Towards Biomimicking Wood: Fabricated Free-standing Films of Nanocellulose, Lignin, and a Synthetic Polycation
Summary
The objective of this research was to form synthetic plant cell wall tissue using layer-by-layer assembly of nanocellulose fibrils and isolated lignin assembled from dilute aqueous suspensions. Surface measurement techniques of quartz crystal microbalance and atomic force microscopy were used to monitor the formation of the polymer-polymer nanocomposite material.
Abstract
Woody materials are comprised of plant cell walls that contain a layered secondary cell wall composed of structural polymers of polysaccharides and lignin. Layer-by-layer (LbL) assembly process which relies on the assembly of oppositely charged molecules from aqueous solutions was used to build a freestanding composite film of isolated wood polymers of lignin and oxidized nanofibril cellulose (NFC). To facilitate the assembly of these negatively charged polymers, a positively charged polyelectrolyte, poly(diallyldimethylammomium chloride) (PDDA), was used as a linking layer to create this simplified model cell wall. The layered adsorption process was studied quantitatively using quartz crystal microbalance with dissipation monitoring (QCM-D) and ellipsometry. The results showed that layer mass/thickness per adsorbed layer increased as a function of total number of layers. The surface coverage of the adsorbed layers was studied with atomic force microscopy (AFM). Complete coverage of the surface with lignin in all the deposition cycles was found for the system, however, surface coverage by NFC increased with the number of layers. The adsorption process was carried out for 250 cycles (500 bilayers) on a cellulose acetate (CA) substrate. Transparent free-standing LBL assembled nanocomposite films were obtained when the CA substrate was later dissolved in acetone. Scanning electron microscopy (SEM) of the fractured cross-sections showed a lamellar structure, and the thickness per adsorption cycle (PDDA-Lignin-PDDA-NC) was estimated to be 17 nm for two different lignin types used in the study. The data indicates a film with highly controlled architecture where nanocellulose and lignin are spatially deposited on the nanoscale (a polymer-polymer nanocomposites), similar to what is observed in the native cell wall.
Introduction
There is great interest to derive additional chemicals and fuels from biomass, as carbon sequestered by plants during photosynthesis is part of the current CO2 cycle. The majority of sequestered carbon (42-44%) is in the form of cellulose, a polymer composed of β 1-4-linked glucopyranose units; when hydrolyzed, glucose can be used as the primary reactant for fermentation into alcohol based fuels. However, cell wall architecture of woody plants has evolved for millennia creating a material that is resistant to degradation in the natural environment1. This stability carries over into the industrial processing of woody materials such as energy crops making cellulose difficult to access, isolate, and breakdown into glucose. A closer look at the ultrastructure of the secondary cell wall reveals that it is a polymer nanocomposite composed of layered paracrystalline cellulose microfibrils embedded in an amorphous matrix of lignin and hemicelluloses2-4. The longitudinally oriented cellulose microfibrils have a diameter of approximately 2-5 nm, which are aggregated together with other hetero-polysaccharides to form larger units of fibril bundles5. The fibril bundles are embedded in a lignin-hemicellulose complex composed of an amorphous polymer of phenylpropanol units with some linkages to other hetero-polysaccharides like glucoronoxylan4. Furthermore, this structure is further organized into layers, or lamellae, throughout the lignified secondary cell wall6-8. Enzymes, like cellulases, have a very difficult time accessing cellulose within the cell wall as it is found in its fibril form and embedded in lignin. The crux of truly making biobased fuels and renewable chemical platforms a reality is to develop processes that economically allow the saccharification of cellulose in its native form.
New chemical and imaging technologies are aiding in the study of the mechanisms involved in the saccharification of cellulose9,10. Much work has centered on Raman confocal imaging11 and atomic force microscopy12 to study the cell wall chemical composition and morphology. Being able to closely follow mechanisms of delignification and saccharification is a significant step forward, impacting the conversion of cellulose to glucose. Saccharification of model cellulose surfaces was analyzed by measuring enzyme kinetic rates with a quartz crystal microbalance with dissipation monitoring (QCM-D)13. However, native cell walls are highly complex as indicated above, and this creates ambiguity of how different conversion processes change the structure of the plant cell wall (polymer molecular weight, chemical linkages, porosity). Free-standing models of the cell wall substances with known structural composition would address this concern and allow the integration of samples into state-of-art chemical and imaging equipment.
There is a dearth of cell wall models and the few available can be categorized as blends of polymer materials and regenerated cellulose or bacterial cellulose14, enzymatically polymerized lignin-polysaccharide composites15-17, or model surfaces18-21. Some models that begin to resemble the cell wall are the samples that contain lignin precursors or analogs polymerized enzymatically in the presence of cellulose in its microfibrillar form. However, these materials suffer from the lack of organized layer architecture. A simple route for the creation of nanocomposite materials with organized architecture is the layer-by-layer (LbL) assembly technique, based on the sequential adsorption of polymers or nanoparticles with complementary charges or functional groups to form organized multilayered composite films22-25. Free-standing hybrid nanocomposites of high strength, made by LbL deposition of polymer and nanoparticles, have been reported by Kotov et al.26-30. Among many other applications, LbL films have also been investigated for their potential use in therapeutic delivery31, fuel cell membranes32,33, batteries34, and lignocellulosic fiber surface modification35-37. The recent interest in nanoscale cellulose based composite materials have led to the preparation and characterization of LbL multilayers of cellulose nanocrystals (CNC) prepared by sulfuric acid hydrolysis of cellulose fibers, and positively charged polyelectrolytes38-43. Similar studies have also been conducted with cellulose nanocrystals obtained from marine tunicin and cationic polyelectrolytes44, CNC and xyloglucan45, and CNC and chitosan46. LbL multilayer formation of carboxylated nanofibrillated celluloses (NFCs), obtained by high-pressure homogenization of pulp fibers with cationic polyelectrolytes has also been studied47-49. The preparation, properties, and application of CNCs and nanofibrillated cellulose have been reviewed in detail50-53.
The present study involves the examination of LbL technique as a potential way to assemble isolated lignocellulosic polymers (such as nanocellulose and lignin) in an ordered fashion as the first step towards a biomimetic lignocellulosic composite with lamellar structure. The LbL technique was selected for its benign processing conditions such as, ambient temperature, pressure, and water as the solvent, which are conditions for natural composite formation54. In this study we report on the multilayer build-up of constitutive wood components, namely cellulose microfibrils from the tetramethylpiperidine 1-oxyl (TEMPO) mediated oxidation of pulp and isolated lignin into free-standing lamellar films. Two different lignins are used from different extraction techniques, one a technical lignin from the organosolv pulping process, and the other a lignin isolated from ball-milling with less modification during isolation. These compounds are combined with a synthetic polyelectrolyte in this initial study to demonstrate the feasibility of making stable free-standing films with architecture similar to the native cell wall.
Protocol
1. Nanofibrillated Cellulose Preparation55
- Setup a 3 L three-neck flask with 2 L of deionized water, an overhead stirrer, and pH probe.
- Add delignified kraft pulp, 88% brightness (20 g, 1% (w/v, dry weight basis)), 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) (0.313 g, 0.1 mmol/g cellulose), and sodium bromide (NaBr, 2.0 g, 1 mmol/g cellulose) to the flask.
- Mix the pulp fiber with the overhead stirrer until the fiber is dispersed and no aggregates can be seen in the reaction.
Note: Dispersion can be aided by blending the slurry in water prior to adding the pulp to the 3 L flask.
- Mix the pulp fiber with the overhead stirrer until the fiber is dispersed and no aggregates can be seen in the reaction.
- Initiate the oxidation by slowly adding a 12% solution of sodium hypochlorite (NaClO, 51.4 ml, 5 mmol per g of cellulose) to the reaction mixture.
Note: For consistency throughout the reaction, use a syringe pump to deliver the NaClO with an injection rate of 1.5 ml/min. - Fill a second syringe with sodium hydroxide (NaOH, 0.5 M) and manually meter the alkali solution into the flask drop-wise to maintain the pH at 10 ± 0.2.
- Monitor the change in pH with time and once all the accessible hydroxyl groups on the cellulose are oxidized the pH will no longer decrease and the reaction is complete.
- Add excess EtOH to consume remaining NaClO. Approximately 6 ml of 200 proof EtOH will consume all 100 mmol of the original NaClO.
- Filter and wash the oxidized fiber thoroughly with purified water to remove the reagents until pH is neutral. Use a basket centrifuge or some filtration device like a Büchner funnel to recover the fiber. Store the fiber at 4 °C until further use.
Note: At the completion of the experiment, the fiber should have a carboxylic acid content, as determined by conductometric titration, between 1.0 to 1.5 mmol per gram of fiber. There should be little difference in appearance of the fiber after the TEMPO oxidation. - Create a 3% (w/v, dry weight basis) slurry of the TEMPO oxidized pulp and blend in a Warring blender until the slurry becomes viscous and the blades start spinning in air because of gelling of the suspension.
Note: Lower concentrations do not work as effectively to fibrillate the cellulose.- Dilute the blended slurry to 0.1% (w/v) and continue blending until suspension becomes transparent.
2. Layer-by-layer Film Deposition for QCM-D Experiments
- Prepare the following aqueous solutions and adjust each solution with 0.1 M NaOH to a pH of 10.5: aqueous buffer solution (water and NaOH); 0.5% (w/v) aqueous solution of polydiallyldimethylammonium chloride (PDDA); and 0.01% (w/v) lignin. Adjust the pH of the 0.1% NFC suspension to 8.0.
Note: The pH for these experiments was elevated because it was previously shown that lignin adsorbs in a less aggregated state in alkaline pH56. - Clean a gold-coated quartz crystal following manufacturers recommendation of using a base piranha solution [CAUTION] (3:1 concentrated NH4OH:H2O2 at 60 °C) for 10 min.
- Rinse the crystals with purified water, blow dry in a stream of N2, and immediately insert into the quartz crystal microbalance flow cell to avoid contamination from the air.
- Pass the buffer through the flow cell to obtain a baseline response of the resonating crystal exposed to the liquid.
- Deposit a layer of PDDA onto the quartz crystal by exposing the quartz crystal to the PDDA solution for 5 min.
- After 5 min switch back to the buffer solution.
Note: This process in step 2.3 creates a single layer response where the amount of polymer deposited can be determined without the effect of the polymer solution viscosity. - Repeat the adsorption of other polymers in the following sequence with a buffer rinse between each step: PDDA (+) (step 2.3.1); lignin (-); PDDA(+); and NFC(-). Repeat the cycle 4x to deposit 16 total layers of polymers and nanoparticles.
3. Layer-by-layer Film Deposition for AFM and Ellipsometry Experiments
- Glue a circular disc of mica to a glass microscope slide using a quick epoxy adhesive. After the adhesive cures, attach a piece of tape to the mica disc. Peel the tape away causing the mica surface to cleave.
- Clean a silicon wafer with acid piranha [CAUTION] (3:1 H2SO4:H2O2) for 20 min followed by significant rinsing in water prior to layer deposition.
- With solutions prepared in 2.1, dip either the freshly cleaved mica that is attached to a glass slide or a freshly cleaned silicon wafer in each solution following the same sequence of protocol outlined in 2.3.3.
Note: This technique will create layers of polymers on each of these surfaces that can be inserted into the AFM or ellipsometer, respectively. - Image the deposited layers with an atomic force microscope. Use the intermittent contact mode and cantilevers with 10 nm radius silicon tips (spring constant 42 N/m) when collecting images of the sample. Set scan size as 2.5 x 2.5 μm, scan point as 512 and integral gain of 10 to collect specific sample images.
- For thickness measurement of the layers with AFM of the dried LbL films, use a soft plastic pipette tip and scar a line across the surface of the prepared LbL films on the mica surface.
- Deposit LBL films for ellipsometry measurement onto silicon wafers. Measure the dry film thickness with a phase modulated ellipsometer at a wavelength of 632.8 nm using the multiple angle of incidence mode. Vary the angles between 85° and 65° at 1° intervals.
4. Preparation of Free-standing LBL Film
- Cut a 25.4 x 7.6 mm rectangle of cellulose acetate (CA) film (DS 2.5) that is 0.13 mm thick and attach to automated dipper arm.
Note: Cellulose acetate of DS 3.0 is not soluble in acetone so DS 2.5 is preferred to recover the layered films. - Fill each 500 ml beaker with solutions of PDDA, lignin, and nanocellulose according to concentration and pH in step 2.1.
- Fill three additional beakers with aqueous buffer to use as a rinse solution for each deposition cycle.
- Program the dipper arm to proceed in same sequence as reported in 2.3.3.
Note: It is important to use a different rinse solution after each respective polymer solution because in the layer-by-layer process, some polymer that is not tightly bound to the surface will desorb. Cross contamination of the rinse solutions quickly causes precipitation of polyelectrolyte complexes, which can adsorb as "defects" to the film surface.
- Change the solutions in the beaker during 250 cycles periodically as they begin to appear cloudy because of colloidal complexes. An option is to automate the renewal of the solution by using peristaltic pump to deliver fresh solution or buffer to custom made polyvinylchloride (PVC) containers with inlets and outlets.
Note: Agitated solutions in the containers help enhance the diffusion of the polyelectrolytes to the surface. - Carefully trim the edges of the dried sample with scissors exposing the CA edge and place into a covered glass Petri dish filled with acetone to dissolve the CA.
Note: Two films are isolated after this experiment from the front and backside of the CA. - Soak isolated films in acetone for 24 hr and rinse films repeatedly with acetone to maximize the removal of residual CA.
Representative Results
QCM-D Analysis of Structured Woody Polymer Film Fabrication
The LbL adsorption of lignin, NFC and PDDA was monitored in real-time with QCM-D in two different experiments involving two types of lignins. This analysis method is very sensitive to detect changes in frequency when molecules adsorb to the surface of the quartz crystal. Figure 1 contains a detailed description of the QCM-D response in one deposition cycle, which involves two bilayers (PDDA:HMWL and PDDA:NC). The data represents the normalized change in frequency and dissipation of the 7th overtone (the instrument detects the fundamental frequency and the 3-13 odd harmonic overtones). A baseline was first obtained with pH 10.5 milli-Q water (termed as buffer), followed by introduction of the cationic polymer, PDDA. The introduction of this polymer (step 1) is associated with a decrease in ΔF, and a corresponding increase in ΔD. This response is attributed to a combination of adsorption of PDDA on the gold coated quartz substrate and the change in the bulk effects of the liquid in contact with the vibrating crystal. Step 1 was followed by a rinse step (step 2) with the buffer to remove the excess/unbound polymer, and to negate the frequency and dissipation response due to the bulk effects of the polymer solution. Therefore, a rinse was performed after each polymer adsorption step. The net change in ΔF and ΔD from the baseline after step 2 is due to the irreversible adsorption of PDDA. In step 3, the lignin solution was introduced, which resulted in a decrease in ΔF and a corresponding increase in ΔD. Step 4, the rinse step caused a slight increase in ΔF, however the ΔD remained unchanged, which suggests that lignin is deposited as a rigid layer over the PDDA layer when in contact with gold coated quartz substrate. To deposit the second bilayer, (PDDA:NC), the PDDA solution was reintroduced over the lignin layer (step 5). The introduction of PDDA solution was associated with a slight decrease in ΔF, and a significant increase in ΔD. However, after the initial drop, there was a gradual increase in ΔF followed by a plateau. After the buffer rinse (step 6), the net change in ΔF and ΔD after the deposition of PDDA on the lignin layer (ΔF = -31.6 Hz; ΔD = 1.3 x 10-6) was found to be slightly lower than the previous layer (ΔF = -33.2 Hz; ΔD = 1.7 x 10-6). This change is the result of a strong interaction between PDDA and lignin56,57, which may have caused partial desorption of loosely bound lignin deposited in step 3 (note in AFM section below, lignin remains in system). In step 7, the NC suspension was introduced on the PDDA layer resulting in an increase in ΔF and a corresponding decrease in ΔD. This change was found to be irreversible after the rinse step (step 8) suggesting that NC has been irreversibly deposited on PDDA. In the current study only four deposition cycles (8 bilayers, 4 cycles) were performed, because the ΔF and ΔD change beyond this number of cycles was found not to be reproducible. Figure 2 shows the normalized change in ΔF and ΔD of the 7th overtone as a result of the sequential adsorption of polymers PDDA, HMWL and NC after four deposition cycles. It should be noted that the adsorption of the polymers did not follow a linear change in ΔF and ΔD with the addition of each bilayer, which has also been noted with other LBL systems49,58. The LBL adsorption of NC, PDDA and OL (NC-PDDA-OL), shown in Figure 3, was found to follow the sequential adsorption process observed with NC-PDDA-HMWL. Nonetheless, the systems were found to differ with respect to the exact amount of the polymers deposited in each layer. The difference between these two systems is due to the type of the lignin used, as the cationic polymer and NC used were the same in both systems.
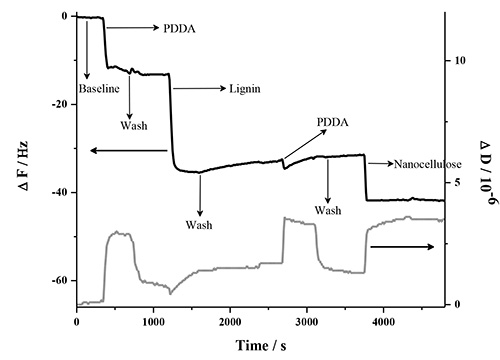
Figure 1. Description of the steps involved in the 1st deposition cycle of LBL adsorption of NC-PDDA-HMWL. The figure shows the normalized change in ΔF and ΔD of the 7th harmonic.
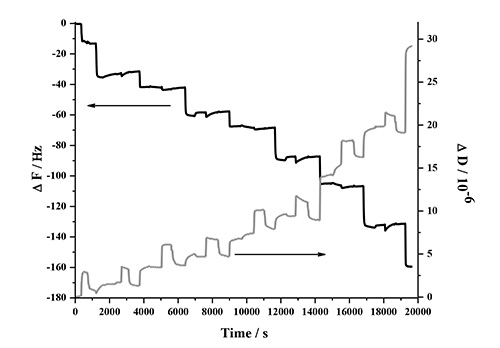
Figure 2. Frequency and dissipation response of the 7th harmonic as a result of the LBL adsorption of NC-PDDA-HMWL in 4 deposition cycles (8 bilayers).
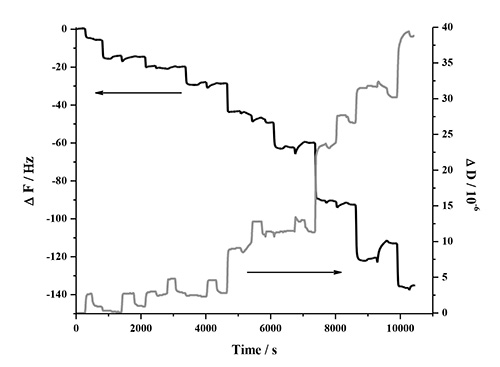
Figure 3. Frequency and dissipation response of the 7th harmonic as a result of the LBL adsorption of NC-PDDA-OL in 4 deposition cycles (8 bilayers).
Imaging Film Build-up with Atomic Force Microscopy
AFM images revealed complete coverage of the surface with lignin in the first PDDA-lignin bilayer for both HMWL and OL (Figure 4). The RMS roughness values for HMWL and OL were 1.6 and 3.8 nm, respectively. AFM images of the first PDDA-NC bilayer showed that the surface was not completely covered with NC fibrils, and revealed scattered NC fibrils in the 2.5 x 2.5 mm images (Figure 5a, RMS roughness 1.6 nm). However, as the layer build-up continued, more uniformity was found for the fibrils deposited, as seen in Figure 5b (RMS roughness 5.3 nm). Similar results were seen with QCM-D data, as ΔF for NC changed greater in magnitude at higher cycle numbers. The adsorbed mass estimated using Johannsmanns's model for the cycles 1 and 4 of NC layer in NC-PDDA-HMWL system was 1.11 ± 0.13 mg/m2 and 5.44 ± 1.78 mg/m2, respectively. A similar trend was also observed with the NC-PDDA-OL system with an estimated NC mass of 1.15 ± 0.09 and 5.46 ± 1.79 mg/m2 for cycles 1 and 4, respectively. These estimates suggest that the hydrated mass of NC layer deposited in the 4th deposition cycle is 4x greater than that of the 1st deposition cycle. The increase in mass associated with NC adsorption can also be attributed to the water entrained in the porous structure created by the fibers. An increase in the amount of trapped water, with the increase in layer thickness has been reported in systems involving MFC multilayers48,59. AFM images additionally show the lignin adsorbed along the PDDA coated fibrils after the lignin deposition step, as seen in the images after 3 cycles (Figures 6a and b).
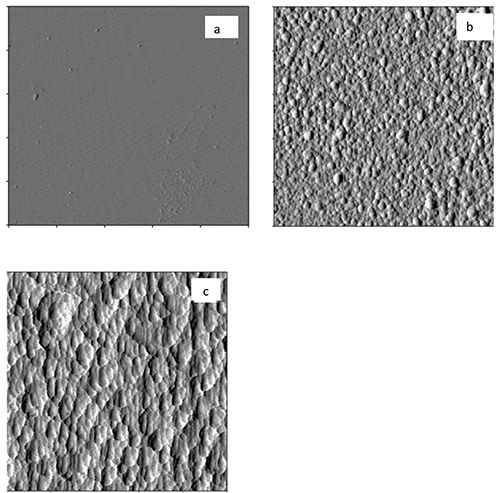
Figure 4. a) AFM Amplitude images of PDDA on mica (5 x 5 μm); b) MWL on PDDA (2.5 x 2.5 μm), and c) OL on PDDA (2.5 x 2.5 μm).
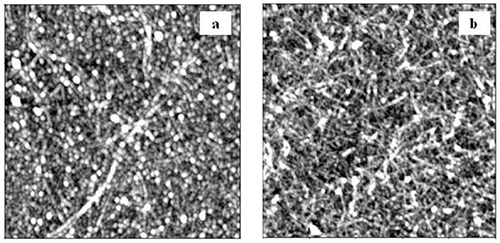
Figure 5. Height images of NC on PDDA after the 1st (a) and 3rd (b) deposition cycle (2.5 x 2.5 μm).
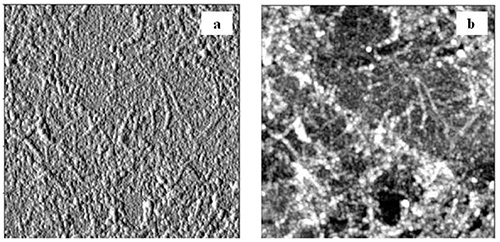
Figure 6. a) Amplitude and b) height images of HMWL after the 4th deposition cycle (2.5 x 2.5 μm). The images show lignin particles deposited on the NC fibrils from the 3rd deposition cycle.
Free-standing LBL Films
Free-standing LBL films were created after 250 deposition cycles with each cycle incorporating two PDDA, one lignin, and one nanocellulose layer. The films were isolated after the cellulose acetate substrate was dissolved in acetone (Figure 7). Initial observation of the film was that it was translucent and bendable. These two properties are rarely associated with lignin-based composites that contain a significant lignin loading. Film samples were dipped in liquid nitrogen for 2 min and applying a bending force with a second set of forceps ruptured the samples. SEM of the cryo-fractured cross-sections of LBL films displays a lamellar structure (Figures 8a and b). The thickness of both NC-PDDA-HMWL and NC-PDDA-OL were found to be approximately 4.3 μm, which implies an average thickness of approximately 17 nm per deposition cycle. The SEM data indicates a significantly higher thickness compared to QCM-D estimates. However, the QCM-D measurements were carried out for only 4 deposition cycles (reaching the limits of the instrument due to the viscoelastic surface). From the QCM-D results, it was noted that the layer build-up was not linear for the four deposition cycles studied. Therefore the data suggests it requires more than 4 deposition cycles for the thickness increase per cycle to plateau.
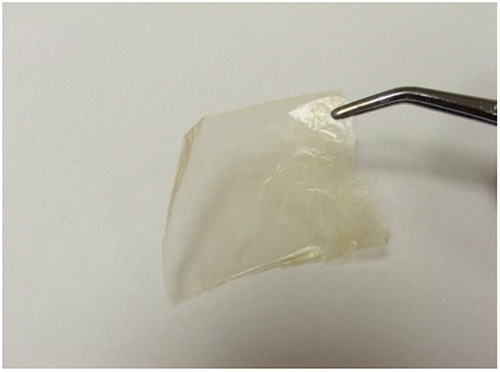
Figure 7. A free-standing film of NC-PDDA-HMWL obtained after 250 deposition cycles. The free-standing films were obtained after dissolving the cellulose acetate substrate in acetone.
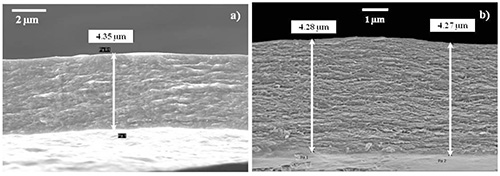
Figure 8. SEM images showing lamellar structure of the cross-sections of cryo-fractured LBL films after 250 deposition cycles. The free standing films of a) NC-PDDA-HMWL and b) NC-PPDA-OL were obtained after the cellulose acetate substrate was dissolved in acetone. Please click here to view a larger version of this figure.
Discussion
Fabrication of Nanocellulose
For nanocellulose fabrication the successful oxidation of the pulp fiber is necessary for facile fibrillation. Oxidation is controlled by available sodium hypochlorite, which should be slowly added at known quantities based on the amount of cellulose. One reason for limited oxidation arises from the storage of the sodium hypochlorite solution for extended periods. This reduced oxidation efficiency can be noted during the reaction; the pulp slurry should turn a pale-yellowish color, part way through the reaction during successful oxidation. If this does not occur, the carboxylic acid content of the fiber is usually below levels that enable easy fibrillation.
The fibrillation of oxidized fiber with carboxylic acid content above 1.0 mmol/g of cellulose can occur by a number of different mechanical treatment methods yielding similar results in nanocellulose particle size. Ultrasonication with a high power sonication horn for short time periods or homogenization with a microfluidic cell are alternatives to blending the oxidized fiber. The former provides a route to prepare single batches of 200 ml of NFC suspensions or less, while the latter provides a route to prepare liters of nanocellulose suspensions. Past experiments using atomic force microscopy have shown that these fibrils have lengths of 530 ± 330 nm and a thickness of 1.4 ± 0.7 nm 60.
QCM-D Viscoelastic Modeling
The mass and thickness of the adsorbed polymer layers can be determined by the Sauerbrey relationship. However, the method is only valid if the deposited layer is rigid, and the whole system performs as a composite resonator. This limitation can be checked by monitoring the frequency dependence of the overtones (ΔF/n). Figure 9 shows that with the increase in the number of layers, the overtones move farther apart, which suggests that as the thickness increases, the response of the films tend to be viscoelastic and less rigid48. For a thicker or viscoelastic film, the nature of propagation of the shear acoustic wave into and through the film affects the estimation of the coupled mass61. Therefore, in such cases, ΔF is not directly proportional to Δm. Also, it is critical to understand that the mass estimated with QCM-D may include coupled water due to hydration and viscous drag. The amount of coupled water varies depending on the nature of the adsorbed film, but can typically range between 1.5-4x the molar mass of the adsorbed material61.
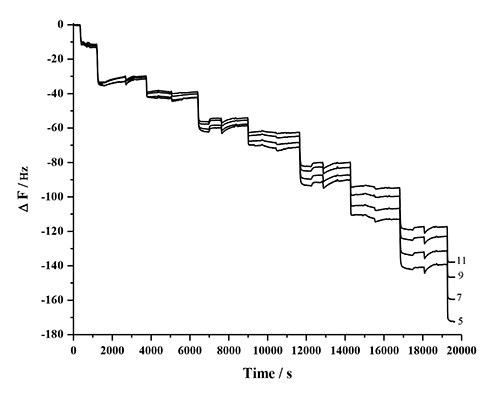
Figure 9. Frequency response of the odd harmonics 5 to 11 as a result of the LBL adsorption of PDDA, HMWL, and NC, showing the frequency dependence of the harmonics as the number of layers increase.
The Johannsmann Model can be used to account for the limitation of the Sauerbrey model. This alternative model determines the true sensed mass of a viscoelastic layer, has been used to study the adsorption characteristics of different systems like polyelectrolyte complexes on polystyrene substrates62, proteins on gold substrate63, and microfibrillated cellulose on polyelectrolytes48,49 . Figure 10 compares the areal mass estimated with Johannsmann model after four deposition cycles for NC-PDDA-HMWL and NC-PDDA-OL systems. The values of the mass adsorbed per cycle and the mass of NC and lignin per cycle are given in Table 1 and 2, respectively. From the comparison, it is seen that the total mass adsorbed after four adsorption cycles is similar for both NC-PDDA-HMWL and NC-PDDA-OL (29.38 ± 2.57 and 31.78 ± 2.44 mg/m2, respectively). From the four adsorption cycles studied, the mass of lignin adsorbed in the first two cycles was seen to differ between the two systems. The mass of HMWL adsorbed in adsorption cycles 1 and 2 were almost twice that of OL (Table 2). However, the mass of the two different lignins adsorbed in cycles 3 and 4 were similar. The mass of NC adsorbed in both systems was similar except for the cycle 2, where the mass of NC adsorbed in NC-PDDA-OL was slightly higher than NC-PDDA-HMWL. This difference caused the beginning of cycle 3 to have the same amount of total mass for the two systems. These results suggest that after the initial cycles there is little difference in assembly for the type of lignin in the composite films. This data suggests that LbL films of model plant walls can be created from lignins of different biological origins and/or isolation protocols. Currently there are not any other methods that can make model stand-alone cell wall materials with select lignins of a particular structure.
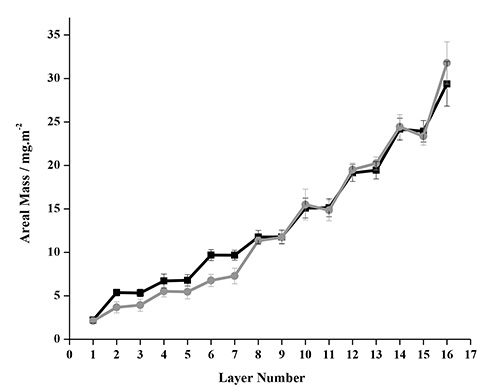
Figure 10. Areal mass estimated with Johannsmann's model for NC-PDDA-HMWL (■) and NC-PDDA-OL (●) after 4 deposition cycles.
| Cycle # | Mass per cycle (mg/m2) | Cumulative mass (mg/m2) | ||
| NC-PDDA-HMWL | NC-PDDA-OL | NC-PDDA-HMWL | NC-PDDA-OL | |
| 1 | 6.72 ± 0.80 | 5.51 ± 0.63 | 6.72 ± 0.79 | 5.51 ± 0.63 |
| 2 | 5.03 ± 0.22 | 5.82 ± 0.50 | 11.76 ± 0.77 | 11.33 ± 0.45 |
| 3 | 7.37 ± 0.37 | 7.52 ± 0.66 | 19.14 ± 0.98 | 19.52 ± 0.73 |
| 4 | 10.23 ± 1.97 | 12.92 ± 1.93 | 29.38 ± 2.57 | 31.78 ± 2.44 |
Table 1. Areal mass estimated from QCM-D data using Johannsmann’s model for 4 deposition cycles.
| Cycle # | Lignin (mg/m2) | Nanocellulose (mg/m2) | ||
| NC-PDDA-HMWL | NC-PDDA-OL | NC-PDDA-HMWL | NC-PDDA-OL | |
| 1 | 3.16 ± 0.26 | 1.56 ± 0.57 | 1.11 ± 0.13 | 1.15 ± 0.09 |
| 2 | 2.91 ± 0.32 | 1.30 ± 0.13 | 2.08 ± 0.36 | 3.18 ± 0.66 |
| 3 | 3.31 ± 0.39 | 3.77 ± 0.14 | 4.00 ± 0.38 | 3.22 ± 1.51 |
| 4 | 4.72 ± 0.64 | 4.22 ± 1.34 | 5.44 ± 1.78 | 5.46 ± 1.79 |
Table 2. Areal mass estimate of lignin and NC using Johannsmann’s model for 4 deposition cycles.
A comparison of the modeled data after four deposition cycles of NC-PDDA-HMWL and NC-PDDA-OL (Figure 11 and Table 3) reveals similar trends observed with Johannsmann’s model. The first trend is the similarity of the final thicknesses between the films with two lignin types for a given film density boundary. The final thicknesses after 4 deposition cycles for NC-PDDA-HMWL and NC-PDDA-OL with an assumed density of 1,000 kg/m2 were 31.5 ± 3.5 and 30.4 ± 5.1 nm, respectively (Figure 12). The final thicknesses for the same, with an assumed density of 1,400 kg/m2, were 23.4 ± 2.8 and 22.1 ± 3.1 nm, respectively. The second trend is revealed at the beginning of the third cycle where the change in thickness is the same for the two lignins, as found for the mass estimate. The thickness of the PDDA layer was not estimated because of negligible or negative changes in the thickness after the adsorption of PDDA. However, it was observed that significant sequential layer build-up was not possible without the adsorption of PDDA following the adsorption of NC or lignin (data not shown). This result indicates that the adsorption of the linking layer is a critical step in the adsorption sequence.
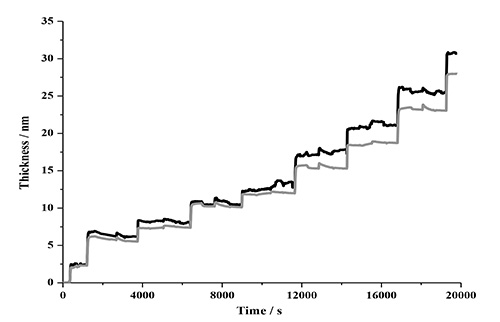
Figure 11. Thickness estimated with Voigt model (black) compared to the thickness estimated with Sauerbrey equation (7th harmonic; grey). The density of the film was assumed to be 1,000 kg/m2.
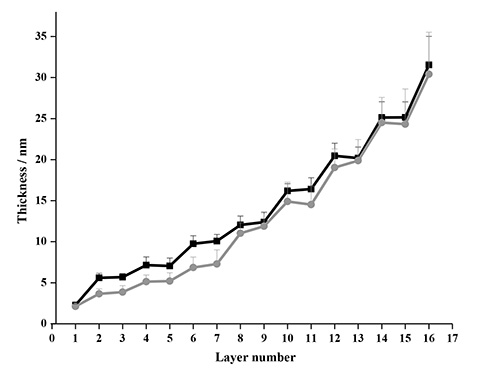
Figure 12. Comparison of thickness estimated with Voigt model for NC-PDDA-HMWL (■) and NC-PDDA-OL (●) after 4 deposition cycles with an assumed density of 1,000 kg/m2.
The thickness of the nanocellulose and lignin films estimated using the viscoelastic model was compared to ellipsometry thickness (dry state) in Table 3. The ellipsometry thickness values from the first deposition cycle gave a close estimate to the Voigt model thickness, with an assumed density of 1,000 kg/m2. The ellipsometry thickness value of the first cycle is almost 2-3x larger than each of the cycles 2-4. This phenomenon is related to the differences in PDDA deposition in the first cycle relative to the other cycles. From the QCM-D experiments, the initial PDDA layer on gold is found to be ~2 nm thick. However, there was negligible or negative change in mass/thickness when PDDA was introduced over NC or lignin. A similar response was seen in a previous study involving LBL adsorption of kraft lignin and PDDA, where a linear buildup of film thickness was observed even though there was negligible PDDA adsorption56. The lower ellipsometry thickness values of the 2nd through 4th deposition cycles compared to the 1st cycle can be attributed to a change in the conformation of the PDDA layer when deposited on gold. However, the relatively small adsorption of PDDA in cycles 2-4 is sufficient to continue the LBL assembly process. There is significant difference between the ellipsometry and QCM-D thickness in the 3rd and 4th cycles, as the estimated Voigt thickness (based on a density of 1,000 kg/m2) was found to be twice that of the ellipsometry thickness for both NC-PDDA-HMWL and NC-PDDA-OL systems. This result relative to the QCM-D data further implicates an increasing viscoelastic nature of the films as the layer build-up proceeds. It is suggested that the topmost layers are more porous compared to the lower layers holding additional trapped water. Moreover, NC used in this study is decorated with carboxyl groups at the C6 position (carboxyl content of 1.0 mmol/g of cellulose), which makes the fibers more hydrophilic. Anionic groups decorating the NC leads to the layer being hydrated and viscous, hence the deviation from the Sauerbrey relation; the relationship is applicable only for thin and elastic films.
| Cycle # | NC-PDDA-HMWL (nm) | NC-PDDA-OL (nm) | ||||
| Voigt | Voigt | Ellipsometry | Voigt | Voigt | Ellipsometry | |
| (1000 kg/m2) | (1400 kg/m2) | (1,000 kg/m2) | (1,400 kg/m2) | |||
| 1 | 7.2 ± 1.0 | 5.0 ± 0.4 | 7.5 ± 0.3 | 5.1 ± 1.0 | 4.0 ± 0.5 | 6.1 ± 0.1 |
| 2 | 12.0 ± 1.1 | 8.8 ± 1.0 | 10.4 ± 0.6 | 11.0 ± 1.3 | 8.0 ± 0.7 | 8.1 ± 0.3 |
| 3 | 20.5 ± 1.5 | 14.4 ± 1.1 | 12.0 ± 0.3 | 19.0 ± 2.3 | 13.2 ± 1.3 | 11.7 ± 0.1 |
| 4 | 31.5 ± 3.5 | 23.4 ± 2.8 | 14.5 ± 0.3 | 30.4 ± 5.1 | 22.1 ± 3.1 | 13.8 ± 0.5 |
Table 3. Cumulative thickness after each adsorption cycle estimated by Voigt model, with assumed densities of 1,000 and 1,400 kg/m3, and thickness estimated by ellipsometry for 4 deposition cycles.
To investigate the validity of the dry film thickness estimated by ellipsometry, an AFM scratch test was performed on both NC-PDDA-HMWL and NC-PDDA-OL after four deposition cycles on a Si wafer. The height profile from the scratch test gave an average thickness of 15.1 ± 0.9 nm and 17.3 ± 3.0 nm respectively for NC-PDDA-HMWL and NC-PDDA-OL, respectively. These values are similar in magnitude to those measured by ellipsometry (Table 3). Ellipsometry resulted in the smallest measurements (optical, dry), followed by AFM (height profile, dry), and QCM-D (sensed mass, estimated from hydrated state).
Lignin Differences
The two types of lignins used in this study were organosolv lignin (OL; Sigma Aldrich, Inc) and hardwood milled wood lignin previously isolated in our laboratories and recently characterized for this current study (HMWL)64. GPC analysis of acetylated samples of HMWL and OL showed an Mn of 5,300 and 1,300 g/mol respectively. The fraction of aromatic:aliphatic acetate hydrogen determined from the 1H NMR analysis of the acetylated lignin samples were found to be 1.16:1, and 0.26:1 respectively for OL and HMWL. Thus, OL was found to have a significantly higher phenolic content, which would account for a greater number of ionizable phenolic groups at an elevated pH. The total acid number of the two lignin determined by conductometric titrations was 0.41 ± 0.02 and 0.34 ± 0.03 mmol/g respectively for OL and HMWL. The acid number determined by conductometric titration represents the contribution from both the phenolic and the carboxylic content present in lignin. Hence the slightly higher charged lignin, which has a lower molecular weight, forms a marginally smaller thickness in the initial frequency change. A difference in deposition is usually noteworthy for polyelectrolyte adsorption onto charged surfaces as segment charge and MW change65. In the first two deposition cycles, organosolv lignin has an areal mass half of the value for the milled-wood lignin. This trend is also observed with the ellipsometry measurements, as thickness of NC-PDDA-OL in the first and second cycle is lower than NC-PDDA-HMWL. However, this study shows that there is minimal change in the third and fourth cycles, as well as when the process is repeated 250x. The great number of cycles will magnify small differences in adsorption. The data suggests that lignin with disparate structure does not greatly impact the fabrication of free-standing films. Hence, either technical lignins, available from biomass conversion into paper, fuels, and chemicals, or model lignins carefully isolated can be used to form free-standing films with nanocellulose. This fact is significant where lignins of different origins can be carefully selected to make model cell wall surfaces.
Future work should integrate hydroxyl rich linker layers (synthetics such as polyvinyl alcohol or biobased like hemicelluloses) to replace or augment the PDDA linker layer used in the current study to derive a structured nanocomposite film that more closely represents the wood cell wall composite. The integration of cellulose microfibrils and lignin across 17 nm is within range of the structures of native cell walls and provides a new model material to serve as an artificial wood cell wall.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported primarily by the Doctoral Scholar’s program of the Institute for Critical Technology and Applied Science (ICTAS) at Virginia Tech, the Virginia Tech Graduate School for supporting the Sustainable Nanotechnology program, and also the United States Department of Agriculture, NIFA grant number 2010-65504-20429. The authors also thank the contributions of Rick Caudill, Stephen McCartney, and W. Travis Church to this work.
Materials
| sulfate pulp | Weyerhaeuser | donated | brightness level of 88% |
| organosolv lignin | Sigma Aldrich | 371017 | discontinued |
| hardwood milled wood lignin | see reference in paper | ||
| polydiallyldimethylammonium chloride | Sigma Aldrich | 409022 | Mn = 7.2×10^4, Mw=2.4×10^5 |
| 2,2,6,6-Tetramethylpiperidine 1-oxyl (TEMPO) | Sigma Aldrich | 214000 | catalytic oxidation of primary alcohols to aldehydes with a purity of 98%, molecular weight is 156.25g/mol |
| sodium bromide | Sigma Aldrich | S4547 | purity ≥99.0%, molecular weight 102.89 |
| sodium hypochlorite | Sigma Aldrich | 425044 | reagent grade, available chlorine 10~15%, molecular weight 74.44g/mol |
| sodium hydroxide | VWR | BDH7221-4 | 0.5N aqueous solution, density 1.02g/ml, molecular weight 40 g/mol |
| sodium hydroxide | Acros Organics | AC12419-0010 | 0.1N aquesous solution, specific gravity 1.0 g/ml, molecular weight 40 g/mol |
| ammonium hydroxide | Acros Organics | AC39003-0025 | 25% solution in water, pH 13.6, density 0.89, molecular weight 35.04 g/mol |
| hydrogen peroxide | Fisher Scientific | H325-100 | 30.0~32.0% certified ACS, pH 3.3, density 1.11 |
| Mica sheets | TED Pella | NC9655733 | Pelco, grade V5, 10×40mm, 23mm T, minimum air and bubbles, very clean |
| sulfuric acid | Fisher Scientific | A300-212 | 95.0~98.0 w/w%, certified ACS plus, molecular weight 98.08 g/mol |
| cellulose acetate | McMaster Carr | 8564K44 | degree of substitution 2.5 |
| ethanol | Decon Laboratories | 04-355-223 | 200 proof (100%), USP |
| acetone | Fisher Scientific | A18-4 | purity ≥99.5%, certified ACS reagent grade, density 0.79 g/ml, molecular weight 58.08 g/mol |
| syringy pump | Harvard Apparatus | 552226 | pump 22 infusion/withdraw with standard syringe holder, flow rate 0.002 ul/h~55.1ml/min |
| Mill-Q water purification system | EMD Millipore | D3-UV | Direct-Q, UV, water conductivity 18.5 MΩ cm with 20 liter reservair |
| pH meter | Mettler Toledo | SeverMulti | |
| balance | Mettler Toledo | AB135-S | accuracy 0.1mg |
| atomic force microscope | Asylum Research | MFP-3D, Olympic fluorescent microscope stage | |
| ellipsometer | Beaglehole Instruments | ||
| fiber centrifuge | unknown | basket style centrifuge | |
| Warring blender | Warring | Commercial | |
| ultrasonic processor | Sonics | Sonics 750W, sound enclosure | |
| Quartz crystal microbalance with dissipation monitoring (QCM-D) | Q-Sense Inc. | E4 | measure fundamental frequency of 5MHz, and monitor odd number overtones/harmonics from 3~13, use gold-coated piezoelectric quartz crystals |
| automatted dipper arm | Lynxmotion |
References
- Fratzl, P., et al. On the role of interface polymers for the mechanics of natural polymeric composites. Phys. Chem. Chem. Phys. 6, 5575-5579 (2004).
- Terashima, N., Fukushima, K., He, L. F., Takabe, K. Forage cell wall structure and digestibity. American Society of Agronomy. , 247-270 (1993).
- Himmel, M. E., et al. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science. 315, 804-807 (2007).
- Terashima, N., et al. Nanostructural assembly of cellulose, hemicellulose, and lignin in the middle layer of secondary wall of ginkgo tracheid. J. Wood. Sci. 55, 409-416 (2009).
- Fahlén, J., Salmén, L. Pore and Matrix Distribution in the Fiber Wall Revealed by Atomic Force Microscopy and Image Analysis. Biomacromolecules. 6, 433-438 (2005).
- Baer, E., et al. Biological and synthetic hierarchical composites. Phys. Today. 45, 60-67 (1992).
- Tirrell, D. A., Aksay, I., Baer, E., Calvert, P. D., Cappello, J., Dimarzio, E. A., Evans, E. A., Fessler, J. Hierarchical structures in biology as a guide for new materials technology. National Academy of Sciences. , (1994).
- Fengel, D., Wegener, G. . Wood: Chemistry, Ultrastructure, Reactions. , (1984).
- Santa-Maria, M., Jeoh, T. Molecular-Scale Investigations of Cellulose Microstructure during Enzymatic Hydrolysis. Biomacromolecules. 11, 2000-2007 (2010).
- Saar, B. G., et al. Label-free, real-time monitoring of biomass processing with stimulated Raman scattering microscopy. Angew. Chem. Int. Edit. 49, 5476-5479 (2010).
- Schmidt, M., et al. Label-free in situ imaging of lignification in the cell wall of low lignin transgenic Populus trichocarpa. Planta. 230, 589-597 (2009).
- Ding, S. -. Y., Himmel, M. E. The maize primary cell wall microfibril: a new model derived from direct visualization. J. Agricul. Food Chem. 54, 597-606 (2006).
- Turon, X., et al. Enzymatic kinetics of cellulose hydrolysis: a QCM-D study. Langmuir. 24, 3880-3887 (2008).
- Dammströem, S., et al. On the interactions between cellulose and xylan, a biomimetic simulation of the hardwood cell wall. BioResources. 4, 3-14 (2009).
- Barakat, A., et al. Studies of xylan interactions and cross-linking to synthetic lignins formed by bulk and end-wise polymerization: a model study of lignin carbohydrate complex formation. Planta. 226, 267-281 (2007).
- Micic, M., et al. Study of the lignin model compound supramolecular structure by combination of near-field scanning optical microscopy and atomic force microscopy. Colloids Surf. B Biointerfaces. 34, 33-40 (2004).
- Li, Z., et al. Nanocomposites prepared by in situ enzymatic polymerization of phenol with TEMPO-oxidized nanocellulose. Cellulose. 17, 57-68 (2010).
- Gradwell, S. E., et al. Surface modification of cellulose fibers: towards wood composites by biomimetics. C. R. Biologies. 327, 945-953 (2004).
- Kaya, A., et al. Surface plasmon resonance studies of pullulan and pullulan cinnamate adsorption onto cellulose. Biomacromolecules. 10, 2451-2459 (2009).
- Gustafsson, E., et al. Direct adhesive measurements between wood biopolymer model surfaces. Biomacromolecules. 13, 3046-3053 (2012).
- Karabulut, E., Wagberg, L. Design and characterization of cellulose nanofibril-based freestanding films prepared by layer-by-layer deposition technique. Soft Matter. 7, 3467-3474 (2011).
- Decher, G., Hong, J. D. Buildup of ultrathin multilayer films by a self-assembly process: II. consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Ber. Bunsen. Phys. Chem. 95, 1430-1434 (1991).
- Decher, G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 277, 1232 (1997).
- Hammond, P. T. Form and function in multilayer assembly: new applications at the nanoscale. Adv. Mater. 16, 1271-1293 (2004).
- Decher, G., Schlenoff, J. B. . Multilayer thin films- sequential assembly of nanocomposite materials. , (2003).
- Mamedov, A. A., Kotov, N. A. Free-standing layer-by-layer assembled films of magnetite nanoparticles. Langmuir. 16, 5530-5533 (2000).
- Mamedov, A. A., et al. Molecular design of strong single-wall carbon nanotube/polyelectrolyte multilayer composites. Nat. Mater. 1, 257-257 (2002).
- Podsiadlo, P., et al. Fusion of seashell nacre and marine bioadhesive analogs: high-strength nanocomposite by layer-by-layer assembly of clay and L-3,4-dihydroxyphenylalanine polymer. Adv. Mater. 19, 949-955 (2007).
- Podsiadlo, P., et al. Ultrastrong and stiff layered polymer nanocomposites. Science. 318, 80-83 (2007).
- Podsiadlo, P., et al. Can nature’s design be improved upon? High strength, transparent nacre-like nanocomposites with double network of sacrificial cross links. J. Phys. Chem. B. 112, 14359-14363 (2008).
- Becker, A. L., et al. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small. 6 (17), (2010).
- Taylor, A. D., et al. Fuel cell membrane electrode assemblies fabricated by layer-by-layer electrostatic self-assembly techniques. Adv. Funct. Mater. 18, 3003-3009 (2008).
- Ashcraft, J. N., et al. Structure-property studies of highly conductive layer-by-layer assembled membranes for fuel cell PEM applications. J. Mater. Chem. 20, 6250-6257 (2010).
- Lee, S. W., et al. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nano. 5, 531-537 (2010).
- Eriksson, M., et al. The influence on paper strength properties when building multilayers of weak polyelectrolytes onto wood fibres. J. Colloid Interf. Sci. 292, 38-45 (2005).
- Lvov, Y. M., et al. Dry and wet strength of paper: layer-by-layer nanocoating of mill broken fibers for improved paper. 21, 552-557 (2006).
- Lin, Z., et al. Nanocomposite-based lignocellulosic fibers 1. Thermal stability of modified fibers with clay-polyelectrolyte multilayers. Cellulose. 15, 333-346 (2008).
- Cranston, E. D., Gray, D. G., Barrett, C. J. Abstracts; 32nd Northeast Regional Meeting of the American Chemical Society. , (2004).
- Podsiadlo, P., et al. Molecularly engineered nanocomposites: layer-by-layer assembly of cellulose nanocrystals. Biomacromolecules. 6, 2914-2918 (2005).
- Cranston, E. D., Gray, D. G. Formation of cellulose-based electrostatic layer-by-layer films in a magnetic field. Sci. Tech. Adv. Mater. 7, 319-321 (2006).
- Cranston, E. D., Gray, D. G. Morphological and optical characterization of polyelectrolyte multilayers incorporating nanocrystalline cellulose. Biomacromolecules. 7, 2522-2530 (2006).
- Jean, B., et al. Structural details of cellulose nanocrystals/polyelectrolytes multilayers probed by neutron reflectivity and AFM. Langmuir. 24, 3452-3458 (2008).
- Renneckar, S., Zink-Sharp, A., Esker Alan, R., Johnson Richard, K., Glasser Wolfgang, G. Cellulose Nanocomposites. ACS Symposium Series. , 78-96 (2006).
- Podsiadlo, P., et al. Layer-by-layer assembled films of cellulose nanowires with antireflective properties. Langmuir. 23, 7901-7906 (2007).
- Jean, B., et al. Non-electrostatic building of biomimetic cellulose-xyloglucan multilayers. Langmuir. 25, 3920-3923 (2009).
- de Mesquita, J. P., et al. Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules. 11, 473-480 (2010).
- Wågberg, L., et al. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir. 24, 784-795 (2008).
- Aulin, C., et al. Buildup of polyelectrolyte multilayers of polyethyleneimine and microfibrillated cellulose studied by in situ dual-polarization interferometry and quartz crystal microbalance with dissipation. Langmuir. 24, 2509-2518 (2008).
- Aulin, C., et al. Self-organized films from cellulose I nanofibrils using the layer-by-layer technique. Biomacromolecules. 11, 872-882 (2010).
- Azizi Samir, M. A., et al. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules. 6, 612-626 (2005).
- Siró, I., Plackett, D. Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose. 17, 459-494 (2010).
- Eichhorn, S., et al. Review: current international research into cellulose nanofibres and nanocomposites. J. Mat. Sci. 45, 1-33 (2010).
- Habibi, Y., et al. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 110, 3479 (2010).
- Teeri, T. T., et al. Biomimetic engineering of cellulose-based materials. Trends Biotechnol. 25, 299-306 (2007).
- Saito, T., et al. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules. 7, 1687-1691 (2006).
- Pillai, K. V., Renneckar, S. Cation-π Interactions as a Mechanism in Technical Lignin Adsorption to Cationic Surfaces. Biomacromolecules. 10, 798-804 (2009).
- Notley, S. M., Norgren, M. Adsorption of a strong polyelectrolyte to model lignin surfaces. Biomacromolecules. 9, 2081-2086 (2008).
- Aulin, C., et al. Buildup of polyelectrolyte multilayers of polyethyleneimine and microfibrillated cellulose studied by in situ dual-polarization interferometry and quartz crystal microbalance with dissipation. Langmuir. 24, 2509-2518 (2008).
- Argun, A. A., et al. Highly conductive, methanol resistant polyelectrolyte multilayers. Adv. Mater. 20, 1539-1543 (2008).
- Li, Q., Renneckar, S. Molecularly thin nanoparticles from cellulose: isolation of sub-microfibrillar structures. Cellulose. 16, 1025-1032 (2009).
- Höök, F., et al. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a auartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 73, 5796-5804 (2001).
- Naderi, A., Claesson, P. M. Adsorption properties of polyelectrolyte-surfactant complexes on hydrophobic surfaces studied by QCM-D. Langmuir. 22, 7639-7645 (2006).
- Kaufman, E. D., et al. Probing protein adsorption onto mercaptoundecanoic acid stabilized gold nanoparticles and surfaces by quartz crystal microbalance and z-potential measurements. Langmuir. 23, 6053-6062 (2007).
- Glasser, W. G., Barnett, C. A., Sano, Y. Classification of lignins with different genetic and industrial origins. J. Appl. Polym. Sci.: Appl. Polym. Symp. , (1983).
- Van de Steeg, H. G. M., et al. Polyelectrolyte adsorption: a subtle balance of forces. Langmuir. 8, 2538-2546 (1992).

