Measuring Spinal Presynaptic Inhibition in Mice By Dorsal Root Potential Recording In Vivo
Summary
GABAergic presynaptic inhibition is a powerful inhibitory mechanism in the spinal cord important for motor and sensory signal integration in spinal cord networks. Underlying primary afferent depolarization can be measured by recording of dorsal root potentials (DRP). Here we demonstrate a method of in vivo recording of DRP in mice.
Abstract
Presynaptic inhibition is one of the most powerful inhibitory mechanisms in the spinal cord. The underlying physiological mechanism is a depolarization of primary afferent fibers mediated by GABAergic axo-axonal synapses (primary afferent depolarization). The strength of primary afferent depolarization can be measured by recording of volume-conducted potentials at the dorsal root (dorsal root potentials, DRP). Pathological changes of presynaptic inhibition are crucial in the abnormal central processing of certain pain conditions and in some disorders of motor hyperexcitability. Here, we describe a method of recording DRP in vivo in mice. The preparation of spinal cord dorsal roots in the anesthetized animal and the recording procedure using suction electrodes are explained. This method allows measuring GABAergic DRP and thereby estimating spinal presynaptic inhibition in the living mouse. In combination with transgenic mouse models, DRP recording may serve as a powerful tool to investigate disease-associated spinal pathophysiology. In vivo recording has several advantages compared to ex vivo isolated spinal cord preparations, e.g. the possibility of simultaneous recording or manipulation of supraspinal networks and induction of DRP by stimulation of peripheral nerves.
Introduction
Presynaptic inhibition is one of the most powerful inhibitory mechanisms in the spinal cord. It inhibits excitatory postsynaptic potentials (EPSPs) in monosynaptically excited motoneurons without changing the postsynaptic membrane potential and the excitability of the motoneurons1-3. Primary afferent depolarization (PAD) induced by GABAergic axo-axonal synapses onto sensory presynaptic fibers is the underlying mechanism4-7 (see also Figure1a). These synapses contain GABAA- and GABAB-receptors (GABAAR and GABABR). GABAAR activity leads to an increase in chloride conductance which elicits PAD due to the local ion distribution. This depolarization blocks the propagation of action potentials into the axon terminals and reduces their strength leading to a decreased Ca2+-influx and a reduction of transmitter release. Activation of GABAB receptors does not contribute to PAD but leads to a reduction of Ca2+-influx thereby enhancing presynaptic inhibition. While the activation of GABAAR seems to be involved in short term inhibition, GABABR are involved in long-term modulation8-10. In addition to GABA, which accounts for the major part of PAD and presynaptic inhibition, other transmitters systems might also modulate and contribute to this mechanism11,12.
Pathological changes in presynaptic inhibition seem to be crucial in several disease states e.g. peripheral inflammation and neuropathic pain13,14, as well as abnormal central pain processing15, spinal cord injury16, and CNS disease with motor hyperexcitability mediated by defective GABAergic transmission17,18. Thus, estimating presynaptic inhibition is worthwhile to investigate experimental pathological conditions on the spinal cord level in vivo. PAD gives rise to volume conducted potentials providing a direct measure of the presynaptic inhibition in the spinal cord. Those potentials are called dorsal root potentials (DRP) and can be measured from spinal cord dorsal roots after stimulation of adjacent dorsal roots7.
First measurements of DRP have been reported in cats and frogs19 and were intensively studied in cats by Eccles, Schmidt, and others in the early 1970s3,4,20,21. While in vivo recordings of DRP in cats22 and rats23 have been widely used, measurements in mice have been almost exclusively performed in ex vivo isolated spinal cord preparations15,24. Here, we describe a method to record DRP in anesthetized mice in vivo allowing a direct measure of presynaptic inhibition in the intact organism.
Protocol
All experimental procedures mentioned in the following protocol were approved by the Thuringian state authorities (Thüringer Landesamt für Verbraucherschutz, Reg.-Nr. 02-044/12).
1. Preparations for Experiment
- Fabrication of suction electrodes
- Pull a micropipette using a standard borosilicate glass capillary with a micropipette puller, e.g. a standard patch electrode.
- Brake the electrode to tip diameter of 0.5-1 mm (slightly larger than the diameter of the dorsal roots) using a diamond file.
- Heat polish the tip so it will not harm the dorsal root when it is sucked in. A standard lab torch will be suitable.
- Mount the glass filament on an electrode holder connected to a syringe through which negative pressure can be applied.
- Preparation of solutions
- Injection anesthesia for mice: Mix 0.75 ml ketamine 10%, 0.24 ml xylazine 2% and 5 ml 0.9% saline. 10 µl/g bodyweight (BW) of the solution will be injected intraperitoneally (i.p.).
- Artificial cerebrospinal fluid (aCSF): Dilute in double distilled water the following salts (in mM): NaCl 134; KCl 3; KH2PO4 1.25; MgSO4•H2O 2; NaHCO3 25; CaCl2 2; D-glucose 10. Add H2O2 to a final concentration of 0.003%. Use always freshly prepared solution.
- Preparation of the recording setup (Figure 2)
- Connect the amplifier to a PC interface for digitizing data.
- Use a PC-based program or an analogue timer to trigger a square pulse stimulator and data acquisition on PC hard drive.
- Use chlorided silverwires connected to standard glass electrode holders as for stimulation and recording.
- Assemble three manipulators for stimulation, recording and reference electrode respectively around a stereotactic frame for mice, so that all electrodes have access to the prepared spinal cord later on.
- Connect tubings and syringes to the electrode holders to be apple to apply negative pressure.
2. General Comments for Animal Experiments and Animal Preparation for Recording Procedure
- Perform all experiments using mice according to the guidelines of the respective institutional animal care and use committee. Perform all surgery and recordings under deep anesthesia ensuring that animal suffering is minimized.
- Anesthetize the animal by i.p. injection of ketamine/xylazine (125mg and 8mg per g BW of ketamine and xylazine, respectively; 10 µl/g BW of the prepared solution as described above). If necessary during long-term recordings, additional injections can be made i.p. or i.m.
Note: Repetitive i.m. injections of 0.05-0.1 ml of ketamine/xylazine solution in the upper thighs have proven to be suitable to keep the animal in deep anesthesia for up to 3 hr. - Use vet ointment on eyes to prevent dryness while under anesthesia.
- Fix the head of the animal in a stereotactic frame and use a heating pad with rectal probe and reflex loop to control body temperature of the animal during the experiment. Fixation of the spinal cord in a stereotaxic frame is not necessary.
- Before starting the preparation, check depth of narcosis by eyeblink reflex and twitching between toes of the mice. Reflexes should be abolished.
- Open the skin along the midline above the spinal cord from the upper thoracic level to lower lumbar areas to get a clear operational field using a scalpel. Do not use scissors to make skin incisions. Carefully loose the skin from the underlying tissue. During subsequent steps keep the wound moistened by 0.9% saline.
- Cut tendons and connective tissue on both side of the vertebrae from lumbar to thoracic levels using scalpel and scissor.
- Remove the spinous processes and rest of connective tissue around the vertebrae using a small nipper.
- Carefully crack vertebrae with the nipper starting from lumbar levels (L4/L5) under a dissecting microscope. Shove the tip of the nipper in the space between the vertebrae and the spinal cord and lift bone pieces apart. Do not harm the dura and avoid pressure to the spinal cord. Both are critical for success. Keep the spinal cord moistened during the whole procedure. Proceed to mid-thoracic levels.
3. Separating of Dorsal Roots and DRP Recording (Figure 2)
- Open the dura mater carefully using a thin needle (30 G) with a bent tip. Use aCSF for moistening from now on.
- Separate the dorsal roots as far as possible using the gauge and cut two adjacent roots as distal as possible. Vigorous pulling on the roots during separation affects the success of the experiment.
- Lower the suction electrode down to the dorsal roots. Add as much aCSF to the spinal cord as possible because liquid helps to suck in the dorsal roots.
- Suck the cut end of one dorsal root into one glass pipettes by applying negative pressure through a syringe. If needed, move the dorsal root in front of the electrode opening using a fine needle.
- If the dorsal root lies dry within the suction electrode, add some aCSF to its tip while carefully sucking until the pipette is sufficiently filled with aCSF.
- After the dorsal root is sucked in, raise the electrode tip from the spinal cord. Take care that no “water bridge” between the pipette tip and the spinal cord short-circuit the recording/stimulation electrode.
- Repeat steps 3.3-3.6 for an ipsilateral adjacent root.
- Position the reference electrode as close as possible to the dorsal roots and keep it moistened by applying aCSF.
- By establishing the recording setup, one root is already chosen for stimulation, the other for recording. Increase voltage stepwise while recording from the second dorsal root is done in current clamp mode. One may notice a short downward deflection which is followed by a slow, long-lasting upward deflection representing the DRP.
- Adjust the stimulus voltage to supramaximal levels and record several sweeps (at least 20 – 30 sweeps/dorsal root at 0.1 Hz, filter between 0.3 Hz-3 kHz).
- Record short trains of three stimuli (100 Hz) to get information about the time-dependent summation of the DRP.
- Switch recording and stimulation site for additional contralateral recordings.
- Animals are not intended to survive the procedure. Sacrifice animals after last recording by decapitation while still under deep anesthesia (ketamine/xylazine, see above, step 2.2).
4. Data Analysis
- Transfer data to analysis program (e.g. Sigma Plot, Igor Pro, or MATLAB).
- Calculate average traces from 20-30 sweeps.
- Take the maximal amplitude of the voltage deflection (from baseline; DRP peak amplitude) for further analysis (Figure 3).
- Calculate the ratio of the DRP peak amplitude after three pulses and single pulse to gain a measure of the time dependent summation of subsequent potentials.
Representative Results
Typical DRP traces are shown in Figure 3. The prominent stimulation artifact is usually followed by a short downward deflection. Thereafter a slow, long-lasting upward deflection, representing the DRP is clearly distinguishable. In a subset of recordings, dorsal root reflexes are visible as small spikes on top of the DRP. In normal wild-type mice, dorsal root reflexes appear most often when stimulation voltage is excessive. As the dorsal root reflexes cannot be elicited with a high reproducibility in this preparation, they were not taken for analysis and care was taken to reduce stimulation voltage to lowest, but still supramaximal strength. For analysis of DRP peak amplitudes, averaged traces of 20-30 subsequent sweeps are used (Figure 3a). The DRP peak amplitudes can be calculated in comparison to baseline levels prior to the stimulation artifact.
The time-dependent summation of the DRP can be analyzed from repetitive stimulations (3 stimuli; 100 Hz; Figure 3b). The peak amplitude is calculated according to the single stimulation experiment. The ratio of the amplitudes after a single and three stimulations at high frequency gives an estimate of the time-dependent summation of PAD.
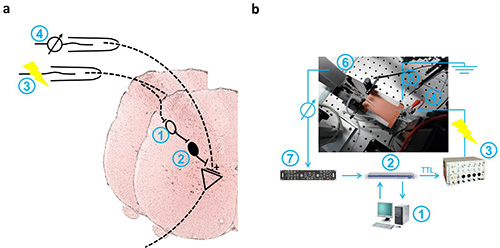
Figure 1. Schematic drawings of PAD-associated spinal cord pathways and recording setup. a. Diagram shows the schematic pathway of presynaptic inhibition in the spinal cord after stimulation of a dorsal root (3). Primary afferent depolarization (PAD) is mediated through activation of a pool of first-order neurons (1) and consecutively GABAergic inhibition by an inhibitory interneuron with an axo-axonal synapse on Ia afferent fibers (2). Dorsal root potentials (DRP) reflect the PAD, spread electronically along the dorsal root and were recorded near its entry to the spinal cord (4). b. Data acquisition is done by a personal computer running patchmaster software (1) using an LIH 8+8 computer interface (2). Voltage signals from the ELC universal amplifier (7) are digitized at 10 kHz. Stimulation is triggered by a TTL signal controlling a S88 dual output square pulse stimulator (3). Voltage pulses are applied through a suction electrode (4). The recording electrode is connected to a preamplifier (6). A chlorided silver wire is used for grounding (5). Please click here to view a larger version of this figure.
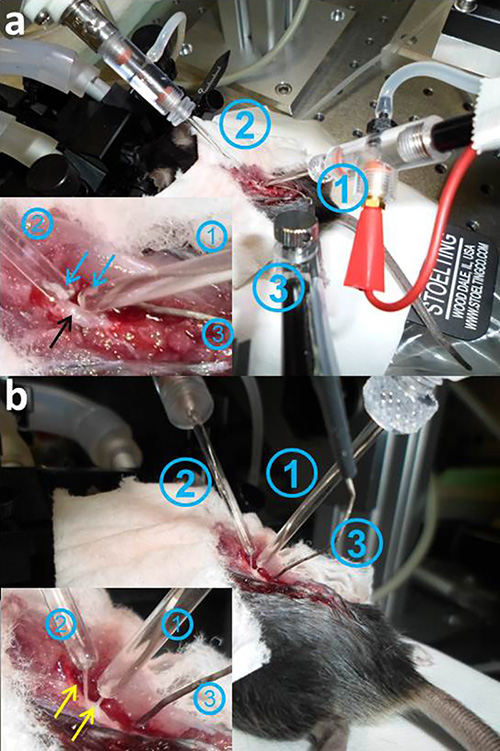
Figure 2. DRP recording in situ. a. The spinal cord of the anesthetized mouse is exposed, the dura mater removed, and moistened with artificial cerebrospinal fluid. The suction electrodes (1: stimulating electrode; 2: recording electrode,) are positioned on the spinal cord. The inset shows the spinal cord (black arrow) and the dorsal roots (blue arrows). The dorsal roots are separated, cut, and sucked into the tips of the electrodes (1,2). b. The tips of the pipettes containing the dorsal roots (inset, yellow arrows) have to be carefully elevated from the spinal cord surface, so that they are not in contact with the surrounding fluid. Stretching of the dorsal roots and touching the spinal cord should be avoided (in a and b: stimulation electrode 1; recording electrode 2; reference electrode 3). Please click here to view a larger version of this figure.
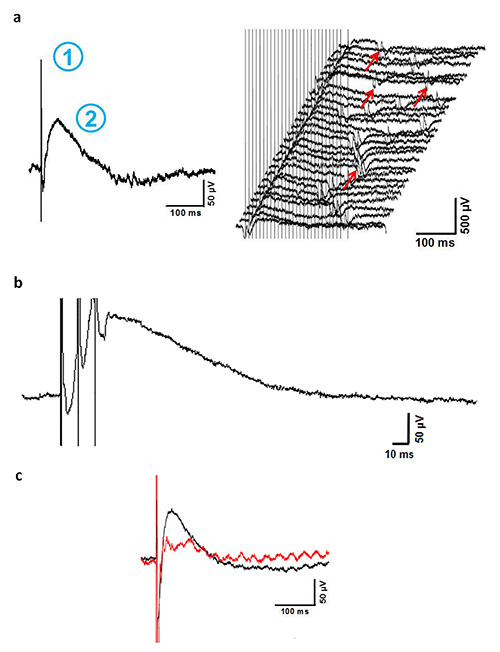
Figure 3. Representative results. a. Averaged recording (left panel) of 28 consecutive traces (right panel) from one pair of dorsal roots. The stimulation artifact (1) is followed by a prolonged negative potential (2, upwards), which reflects the DRP. In some recordings, heartbeat (ECG) is seen as an artifact (right panel, red arrows) but can be clearly differentiated from the DRP. Traces with overlapping ECG artifacts superimposed to the DRP have to be excluded from the analysis. b. Repetitive stimulation (3 stimuli, 10 Hz) leads to a time-dependent summation of the DRP (abscissa is enlarged to clarify temporal summation). c. Example recordings of DRP before (black line) and 5 min after (red line) local application of the GABAergic blocker bicucullin (0.5 mM, 500 µl) onto the exposed spinal cord. Blocking of local GABAergic interneurons leads to a marked decline of DRP peak amplitude confirming GABAergic origin of the recorded potential (note the artifact of 50 Hz sine wave in the red curve which occasionally occurs during DRP in vivo recording and sometimes cannot be prevented even by proper grounding and shielding).
Discussion
Extra- and intracellular electrophysiological recordings of neuronal activity and synaptic potentials in vivo are state of the art techniques in investigating CNS neuronal functions and pathophysiology. Spinal integration is critical for motor function, e.g. limb movement and for multimodal sensory perception. Presynaptic inhibition is one critical mechanism in this computational process ensuring appropriate responses to sensory inputs. GABAergic synapses on Ia afferent fibers inhibit the excitation of motoneurons by PAD. Dorsal root potential-recording in vivo opens the possibility to directly measure PAD in the intact animal. This technique might be of particular interest for research related to disease states in which abnormal spinal inhibition is relevant such as pain, spinal cord injury, peripheral nerve injury, or inflammation. In combination with the use of genetically modified mouse models this technique can be powerful to investigate mechanisms underlying disturbed spinal inhibition. The method here overcomes some limitations of the alternative form of DRP recordings in mice using isolated spinal cord preparations. So, pathways to supraspinal networks are preserved and also combined analysis of spinal and supraspinal neuronal activity is possible. The effect of supraspinal activity on PAD can be investigated by parallel electric stimulation of respective brain centers. In addition, DRP can be evoked by stimulation of peripheral nerve directly, which might be of relevance in animal models of peripheral nerve lesion or inflammation. Using proper anesthesia, vigilance and temperature control, recordings can be performed for hours in the intact animal under physiological conditions.
In contrast, in comparison to the use of isolated spinal cord preparations, in vivo recording of DRP is to some extend limited regarding controlled local drug application and perfusion of bath solution. Drugs can be applied topically but due to the uncertain diffusion into surrounding tissue, the variable solution exchange and amount of solution in the recording preparation it is not possible to control the drug concentration within the spinal cord and the recording site. When acute effects of surface application are studied, these limitations can be partly resolved by local application through pipettes.
The spinal cord of mice is small, highly sensitive to pressure and torsion, and surgery is difficult. Therefore, preparation of mouse spinal cord for in vivo electrophysiology needs some training. There are certain critical points. It is essential that during preparation the dura mater is not harmed and that preferably no or at least very few pressure is applied to the spinal cord. The spinal cord must be kept moistened during the whole procedure, before and after the opening of the dura with NaCl and aCSF, respectively. The signal to noise ratio of DRP recordings can be increased by stimulating and recording as close as possible to the entry point of the respective dorsal root. Diligent grounding and shielding of the recording setup helps to reduce unspecific noise, lamps of the dissection microscope should be turned off or removed during recording.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Manfred Heckmann for helpful discussions during establishing of the method. Further, we thank Claudia Sommer for technical assistance and Frank Schubert for support producing the video. The work was supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1002 and the Interdisciplinary Center for Clinical Research (IZKF) of Jena University Hospital.
Materials
| Glass tubing (inner diameter 1.16 mm) | Science Products (Hofheim, Germany) | GB200F-10 | Other glass tubing might also be suitable |
| Superfusion solution (sterile, 0,9% NaCl) | Braun Melsungen AG | 3570350 | |
| (Melsungen, Germany) | |||
| Rompun 2% (Xylazine) | Bayer Animal Health GmbH (Leverkusen, Germany) | ||
| Ketamin 10% | Medistar GmbH (Ascheberg, Germany) | KETAMIN 10% | |
| 30G micro needle/ Sterican | Braun Melsungen AG | 4656300 | |
| (Melsungen, Geramny) | |||
| Salts for aCSF | Sigma-Aldrich | Diverse | |
| S88 Dual Output Square Pulse | Grass Technologies (Warwick, USA) | S88X | |
| Stimulator | |||
| SIU5 RF Transformer Isolation Unit | Grass Technologies (Warwick, USA) | SIU-V | |
| InstruTECH LIH 8+8 | HEKA (Lambrecht, Deutschland) | LIH 8+8 + Patchmaster software | |
| Data acquisition | |||
| Universal amplifier | npi (Tamm, Deutschland) | ELC-03X | |
| Micropipette puller | Sutter Instruments (Novato, USA) | P-1000 | |
| Dissecting microscope | Olympus (Tokyo, Japan) | ||
| Micromanipulator | Sutter Instruments (Novato, USA) | MPC-200/MPC-325 | Mechanical micromanipulators also possible |
| Homeothermic Blanket System | Stoelting (Wood Dale, USA) | 50300V | |
| Intra-/extracellular recording electrode holder | Harvard Apparatus (Holliston, USA) | 641227 |
References
- Eccles, J. C., Eccles, R. M., Magni, F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J. Physiol. 159, 147-166 (1961).
- Levy, R. A. The role of gaba in primary afferent depolarization. Prog. Neurobiol. 9, 211-267 (1977).
- Eccles, J. C., Magni, F., Willis, W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J. Physiol. 160, 62-93 (1962).
- Eccles, J. C., Schmidt, R., Willis, W. D. Pharmacological Studies on Presynaptic Inhibition. J. Physiol. 168, 500-530 (1963).
- Maxwell, D. J., Bannatyne, B. A. Ultrastructure of muscle spindle afferent terminations in lamina VI of the cat spinal cord. Brain Res. 288, 297-301 (1983).
- Barber, R. P., Vaughn, J. E., Saito, K., McLaughlin, B. J., Roberts, E. GABAergic terminals are presynaptic to primary afferent terminals in the substantia gelatinosa of the rat spinal cord. Brain Res. 141, 35-55 (1978).
- Wall, P. D., Lidierth, M. Five sources of a dorsal root potential: their interactions and origins in the superficial dorsal horn. J. Neurophysiol. 78, 860-871 (1997).
- Rudomin, P. In search of lost presynaptic inhibition. Exp. Brain Res. 196, 139-151 (2009).
- Rudomin, P., Schmidt, R. F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 129, 1-37 (1999).
- Kullmann, D. M., et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why?. Prog. Biophys. Mol. Biol. 87, 33-46 (2005).
- Hochman, S., Shreckengost, J., Kimura, H., Quevedo, J. Presynaptic inhibition of primary afferents by depolarization: observations supporting nontraditional mechanisms. Ann. N.Y. Acad. Sci. 1198, 140-152 (2010).
- Thompson, S. W., Wall, P. D. The effect of GABA and 5-HT receptor antagonists on rat dorsal root potentials. Neurosci. Lett. 217, 153-156 (1996).
- Enriquez-Denton, M., Manjarrez, E., Rudomin, P. Persistence of PAD and presynaptic inhibition of muscle spindle afferents after peripheral nerve crush. Brain Res. 1027, 179-187 (2004).
- Wall, P. D., Devor, M. The effect of peripheral nerve injury on dorsal root potentials and on transmission of afferent signals into the spinal cord. Brain Res. 209, 95-111 (1981).
- Witschi, R., et al. Presynaptic α2-GABAA Receptors in Primary Afferent Depolarization and Spinal Pain Control. J. Neurosci. 31, 8134-8142 (2011).
- Calancie, B., et al. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in. 89, 177-186 (1993).
- Geis, C., et al. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 133, 3166-3180 (2010).
- Geis, C., et al. Human IgG directed against amphiphysin induces anxiety behavior in a rat model after intrathecal passive transfer. J. Neural Transm. 119 (8), 981-985 (2012).
- Barron, D. H., Matthews, B. H. The interpretation of potential changes in the spinal cord. J. Physiol. 92, 276-321 (1938).
- Schmidt, R. F., Trautwein, W., Zimmermann, M. Dorsal root potentials evoked by natural stimulation of cutaneous afferents. Nature. 212, 522-523 (1966).
- Eccles, J. C., Schmidt, R. F., Willis, W. D. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J. Physiol. 161, 282-297 (1962).
- Manjarrez, E., Rojas-Piloni, J. G., Jimenez, I., Rudomin, P. Modulation of synaptic transmission from segmental afferents by spontaneous activity of dorsal horn spinal neurones in the cat. J. Physiol. 529 Pt 2, 445-460 (2000).
- Geis, C., et al. Human Stiff-Person Syndrome IgG Induces Anxious Behavior in Rats. PLoS One. 6, e16775 (2011).
- Martinez-Gomez, J., Lopez-Garcia, J. A. Electrophysiological and pharmacological characterisation of ascending anterolateral axons in the in vitro mouse spinal cord. J. Neurosci. Methods. 146, 84-90 (2005).

