out small (~2 x 2 cm2) sheets of high purity unpolished aluminum (99.997%) with thickness 250 μm.
- In lieu of electropolishing, a simpler chemical polishing process 18 is used. Submerge a small number of 2 x 2 cm2 sheets in nitric-phosphoric acid etchant at 80 °C on a hotplate for 5 min.
Note: The nitric-phosphoric acid solution used to pre-treat the aluminum sheets is 15 parts 68% nitric acid and 85 parts 85% phosphoric acid. A polishing step is necessary prior to anodization because surface roughness of as-purchased aluminum is of the order of few microns, which creates a non-uniform electric field at the surface and prevents formation of an ordered pore array. In literature, electropolishing has been used extensively for this purpose 2,3. However, chemical polishing is a cheap and easy alternative, which also yields polished surfaces with comparable (or better) smoothness 18.
- After etching, neutralize the foils in 1 M sodium hydroxide for 20 min. These “chemically polished” foils are now ready to be anodized.
- Load the polished aluminum sheets in to flat cells and anodize them for 15 min with 3% oxalic acid at 40 V DC bias.
Note: For foil samples a two-step anodization process is performed to improve pore ordering 2,3,19. This first step will create a porous oxide layer on the Al surface and nano-scale dimples at the aluminum/alumina interface, which act as the initiation sites for pore growth during the second step of anodization.
- Etch the sample in chromic-phosphoric acid by removing it from the flat cell. Immerse the sample in a beaker of the etchant on a hot plate at 60 °C for ~30 min to remove the initial oxide layer.
- Repeat the anodization process (step 1.4) for 2.5 min while keeping all other parameters unchanged. Try to realign the foil in the flat cell such that the same area anodized in step (1.4) will again be exposed to the electrolyte.
Note: The time of the final anodization step determines the thickness of the final oxide layer and can be altered accordingly. Duration of 2.5 min. corresponds to a film thickness (pore length) of ~500 nm. At the end of the second step a well-ordered nanopore array is created in the anodic alumina layer. The anodization and etching cycle can be repeated to further improve pore ordering.
- Submerge the template in 5% phosphoric acid at room temperature for 40 min to thin the barrier layer at the bottom of the nanopores and widen the nanopore diameter. Final nanopore diameter after this step is ~60-70 nm.
2. Growth of AAO Templates on Transparent Substrates (Glass)
- Deposit the following multilayer system sequentially on cleaned glass slides: TiO2 (20 nm, atomic layer deposition), Au (7 nm, sputtering), Al (1 μm, sputtering).
Note: The Au layer acts as an electrode, required for anodization, and does not deteriorate transparency 20. The TiO2 acts as a transparent adhesion layer between the Au and glass substrate.
- Attach a foil electrode to the surface of the thin film of aluminum to be anodized using a conductive silver epoxy. This will result in a proper connection from power source to sample while improving current distribution.
Note: Since there is very little aluminum deposited on the glass substrate, polishing techniques mentioned before are not viable to flatten the aluminum surface. Instead, this protocol modifies the anodization procedure to incorporate another anodization/etching step.
- Load the sample in to the flat cell and anodize the aluminum thin film for 4 min using 3% oxalic acid at 30 V DC bias.
- Without removing the sample from the flat cell, rinse the cell out with DI water and etch the template in chromic-phosphoric acid at 60 °C for 1 hr by pouring the hot etchant in to the cell.
Note: The temperature of the etchant will immediately start to decrease once it has been poured in to the cell. Therefore, the duration of etching is increased to 1 hr from 30 min for the foil samples to ensure all oxidized film is removed.
- Rinse out the cell again and anodize a second time under the same conditions as the first; for 4 min, using 3% oxalic acid at 30 V DC bias.
- Repeat step (2.4). Without removing the sample from the flat cell, rinse the cell out with DI water and etch the template in chromic-phosphoric acid at 60 °C for 1 hr by pouring the hot etchant in to the cell.
- Rinse the cell out for the final time and perform the third (and last) anodization step, using 3% oxalic acid at 30 V DC. Monitor the current of the system to determine when to stop.
Note: The current needs to be monitored during the final anodization. After the first few seconds, the current stabilizes at around 1-2 mA. This indicates uniform anodization is taking place. Once the anodization process has consumed the remaining aluminum, the electrolyte solution (3% oxalic acid) will come in to contact with the underlying gold layer, which will cause a sharp increase in the anodization current (Figure 3). At this point, the anodization is stopped. The time should be roughly around the 4 min mark. This rise in current is not observed in foil samples (Figure 3) because a uniform barrier layer separates the solution and metal substrate.
- Perform a pore widening step, similar to the foil sample protocol by submerging the template in 5% phosphoric acid at room temperature for 40 min.
Note: This will widen the pores but since the anodization process has eaten through the barrier layer there is none left to thin. Figure 4 shows the layered structure of glass substrate / 20 nm TiO2 / 7 nm Au / 500 nm porous Al2O3 with the absence of a barrier layer and pores clearly exposed to underlying Au thin film. Figure 5a and 5b shows empty AAO templates on foil and glass substrates respectively.
3. Centrifuge Assisted Growth of Small Molecular Organic Nanowires Within the Pores of AAO Template
- Prepare a saturated solution of the small molecular organic in a suitable solvent.
Note: The following organic molecules and solvents have been used: rubrene in acetone, Alq3 in chloroform and PCBM in toluene. From here on PCBM is referred to as the molecule of interest.
- Load the templates in to the bottom of a centrifuge test tube such that the anodized area is facing the top of the test tube. The tube must be large enough to fit the sample inside.
Note: For foil samples, it is helpful to use a wafer of similar size to support the foil and prevent bending during centrifugation as described below. Figure 2 shows a schematic description of how the sample is mounted in the centrifuge.
- Use a pipette to fill the test tube with enough PCBM solution such that the template is completely submerged.
- Load the test tube in the centrifuge and run for 5 min at 6,000 rpm.
Note: If the samples were mounted in the test tube at an angle, ensure the test tube is mounted in such a way that the anodized surface is pointing towards the center of the centrifuge (Figure 2).
- Once the centrifuge has stopped, unload the test tubes and pour out the PCBM solution from the tubes.
- Remove the templates from the test tubes, or leave them at the bottom, for ~1 min to dry.
- Repeat steps 3.2-3.6 so that a total of 5-10 centrifuge runs have been performed.
Note: In situations where there is low solubility of the small molecule in its solvent, more centrifuge runs will help deposit more material in the nanopores.
- Remove the sample from the bottom of the test tube and use a cotton swab soaked in toluene (or the respective solvent) to gently clean the surface of the template, removing any material that is left over on the surface of the template.
Representative Results
As evidenced by the figures shown below (Figures 5 and 6), this centrifuge assisted drop casting method produces continuous nanowires. The nanowires, fabricated inside the pores of the AAO template, are vertically aligned, uniform, and electrically isolated from one another with capped bottoms. The diameter of the nanowires is determined by the diameter of pores in the template. They can be successfully fabricated on several different substrates, which lead to the potential application of these structures in many devices outlined later.
Because these results are in such high aspect ratio features, it stands to reason this method of deposition can also be expanded to other drop casting/coating methods of soluble materials such as coating textured substrates with PEDOT:PSS or PCBM for photovoltaic cell applications.
Figure 2, a schematic of the centrifuge before and during centrifugation, helps to visualize what is happening inside the centrifuge tube. Under centrifugation, the solution is forced against the substrate at a near perpendicular angle. This increases the “effective gravity” on the solution, forcing it in the pores. The result of this process is the filling of empty pores (Figure 4 and 5) with organic small-molecule material such that they form nanowires (Figure 6).
To further verify that the material inside the pores of Figure 6 is in fact PCBM nanowires, Raman spectroscopy of the filled templates has been performed. Studies are limited on the Raman spectrum of PCBM thin films and not existent, to our knowledge, on PCBM nanowires and nanotubes. However, we can compare the Raman data from our experiments to the limited literature results available as well as that of fullerene (C60) as the molecules are very similar in structure and show comparable vibrational modes from literature. We observe peaks at 1,430, 1,463, and 1,577 cm-1 (Figure 7), which correspond to the T1u(4), Ag(2), and Hg(8) modes, respectively. This matches well with literature values of 1,429, 1,470 and 1,575 cm-1 for pristine C60 21 and 1,429, 1,465 and 1,573 cm-1 for pristine PCBM for the same respective modes 22. This shows that there is no significant shift in the Raman peaks due to nanowire geometry and supports the fact that we do in fact have PCBM nanowires existent within our pores.
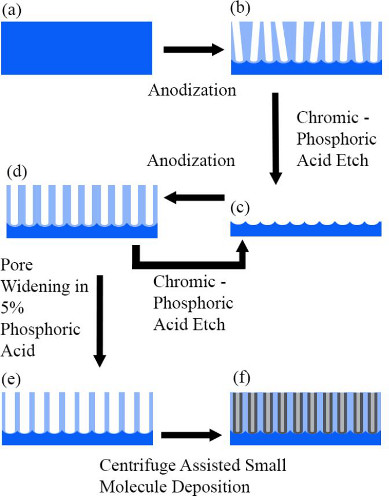
Figure 1. Schematic description of the organic nanowire synthesis. Steps (a) – (e) represents multi-step anodization and etching for fabrication of well-ordered nanopores. Step (f) represents organic nanowire growth.
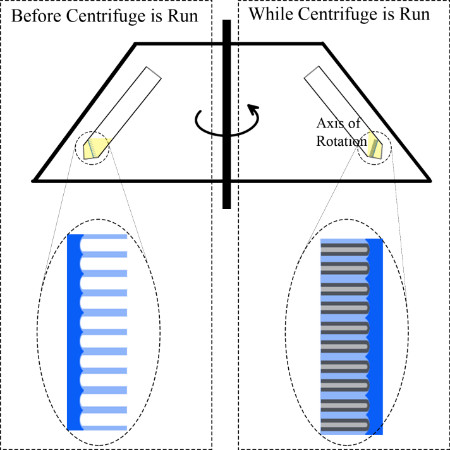
Figure 2. Schematic of the centrifuge and loading of the empty template in the test tube for organic nanowire growth.
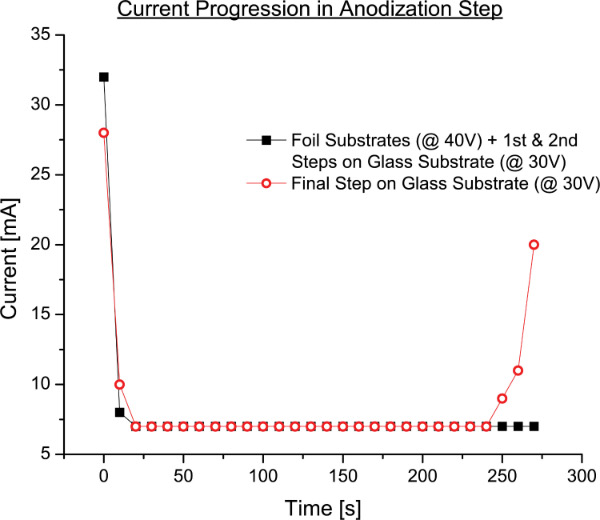
Figure 3. Anodization current as a function of time. For the final step of anodization on glass substrate, current rises when the entire aluminum is consumed and the electrolyte comes in contact with the underlying Au layer.
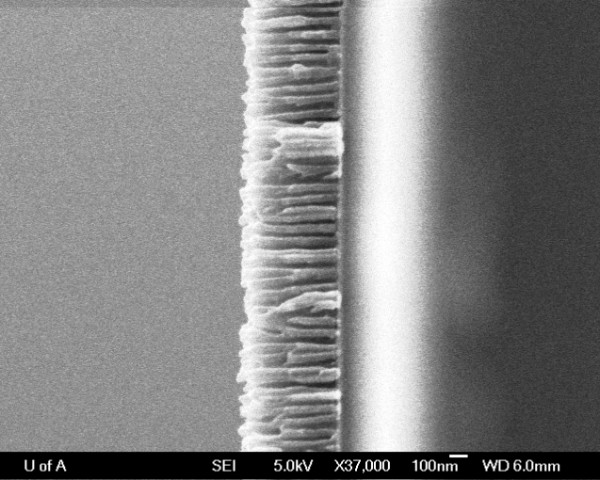
Figure 4. FESEM of layered structure (500 nm porous Al2O3 / 7 nm Au / 20 nm Ti02 / glass substrate) prior to Ag nanowire deposition.
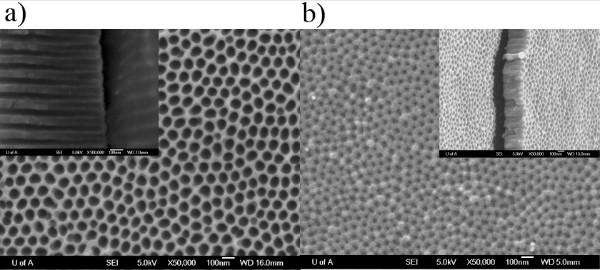
Figure 5. FESEM images of the empty template grown on (a) Al foil, (b) glass. The insets show the cross-sectional view and the main images show the top view. Click here to view larger figure.
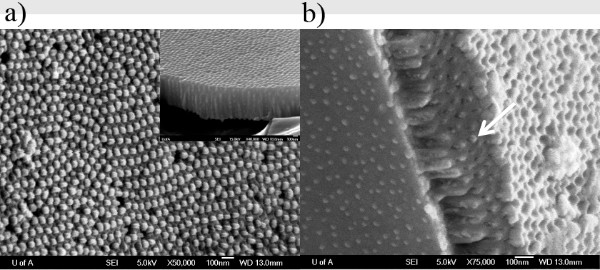
Figure 6. FESEM images of the filled template. (a) The main image shows the PCBM nanotube tips exposed from the AAO matrix. The PCBM nanotubes are closed at the bottom. The inset shows the cross-sectional view of the PCBM nanotubes grown within the AAO pores. (b) Cross sectional image of Alq3 nanowires (shown by arrow) protruding from the pores of the AAO template. Click here to view larger figure.
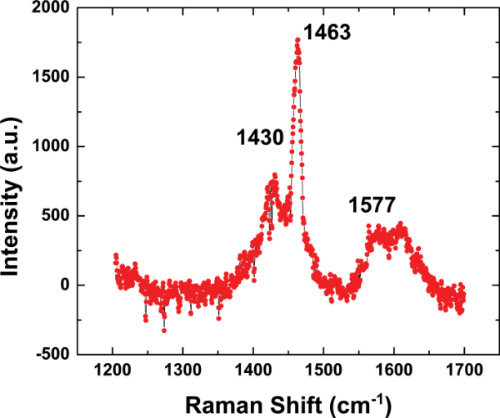
Figure 7. Raman Spectrum of PCBM nanowires imbedded in Al2O3 template.
Discussion
Physical Picture for Nanowire Growth
It is first important to fully understand the growth method of the organic nanowires. Once we know exactly how they grow and form themselves in the pores we can use this deposition method to engineer nanostructures, devices and materials. In the past, polymer nanowires have been fabricated using the template wetting procedure without the assistance of a centrifuge, but for some materials such as organic small molecules, we have found this to be ineffective. Due to surface chemistry between the solution and template as well as the air pocket trapped in the nanopore, the solution is unable to freely enter the pore. When the solution is under the influence of the centrifuge’s centrifugal force, it is in essence adding to the gravitational force the sample was already experiencing. Because the organic solution is obviously denser than the air occupying the pore it is forced to the pore bottom under the increased centrifugal force. Once the solution has overcome the forces keeping it from naturally entering the pores, it will continue to occupy the pore even after the centrifuge is stopped. The sample is then removed from the centrifuge and left out to dry. Because organic solvents evaporate relatively fast, the drying process only takes about a minute at room temperature. The solution in the pore nearest to the pore opening will evaporate first and progress lower and lower until the solution at the pore bottom has evaporated and all that is left in the pore is the organic small molecules. As the solution evaporates and exits the pore nearest to the pore opening, the small molecules that were dissolved in that volume of solvent get pushed to the pore walls and remain there under Van der Waals forces. The solvent continues to evaporate down the length of the pore continually depositing material on the pore walls through the entire length of the pore, creating a continuous and hollow nanotube inside the pore. Once this process reaches the pore bottom, there will be a slight excess of small molecules, which will coat the pore walls at the bottom as well as the barrier layer at the pore bottom. This will create a “capped” end to the nanotube at the pore bottom that can be very beneficial to devices needing proper electrical contact to the nanotube material. Repeated centrifugation will result in solid nanowires instead of hollow nanotubes.
Critical parameters
One critical parameter that needs to be considered in the deposition process is the RPM of the centrifuge. If the RPM is too low, the centrifugal force will not be strong enough to replace the trapped air pockets with organic solution. For most centrifuge setups, the maximum RPM setting should be able to be used. As long as foil substrate samples are supported with a strong enough backing (wafer, glass or other substrate), there should be no damage done to the template even in conical shaped centrifuge tubes.
The concentration of the small molecule in the solvent of choice is also an important factor in the process. The more soluble a material is in its solvent, the more material will be deposited in the pore. For most applications, researchers should use a saturated solution of the material in solvent to maximize the amount of material in the pore. However, one should theoretically be able to control the wall thickness of the nanotube by manipulating the solution concentration. A lower concentration would limit the number of molecules available to form a tube and result in a thinner tube wall.
Run time or length of centrifugation is another parameter we can control. This parameter does affect the final structure that is formed. Run time needs to be long enough to ensure all pores have been filled with solution, which could be different for different setups (solvent and template combinations). For our particular setup, we have found that run times of 5 min will suffice. For solutions that have low solubility in solvents, we can repeat the deposition procedure a few times. The more centrifuge runs we perform, the more material there should be deposited in the pore. Increasing the number of runs can help deposit more material in the pores and increase the chances of nanotube formation in low concentration solutions.
Anodization on aluminum foils has been explored extensively and is a well-known process 2,3. While anodization on glass in nothing fundamentally new, it is less developed than foil anodization and incorporates more challenges. Due to the thin gold electrode, a high current density can result when the aluminum is completely anodized and the acid comes in contact with the electrode (Figure 3). It is important to keep the voltage at a level lower than that of foil anodization to avoid pore merging and overheating/burning of the alumina template.
Potential Benefits and Drawbacks
The main benefits this technique has over other forms of organic small molecule deposition are that it is low-cost, simple and does not require any complex experimental setup. The only equipment needed for this technique is a centrifuge, which is relatively inexpensive and readily available in most nanofabrication facilities when compared with complex vacuum chambers, pumps and power sources needed for evaporation of organic materials in PVD techniques. This technique also allows for extremely high aspect ratio features to be deposited in and features where there is not a direct line of sight from crucible or source material to the location of deposition, which is needed in all PVD type deposition techniques. It is also compatible with other solution processing techniques, which will become more and more common as organic electronic devices become more commercially viable.
While this is a new deposition technique that enables users to easily deposit organic molecules in to high aspect ratio features, it does have some drawbacks. Using this technique, we are limited to molecules that can be processed in solution. If the material does not have the ability to dissolve in some solvent, we will not have a carrier to transfer it in to the pore. Also, because this is a template fabrication technique, the limitations we encounter for producing the template will also limit the structures we can grow inside them. This technique does not have the ability to control nanowire length within the pore or vary any other parameter of the wire after the template is grown. Once the template is formed, the entire length of the pore will be deposited in, which will determine the nanotube length. The final pore diameter will determine the nanotube diameter. However, fortunately the AAO template growth process is heavily investigated 2,3 and enormous control over nanopore geometry is available, including the possibility of creating branched and modulated diameter pores 23. Therefore this is presumably not a very serious limitation.
Future Directions, Modifications and Potential Applications
This is a novel deposition technique with many features that need to be characterized and investigated. There is still a lot of work to do to determine the capabilities and limitations of this technique. To this point, only a fixed angle centrifuge has been used for deposition. This type of centrifuge makes mounting the substrate at the proper angle a challenge. One way to circumvent this problem is to use a variable angle centrifuge with flat bottom test tubes. As the centrifuge picks up speed, the arms of the centrifuge that hold the test tubes will swing out such that the centrifugal force will remain perpendicular to the flat bottom of the test tube. This will ensure that the solution will always be directed parallel to the pore length and that no component of the force will push the solution to the side of the template. Further work also needs to be done to better understand how manipulating the critical steps of the process affect the final structure. The effect of annealing on crystallinity should also be examined to better understand the physical properties of the resulting nanotubes.
In future, this versatile deposition technique may find application in diverse areas such as memory devices 24,25, organic photovoltaics 26-31, plasmonics 32, chemical sensors 33,34, OLEDs 35 and organic nanowire FETs 36,37. Two structures that are currently being explored in our group are axially and radially heterostructured organic nanowire devices. We have already fabricated axially heterostructured metal-organic hybrid nanowire structures by electrodepositing metal nanowire in the bottom of the pore and filling the remaining portion with organics 5,6. The work on fabricating coaxial organic nanowires is currently in progress and such structures are promising candidates for high-efficiency organic photovoltaic devices 31,38-40.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work has been financially supported by NSERC, CSEE, nanoBridge and TRLabs.
Materials
| Reagents | |||
| Toluene | Fisher Scientific | T324-4 | |
| 68% Nitric Acid | Fisher Scientific | A200-212 | |
| 85% Phosphoric Acid | Fisher Scientific | A242-4 | |
| 10% Chromic Acid | RICCA Chemical Company | 2077-32 | |
| 10% Oxalic Acid | Alfa Aesar | FW.90.04 | |
| Chloroform | Fisher Scientific | C607-4 | |
| Aluminum Sheets | Alfa Aesar | 7429-90-5 | |
| PCBM | Nano-C | Nano-CPCBM-BF | |
| Alq3 | Sigma Aldrich | 444561-5G | |
| Rubrene | Sigma Aldrich | 551112-1G | |
| Equipment | |||
| FlexAL Atomic Layer Deposition (ALD) | Oxford Instruments | For deposition of TiO2 | |
| PVD Sputter System | Kurt J. Lesker | For deposition of Au & Al | |
| Flat Cell | Princeton Applied Research | K0235 | For anodization of Al |
| Centrifuge | HERMLE Labnet | Z206 A | For deposition of organic nanowires |
References
- Martin, C. R. Nanomaterials: a membrane-based synthetic approach. Science. , (1994).
- Pramanik, S., Kanchibotla, B., Sarkar, S., Tepper, G., Bandyopadhyay, S. Electrochemical Self-Assembly of Nanostructures: Fabrication and Device Applications. Encyclopedia of Nanoscience and Nanotechnology. 13, 273-332 (2011).
- Kanchibotla, B., Pramanik, S., Bandyopadhyay, S. Self-assembly of nanostructures using nanoporous alumina template. Nano and Molecular Electronics Handbook. Chapter 9, (2007).
- Pramanik, S., Stefanita, C. -. G., et al. Observation of extremely long spin relaxation times in an organic nanowire spin valve. Nat. Nano. 2 (4), 216-219 (2007).
- Alam, K. M., Bodepudi, S. C., Starko-Bowes, R., Pramanik, S. Suppression of spin relaxation in rubrene nanowire spin valves. Applied Physics Letters. 101 (19), 192403 (2012).
- Alam, K. M., Singh, A. P., Starko-Bowes, R., Bodepudi, S. C., Pramanik, S. Template-Assisted Synthesis of π-Conjugated Molecular Organic Nanowires in the Sub-100 nm Regime and Device Implications. Advanced Functional Materials. 22 (15), 3298-3306 (2012).
- Zhang, D., Luo, L., Liao, Q., Wang, H., Fu, H., Yao, J. Polypyrrole/ZnS Core/Shell Coaxial Nanowires Prepared by Anodic Aluminum Oxide Template Methods. The Journal of Physical Chemistry C. 115 (5), 2360-2365 (2011).
- Kim, F. S., Ren, G., Jenekhe, S. A. One-Dimensional Nanostructures of π-Conjugated Molecular Systems: Assembly, Properties, and Applications from Photovoltaics, Sensors, and Nanophotonics to Nanoelectronics. Chem. Mater. 23 (3), 682-732 (2010).
- Brock, T. D. . Membrane filtration: a user’s guide and reference manual. , (1983).
- Valizadeh, S., George, J., Leisner, P., Hultman, L. Electrochemical deposition of Co nanowire arrays; quantitative consideration of concentration profiles. Electrochimica Acta. 47 (6), 865-874 (2001).
- Nasirpouri, F., Southern, P., Ghorbani, M., Iraji zad, A., Schwarzacher, W. GMR in multilayered nanowires electrodeposited in track-etched polyester and polycarbonate membranes. Journal of Magnetism and Magnetic Materials. 308 (1), 35-39 (2007).
- Diggle, J. W., Downie, T. C., Goulding, C. W. Anodic oxide films on aluminum. Chemical Reviews. 69 (3), 365-405 (1969).
- Steinhart, M., Wehrspohn, R. B., Gösele, U., Wendorff, J. H. Nanotubes by Template Wetting: A Modular Assembly System. Angewandte Chemie International Edition. 43 (11), 1334-1344 (2004).
- Al-Kaysi, R. O., Ghaddar, T. H., Guirado, G. Fabrication of One-Dimensional Organic Nanostructures Using Anodic Aluminum Oxide Templates. Journal of Nanomaterials. 2009, 1-14 (2009).
- Lee, J. W., Kim, K., et al. Light-Emitting Rubrene Nanowire Arrays: A Comparison with Rubrene Single Crystals. Advanced Functional Materials. 19 (5), 704-710 (2009).
- Pramanik, S., Bandyopadhyay, S., Garre, K., Cahay, M. Normal and inverse spin-valve effect in organic semiconductor nanowires and the background monotonic magnetoresistance. Physical Review B. 74 (23), 235329 (2006).
- Alam, K. M., Pramanik, S. High-field magnetoresistance in nanowire organic spin valves. Physical Review B. 83 (24), 245206 (2011).
- Alam, K. M., Singh, A. P., Bodepudi, S. C., Pramanik, S. Fabrication of hexagonally ordered nanopores in anodic alumina: An alternative pretreatment. Surface Science. 605 (3-4), 441-449 (2011).
- Masuda, H., Hasegwa, F., Ono, S. Self-Ordering of Cell Arrangement of Anodic Porous Alumina Formed in Sulfuric Acid Solution. Journal of The Electrochemical Society. 144 (5), L127-L130 (1997).
- Stec, H. M., Williams, R. J., Jones, T. S., Hatton, R. A. Ultrathin Transparent Au Electrodes for Organic Photovoltaics Fabricated Using a Mixed Mono-Molecular Nucleation Layer. Advanced Functional Materials. 21 (9), 1709-1716 (2011).
- Schettino, V., Pagliai, M., Ciabini, L., Cardini, G. The Vibrational Spectrum of Fullerene C60. J. Phys. Chem. A. 105, 11192-11196 (2001).
- Lee, Y., Lee, S., Kim, K., Lee, J., Han, K., Kim, J., Joo, J. Single nanoparticle of organic p-type and n-type hybrid materials: nanoscale phase separation and photovoltaic effect. J. Mater. Chem. 22, 2485-2490 (2012).
- Bodepudi, S. C., Bachman, D., Pramanik, S. Fabrication of Highly Ordered Cylindrical Nanopores with Modulated Diameter Using Anodic Alumina. , 1-4 (2011).
- Vlad, A., Melinte, S., Mátéfi-Tempfli, M., Piraux, L., Mátéfi-Tempfli, S. Vertical Nanowire Architectures: Statistical Processing of Porous Templates Towards Discrete Nanochannel Integration. Small. 6 (18), 1974-1980 (2010).
- Jo, S. H., Kim, K. -. H., Lu, W. High-Density Crossbar Arrays Based on a Si Memristive System. Nano Letters. 9 (2), 870-874 (2009).
- Haberkorn, N., Gutmann, J. S., Theato, P. Template-Assisted Fabrication of Free-Standing Nanorod Arrays of a Hole-Conducting Cross-Linked Triphenylamine Derivative: Toward Ordered Bulk-Heterojunction Solar Cells. ACS Nano. 3 (6), 1415-1422 (2009).
- Aryal, M., Buyukserin, F., et al. Imprinted large-scale high density polymer nanopillars for organic solar cells. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures. 26 (6), 2562 (2008).
- Lee, J. H., Kim, D. W., et al. Enhanced solar-cell efficiency in bulk-heterojunction polymer systems obtained by nanoimprinting with commercially available AAO membrane filters. Small (Weinheim an Der Bergstrasse, Germany). 5 (19), 2139-2143 (2009).
- Allen, J. E., Black, C. T. Improved Power Conversion Efficiency in Bulk Heterojunction Organic Solar Cells with Radial Electron Contacts. ACS Nano. 5 (10), 7986-7991 (2011).
- Slota, J. E., He, X., Huck, W. T. S. Controlling nanoscale morphology in polymer photovoltaic devices. Nano Today. 5 (3), 231-242 (2010).
- Chidichimo, G., Filippelli, L. Organic Solar Cells: Problems and Perspectives. International Journal of Photoenergy. 2010, 1-11 (2010).
- O’Carroll, D. M., Fakonas, J. S., Callahan, D. M., Schierhorn, M., Atwater, H. A. Metal-Polymer-Metal Split-Dipole Nanoantennas. Advanced Materials. 24 (23), (2012).
- Zheng, J. Y., Yan, Y., et al. Hydrogen Peroxide Vapor Sensing with Organic Core/Sheath Nanowire Optical Waveguides. Advanced Materials. 24 (35), (2012).
- Zhang, L., Meng, F., et al. A novel ammonia sensor based on high density, small diameter polypyrrole nanowire arrays. Sensors and Actuators B: Chemical. 142 (1), 204-209 (2009).
- Cui, Q. H., Jiang, L., Zhang, C., Zhao, Y. S., Hu, W., Yao, J. Coaxial Organic p-n Heterojunction Nanowire Arrays: One-Step Synthesis and Photoelectric Properties. Advanced Materials. 24 (17), 2332-2336 (2012).
- Duvail, J. L., Long, Y., Cuenot, S., Chen, Z., Gu, C. Tuning electrical properties of conjugated polymer nanowires with the diameter. Applied Physics Letters. 90, 102114 (2007).
- Briseno, A. L., Mannsfeld, S. C. B., Jenekhe, S. A., Bao, Z., Xia, Y. Introducing organic nanowire transistors. Materials Today. 11 (4), 38-47 (2008).
- Kippelen, B., Brédas, J. -. L. Organic photovoltaics. Energy & Environmental Science. 2 (3), 251-261 (2009).
- Günes, S., Neugebauer, H., Sariciftci, N. S. Conjugated polymer-based organic solar cells. Chemical Reviews. 107 (4), 1324-1338 (2007).
- Coakley, K. M., McGehee, M. D. Conjugated Polymer Photovoltaic Cells. Chem. Mater. 16 (23), 4533-4542 (2004).

