An Assay for Measuring the Effects of Ethanol on the Locomotion Speed of Caenorhabditis elegans
Summary
C. elegans is a useful model for studying the effects of ethanol on behavior. We present a behavioral assay that quantifies the effects of ethanol on the locomotion speed of crawling worms; both initial sensitivity and the development of acute functional tolerance to ethanol can be measured with this assay.
Abstract
Alcohol use disorders are a significant public health concern, for which there are few effective treatment strategies. One difficulty that has delayed the development of more effective treatments is the relative lack of understanding of the molecular underpinnings of the effects of ethanol on behavior. The nematode, Caenorhabditis elegans (C. elegans), provides a useful model in which to generate and test hypotheses about the molecular effects of ethanol. Here, we describe an assay that has been developed and used to examine the roles of particular genes and environmental factors in behavioral responses to ethanol, in which locomotion is the behavioral output. Ethanol dose-dependently causes an acute depression of crawling on an agar surface. The effects are dynamic; animals exposed to a high concentration demonstrate an initial strong depression of crawling, referred to here as initial sensitivity, and then partially recover locomotion speed despite the continued presence of the drug. This ethanol-induced behavioral plasticity is referred to here as the development of acute functional tolerance. This assay has been used to demonstrate that these two phenotypes are distinct and genetically separable. The straightforward locomotion assay described here is suitable for examining the effects of both genetic and environmental manipulations on these acute behavioral responses to ethanol in C. elegans.
Introduction
Alcohol use disorders (AUD) are widespread and produce serious health, social, and economic problems. In humans, the susceptibility to developing an AUD is heavily influenced by both genetics and the environment1,2. A strong physiological predictor of abuse liability is the initial level of response (LR) to alcohol (ethanol) that is exhibited by naïve drinkers3-5. This LR phenotype is influenced by genetics and non-genetic components6. Determining the molecular mechanisms that influence the LR to ethanol is an important goal of the study of ethanol response behaviors.
The nematode, Caenorhabditis elegans, has been increasingly used as a model for studying the effects of ethanol on behavior7-9. There is strong molecular conservation in the machinery of nervous system function between worms and mammals, and several genes that have been shown to influence the LR to ethanol in worms have been shown to influence LR to ethanol in mammals10-16, and have been implicated in abuse liability in humans17-19.
Ethanol intoxicates worms, which is reflected in a decrease in their locomotion speed. Several different laboratories have developed behavioral assays that differ in several ways, for example, the locomotion behavior that they study (crawling versus swimming11,12,14,20,21) or in the composition of the solutions in which the assays are performed (nematode growth medium versus Dent’s saline20,22). Interestingly, these diverse assays have yielded somewhat different dose response profiles for the effects of ethanol. These results have pointed to important differences in the underlying behaviors of crawling and swimming9,23, as well as a role for the environmental variable osmolarity in ethanol responses20, and have highlighted the importance of describing experimental detail of the various assays.
An assay to measure the acute effects of ethanol on crawling behavior is presented here. This assay has been used extensively to study the genetic and environmental influences on the LR to ethanol8,10,20,24,25. The mammalian LR phenotype is a composite of at least two components, initial sensitivity to ethanol and acute functional tolerance to ethanol26,27. In worms, the LR phenotype has been shown to be separable into these two components through the use of this behavioral assay. The influences of genetic and environmental manipulations on both phenotypes can be examined using this single assay. Importantly, these two phenotypes are genetically separable.
Protocol
1. Steps to Perform on the Day before the Assay
- Pick L4 stage worms to fresh nematode growth medium (NGM) plates seeded with a lawn of OP50 E. coli, and culture them at 20 ˚C O/N. Each assay condition requires 10 worms; pick an excess of worms to allow for O/N loss of worms.
- Only assay animals that are first-day adults; many mutants grow at a slower speed than wild type. Adjust the timing of picking for strains that have developmental delays so that all animals tested are first-day adults.
2. Steps to Perform on the Day of the Assay
- Preparation for the assay:
- Perform assays on standard unseeded 60 x 15 mm NGM plates. Dry all of the plates to be used at 37 ˚C for 2 hr, with lids off. For each experimental trial, use four dried NGM plates; these will be the 0 mM and 400 mM ethanol assay plates and their accompanying acclimation plates.
NOTE: The use of NGM plates is important here; differences in the composition of the plates, in particular their osmolarity, can strongly affect the dose response of ethanol on behavior, this is due, at least in part, to changes in the amount of ethanol accumulated by the animals20. NGM agar is 160 mOsm. - After drying, weigh each of the unseeded NGM plates to be used in the assay and note the weight. Determine the volume of media in the plates based on the weight of an empty plate. To approximate the conversion of weight of the agar to volume, assume that the media weighs the same as an equal volume of water.
NOTE: Our most consistent results have been found with NGM media that has dehydrated sufficiently that an original 10 ml volume has a post-drying volume between 8.3–8.9 ml. An alternative to the 2 hr dry time is to dry until the plates reach this volume range to account for differences in incubators. - Melt 4 copper rings (inner diameter of 1.6 cm) into the surface of each of the plates to act as corrals for the different genotypes or treatment groups of worms.Grasp each ring with forceps, and heat in a flame (a strong flame from a Bunsen burner works well) for approximately 3 sec. Immediately place the ring on a plate while still holding the ring with the forceps to prevent it from ‘skipping’.
- Ensure that the ring rests flat against the surface of the agar by pressing down gently with the forceps at several points on the ring. When placing the ring, be careful to leave room for an additional three rings.
- Melt the three additional copper rings into the surface of the plate, taking care to place them as close together as possible so that all four will be in the field of view of the camera.
NOTE: Making a good seal with the agar is essential to keep the worms in the rings during the assay. Rings that skip around on the plate are unlikely to make the correct seal with the agar and may scar the agar, which allows worms to burrow and interferes with visualizing the worms. - For each assay plate prepare an accompanying “acclimation” plate, which should be dried and unseeded and will receive no ethanol. Place four copper rings on these plates.
- Label the bottom of the plates next to each ring with the worm strain to be used in the ring, taking care to not write in the field of view of the ring itself. Match the labels on the assay plate with its accompanying acclimation plate.
- Calculate the amount of 100% ethanol to add to each assay plate so that the final concentration is 0 mM or 400 mM ethanol (weight to volume). For each experiment (n = 1), there will be one 0 mM and one 400 mM ethanol plate; acclimation plates do not receive ethanol. Add the ethanol, pipetting it around the surface of the plate. Use laboratory film to seal the plate and allow it to equilibrate on the bench top for 2 hr.
- When 1.5 hr has elapsed, begin the acclimation step of the assay, step 2.2.
- Perform assays on standard unseeded 60 x 15 mm NGM plates. Dry all of the plates to be used at 37 ˚C for 2 hr, with lids off. For each experimental trial, use four dried NGM plates; these will be the 0 mM and 400 mM ethanol assay plates and their accompanying acclimation plates.
- Perform locomotion assay: Assay plates
- Carefully transfer 10 worms of each experimental group to the center of a copper ring on the acclimation plate. Remove any food that is visible on the agar at this point by scraping gently with the worm pick. Vary the order in which the experimental groups are put on the plates across experimental trials so that the same strains are not put on the plates in the same order across trials.
NOTE: The goal is to transfer the animals to the plate with minimal quantities of food, because if food on the acclimation plate is transferred to the assay plate the worms will aggregate around the food and the effect of the drug on locomotion will be obscured. - Be careful not to break the surface of the agar with the pick, as this will allow the worms to burrow and will disrupt the assay. Incubate the worms at RT for 30 min.
- Ensure that there is an appropriate interval between the initiations of each plate. An experienced experimenter can move 40 animals, 10/ring for 4 rings in < 1.5 min, but any interval up to 2 min is acceptable. The standard assay has movies recording at 10-12 min and 30-32 min of exposure for both 0 mM and 400 mM ethanol.
- Initiate the acclimation plate for the 0 mM exposure and the acclimation plate for the 400 mM exposure approximately 2 min and 30 sec apart to allow for saving of the first movie file before the second movie must begin.
- After the 30-min acclimation period, transfer the worms from the acclimation plates to the assay plates. Transfer the worms to the assay plates (0 mM or 400 mM ethanol) in the same order that they were added to the acclimation plate, keeping track of the timing between the completions of each plate. Seal the plate with laboratory film to minimize loss of ethanol to vaporization.
- Use a scooping motion with a thin-edged flattened worm pick to collect worms on top of the flattened pick. Perform the transfer of worms from the unseeded acclimation plate to the assay plate without the use of bacteria, which is commonly used in transferring worms as it helps to glue the worm to the pick.
NOTE: The speed at which animals are transferred at this step is very important because the first worms on the plate will be exposed to ethanol longer than the last worms added to the plate, and earlier time points may show some time-dependent effects. This is the major reason for rotating which strains are placed on a plate first across experimental replicates.
- Carefully transfer 10 worms of each experimental group to the center of a copper ring on the acclimation plate. Remove any food that is visible on the agar at this point by scraping gently with the worm pick. Vary the order in which the experimental groups are put on the plates across experimental trials so that the same strains are not put on the plates in the same order across trials.
- Perform Locomotion Assay: Filming
- Use a microscope/camera combination that allows for simultaneous imaging of all four rings in the field of view (approximately 42×42 mm2 square), such as a 0.5x microscope objective, 0.8x magnification and a camera with a 2048×2048 7.4 µm pixel CCD.
- Use even illumination across the field of view, which aids in object recognition at the intensity threshold step (see below). A 3”x3” backlight works well.
- Image the worms on a plate positioned media-side up (lid down), which generates contrast that is lost when using the backlight compared with a traditional microscope transmitted light source.
- Prepare the image analysis software to capture time-lapse movies of the moving worms. Set the software to capture 12-bit gray-scale images every 1 sec, as a 2-min (120 frame) movie. To reduce the size of the output file, while still retaining sufficient image resolution, use a 2×2 binning mode to capture 1024×1024 pixel images.
- Record the movies. Begin recording a 120-frame movie of the first plate (0 mM ethanol) 10 min after the last worms were placed on that plate. Save the movie file.
- Record the second plate (400 mM ethanol). Repeat this process for both plates beginning 30 min after the last worms were placed on the first plate (0 mM ethanol) to capture the 30–32 min time points for each exposure.
NOTE: Allow sufficient time to save the image files after each capture session before beginning recording of the next plate to be analyzed. - For future-proofing of archived movies and to allow these movies to be opened and analyzed by other public domain open source imaging software programs, such as ImageJ28, convert a copy of each movie to 8-bit 256 gray scale TIFF files.
NOTE: The image analysis software described here uses a proprietary file format.
- Analysis of movies using ImagePro software.
- Once captured, analyze the movies using Image-Pro Plus software or its equivalent.
NOTE: A variety of other object tracking software is available that has been used to successfully track multiple C. elegans at one time29 and such software could reasonably substitute for that described here as the object identification steps are based on similar principles. The method described relates to Image-Pro Plus software versions 6.0-6.3 and 7.0, newer versions of Image-Pro Plus software may have minor differences. - Analyze movies in 2-min (120 frame) segments. First, apply a filter to the images to flatten the background and enhance the contrast of the worm objects. Select the filter as follows: Menu>Process>Filters>Enhancement>Flatten: Parameters: Background = Dark; Feature Width = 20 Pix.
- Re-save the movies after the filtering step but always retain the original movie in its unfiltered form.
- Analyze the locomotion of animals in each ring separately with a circular region of interest (ROI) that is placed and sized to overlap with the copper ring. Identify and track the worms with the Menu>Measure>Track Objects… command; this brings up the Tracking Data Table window. The Tracking Options button allows specific tracks to be excluded and to limit any experimental artifacts.
- Under the Auto Tracking tab, use the following parameters: Track Parameters: Velocity limit (search radius) = 400 µm/frame, Acceleration limit = Auto, Minimum total track length = 400 µm, Predominant motion type = chaotic; Objects in tracks parameters: Allow partial tracks = yes, Min length = 21 frames, Tracking prediction depth = 1 frame.
- To initiate the tracking process, click the Find All Tracks Automatically function button to bring up the Count/Size options dialog box and the Tracking dialog box. Select the Manual option for the Intensity Range Selection in the Count/Size dialog box, which provides the important threshold step that uses the gray-scale intensity of the worm pixels to highlight a worm object for analysis and to ignore the lighter background pixels.
- Adjust the intensity threshold sliders (one to set the upper limit, one to set the lower limit) to create an inclusive range that highlights all dark objects. A range of 0–1,500 on a scale of 0–4,095 is a good starting point for finer tuning.
- Apply a size filter to exclude objects that are larger and smaller than a single worm object, such as the copper ring and any debris on the plates. Set two size parameters to do this that cover sizes for individual worms in a variety of postures with the Measure>Select Measurements menu item on the Count/Size options dialog box. (Area range: 28,000–120,000 µm2 and Perimeter range: 600–2,500 µm).
- If a mutant strain is significantly smaller or larger than other animals on the plate to be analyzed, broaden the range of the filter settings for object recognition to accommodate the different sizes of the strains first before tracking animals so that settings do not need to be changed mid-analysis.
- Complete the tracking process by clicking Continue in the Tracking dialog box. Visually compare the output tracks with the progress of each worm in the movie to ensure that every worm is represented unless there is a valid reason to exclude it based on the automatic filter settings. Manually delete tracks that were produced by the presence of confirmed non-worm objects that met the size filter criteria.
- Use the software to calculate the velocity of each worm between each frame (distance traveled for the centroid of an object per 1 sec between frames) and display the average velocity for each track and the average velocity across all tracks for the population of worms in the copper ring. Consider this final average to be n = 1 in terms of experimental trials. Export the data to a spreadsheet program for statistical analyses and data archiving.
- Once the data has been recorded for the first ring on the plate, move the ROI to the next ring and repeat the tracking process, without changing any of the parameters.
- Calculate relative speed of locomotion through:
- Relative speed (%) = treated (400 mM) speed/ untreated (0 mM) speed x 100.
NOTE: Different genetic manipulations often alter the basal locomotion rate of animals. In order to determine the effect of ethanol on the locomotion speed of animals and to be able to compare these effects across different genotypes or conditions, calculate a relative speed.
- Once captured, analyze the movies using Image-Pro Plus software or its equivalent.
Representative Results
Representative data (Figure 1) from several different genotypes and their paired controls are presented8,24; data were specifically chosen that highlight differences in the assayed animals. The degree of effect at 10 min of exposure is considered the initial sensitivity of a strain, which is shown on the left axes in Figure 1B-G. Mutant strains with a relative speed larger than the control at 10 min are considered to be ethanol resistant (Figure 1F, G), while mutant strains with a relative speed that is less than the control are considered to be hypersensitive to ethanol (Figure 1D, E). The right axes in Figure 1 show the degree of recovery of speed between the 10- and 30-min time periods. This represents a measure of the rate of development of acute functional tolerance (AFT) and is calculated as the relative speed at 10 min subtracted from the relative speed at 30 min. Strains with larger recovery values are considered to have a more rapid rate of development of AFT compared with control animals (Figure 1D, F) and strains with lower recovery values than the control animals are considered to have lower rates of development of AFT (Figure 1C, E). Note that the measured AFT for sbp1(ep79) (Figure 1G) is trending toward a negative recovery but was not statistically different from wild type due to large variance (p =0.08 for comparison against N2 recovery). tub-1 encodes a TUBBY homolog; bbs-1 encodes a homolog to human BBS1; lips-7 encodes a triacylglycerol lipase; fat-1 encodes an omega-3 fatty acyl desaturase; fat-3 encodes a delta-6 fatty acid desaturase; sbp-1 encodes a homolog to human Sterol Regulatory Element Binding Proteins (SREBPs).
There are several important characteristics of the data to note: First, the N2 (wild-type) control data differs across experiments; this is likely to be due to environmental factors that are difficult to control, such as absolute water content or salt concentration of the dried media and temperature in the laboratory. Typically, 400 mM exogenous ethanol depresses the speed of N2 to approximately 30% of the untreated controls; however the absolute degree of depression varies from experiment to experiment. Further, while approximately 12% recovery of locomotion speed over 30 min in N2 is usually expected, here again the numbers vary. Importantly, while the absolute levels of sensitivity and AFT exhibit day-to-day variation, the comparisons between genotypes or conditions do not generally differ. That is, wild-type and a mutant will always have similar effects relative to each other; if a mutant causes a decrease in AFT relative to wild-type, that decrease will scale with the amount of AFT demonstrated by wild type. This highlights the importance of performing paired controls on the same plates at the same time for strains or conditions that are being compared.
Second, the phenotypes of initial sensitivity and AFT are genetically separable. Note that initial sensitivity and acute functional tolerance can vary together or separately; examples have been included of a variety of phenotypes in which initial sensitivity and AFT differ independently. An alteration in one of the phenotypes does not predict a change in the other phenotype, nor does it predict the direction of change of the second phenotype (either increased or decreased response to ethanol).
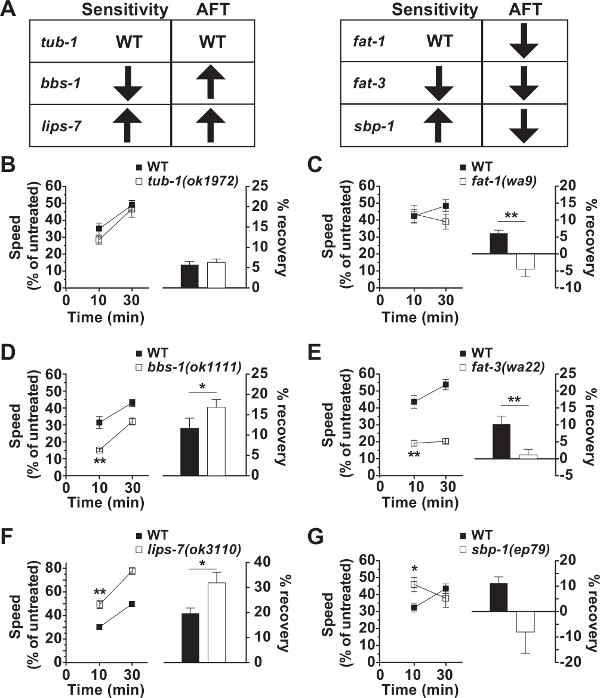
Figure 1. Initial ethanol sensitivity and the rate of development of acute functional tolerance (AFT) are separable behavioral responses. (A) Summary table for the direction of effects of mutations in the named genes on the level of initial sensitivity to ethanol and the rate of development of AFT, both are in comparison with the wild-type controls (N2 in each case). (B–G) Acute locomotion responses to 400 mM exogenous ethanol are measured at 10-12 min and 30-32 min of continuous ethanol exposure. Relative speeds (% of untreated speed) are shown on the left axes. The degrees of recovery in speed are shown on the right axes and represent a measure of the rate of AFT during that 20-min interval. The number of trials compared is as follows: B, C, E: n = 6; G: n = 7; D: n = 9; F: n = 11. Statistically significant comparisons (paired t-tests) are shown: *, p < 0.05; **, p < 0.01. 8,24 The data presented in this figure are modified from 8,24. Please click here to view a larger version of this figure.
Discussion
The simple neurobiology and genetic tools available in C. elegans make the worm an excellent model in which to study the molecular bases of the effects of ethanol on behavior. Here, we describe an assay that has been used to identify several molecular and environmental mediators of the acute behavioral response to ethanol8,10,20,24,25. This method allows the differentiation and simultaneous examination of two different ethanol response behavior phenotypes, initial sensitivity and the development of acute functional tolerance, that together model the composite phenotype of level of response in mammals. The same assay could be easily adapted to study other pharmacological agents, thereby taking advantage of the simultaneous testing of multiple strains in the assessment of the behavioral effects of other drugs.
The 10-12 min time window provides a useful time point to measure initial sensitivity of a strain to ethanol. Analysis of the effects of 400 mM ethanol across the first 10 min of exposure shows speed effects beginning in the second minute of exposure but a steady state of effect is not achieved until the seventh minute of ethanol exposure 20. Due to this time-dependent effect on locomotion speed, the order in which particular strains are placed on the plate will be important for any speed measurements prior to the eighth or ninth minute (assuming it takes less than 2 min to place all of the worms on the ethanol plate). At these earlier time points some worms will be more affected by ethanol simply because they were placed on the plate earlier. Even though a stable initial effect of ethanol is achieved by the seventh minute, it is recommended that the order in which strains are placed on plates be varied across replicate trials to minimize any systematic effect across strains due to this time-dependent effect of ethanol and the time required to move worms to the plate.
Specific parameters were chosen in the object tracking steps to minimize bias from worms that repeatedly collide with the copper ring or with other worms. The object size filter step should eliminate any object from tracking that is larger than a single worm, including two touching worms. If a worm touches another worm or the copper ring then the track ends and it should no longer be recognized as a valid object while it remains in contact. When the worm loses contact with the other object, it becomes a new object and initiates a new track. If the track made by a worm is less than 20 sec in length, then it is automatically excluded by defining a minimum track length of 21 frames. This filter prevents instances of a worm moving forward into another object (worm or ring), reversing and then going forward to touch that object again, as short bouts of quick reversals may bias the overall average speed of the population of animals. Using these settings, it is possible and not uncommon for a single worm to contribute more than one track that is greater than 20 sec in length (but less than 99 sec) to the overall average. The settings could be altered so that each worm only contributes a single track by making the minimum track length 61 sec (out of a total of 120 sec), but empirical observation determined that this eliminated a significant number of tracks and it was found that slower worms that collided with other worms less frequently would be over-represented. Alternatively, new tracks could be disallowed to begin after the first frame of the movie but then worms that collide early in the 2-min period are removed from the analysis and useful data would be lost. Finally, the use of 10 worms per copper ring represents a good compromise between having multiple representative tracks contributing to an average and the problem of too many worm collisions.
This behavioral assay has several important advantages for the study of ethanol response behaviors in worms. Simultaneous testing of several genotypes on the same plate is possible through the use of copper “corrals.” This innovation has several distinct advantages. First, in this assay, the control condition or strain is always tested on the same plate at the same time as the experimental condition or strain, and only animals tested simultaneously are directly compared. This is of particular importance because behavioral assays are often influenced by the environment, and day-to-day variation in behavioral responses can increase the noise in the assay and decrease the ability to detect signals. The side-by-side comparison of experimental groups in identical conditions substantially decreases the impact of day-to-day environmental variation, which is a major advantage of this approach. Second, the use of copper corrals contains animals in the field of view, which allows the locomotion assays to be performed in the absence of food. When deprived of food, worms move quickly and tend to disperse in search of bacteria; without the rings, worms would leave the field of view during the course of the assay. In addition, the worms are repelled by copper and tend to stay closer to the middle of the rings, making them easy to visualize. The fact that the worms move quickly in these assay conditions substantially increases the resolution to detect depressive effects of ethanol; when worms move slowly, the speeds reach the floor of the ability to detect them earlier. Note that copper is toxic to the worms; while the rings do not appear to affect the acute responses to ethanol assayed in this protocol, extended incubation of animals in the rings (over several hours) is not recommended. Third, with this experimental design, a single assay can assess the behavioral responses of up to four different experimental groups. Therefore, the time taken to accumulate statistically meaningful data is decreased. In addition, since a single digital movie is made, this decreases memory space requirements for long-term storage of primary data. Finally, in each experiment, the behavior of 10 individual animals of each experimental group is examined. The speeds of those 10 individuals are averaged to generate a composite speed for a single assay, so that n = 1 trial. This averaging of the behavior of many animals further decreases variability in these sensitive behavioral assays, and further increases the resolution to detect subtle effects on ethanol response behaviors.
There are limitations to the methods described here, some of which apply to behavioral analyses of any mutant animals. One significant limitation to this approach is that mutant worms that have very significantly reduced basal speeds, due to incoordination or near paralysis, may be identified as falsely positive for resistance to the effects of ethanol because their measured speed decreases to a value that is below the threshold of accurate detection for a particular imaging setup. As most object tracking software programs use a centroid (center of mass) as the position from which to measure distance traveled between frames, a stationary worm that moves its head can still generate a shift in the position of the centroid and that shift would be detected as movement. Therefore a worm needs to be moving faster than is caused by subtle shifts in body posture in order to measure as true locomotion. Worms exposed to ethanol are not paralyzed but they are very uncoordinated, so with worms that already have a deficit in locomotion it is possible that ethanol exposure will decrease their rates of speed below this technical floor effect. Increasing the time between frames so that larger changes in body position would be separable from subtle shifts in centroid position does not solve this problem, if the time between frames is lengthened much more than 1 sec it becomes more difficult for object tracking software to ensure that the same worm is contributing to a track rather than a track jumping between close but not touching worms.
Disclosures
The authors have nothing to disclose.
Acknowledgements
These studies were supported by grants from the National Institutes of Health, National Institute for Alcoholism and Alcohol Abuse: R01AA016837 (JCB) and P20AA017828 (AGD and JCB).
Materials
| C. elegans strains | Caenorhabditis Genetics Center | ||
| 60 x15 mm Petri plates, triple vented | Greiner Bio-One | 628161 | Other plate brands will suffice. |
| NGM agar | Various | NaCl (3g/L), agar (17g/L), peptone (2.5g/L), 1 mL cholesterol (5mg/mL in ethanol), 1 mL (1M) MgSO4, 1 mL (1M) CaCl2, 25 mL (1M) KPO4, pH=6, 975 mL H2O | |
| Forceps | Various | e.g. Fisher Scientific #10300 | |
| 37°C Incubator | Various | For drying agar | |
| Digital balance | Various | For determining plate weights and agar volume | |
| Copper rings | Plumbmaster | STK#35583 (48 cap thread gasket) | 1.6 cm inner diameter, 1.8 cm outer diameter copper rings |
| 100% ethanol | Various | ||
| Parafilm M | Bemis | PM996 | |
| CCD camera | QImaging | RET-4000R-F-M-12 | This camera has a large field of view. |
| Stereomicroscope with C-mount and 0.5X objective | Leica | MZ6 | Discontinued model, M60 is current equivalent. |
| Light source | Schott | A08923 | 3”x3” backlight for even illumination across the field of view |
| Imaging and tracking software | Media Cybernetics | ImagePro-Plus v6.0-6.3 | Newer versions of the software have tracking functions. |
References
- Prescott, C. A., Kendler, K. S. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am. J. Psychiatry. 156, 34-40 (1999).
- Schuckit, M. A. Genetics of the risk for alcoholism. Am. J. Addict. Article Review. 9, 103-112 (2000).
- Heath, A. C., et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol. Med. 29, 1069-1081 (1999).
- Rodriguez, L. A., Wilson, J. R., Nagoshi, C. T. Does psychomotor sensitivity to alcohol predict subsequent alcohol use. Alcohol. Clin. Exp. Res. 17, 155-161 (1993).
- Schuckit, M. A., Smith, T. L. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch. Gen. Psychiatry. 53, 202-210 (1996).
- Kalu, N., et al. Heritability of level of response and association with recent drinking history in nonalcohol-dependent drinkers. Alcohol. Clin. Exp. Res. 36, 522-529 (2012).
- Davis, S. J., Scott, L. L., Hu, K., Pierce-Shimomura, J. T. Conserved single residue in the BK potassium channel required for activation by alcohol and intoxication in. C. elegans. J. Neurosci. 34, 9562-9573 (2014).
- Raabe, R. C., Mathies, L. D., Davies, A. G., Bettinger, J. C. The Omega-3 Fatty Acid Eicosapentaenoic Acid Is Required for Normal Alcohol Response Behaviors in C. elegans. PLoS ONE. 9, e105999 (2014).
- Topper, S. M., Aguilar, S. C., Topper, V. Y., Elbel, E., Pierce-Shimomura, J. T. Alcohol disinhibition of behaviors in C. elegans. PLoS ONE. 9, e92965 (2014).
- Bhandari, P., et al. Chloride intracellular channels modulate acute ethanol behaviors Drosophila,Caenorhabditis elegans and mice. Genes Brain Behav. 11, 387-397 (2012).
- Davies, A. G., et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 115, 655-666 (2003).
- Davies, A. G., Bettinger, J. C., Thiele, T. R., Judy, M. E., McIntire, S. L. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron. 42, 731-743 (2004).
- Kapfhamer, D., et al. Loss of RAB-3/A in Caenorhabditis elegans and the mouse affects behavioral response to ethanol. Genes Brain Behav. 7, 669-676 (2008).
- Speca, D. J., et al. Conserved role of unc-79 in ethanol responses in lightweight mutant mice. PLoS Genet. 6, e1001057 (2010).
- Thiele, T. E., Badia-Elder, N. E. A role for neuropeptide Y in alcohol intake control: evidence from human and animal research. Physiol. Behav. 79, 95-101 (2003).
- Treistman, S. N., Martin, G. E. BK Channels: mediators and models for alcohol tolerance. Trends Neurosci. 32, 629-637 (2009).
- Han, S., et al. Integrating GWASs and human protein interaction networks identifies a gene subnetwork underlying alcohol dependence. Am. J. Hum. Genet. 93, 1027-1034 (2013).
- Kendler, K. S., et al. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcohol. Clin. Exp. Res. 35, 963-975 (2011).
- Schuckit, M. A., et al. Autosomal linkage analysis for the level of response to alcohol. Alcohol. Clin. Exp. Res. 29, 1976-1982 (2005).
- Alaimo, J. T., et al. Ethanol metabolism and osmolarity modify behavioral responses to ethanol in C. elegans. Alcohol. Clin. Exp. Res. 36, 1840-1850 (2012).
- Morgan, P. G., Sedensky, M. M. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol. Clin. Exp. Res. 19, 1423-1429 (1995).
- Mitchell, P. H., et al. The concentration-dependent effects of ethanol on Caenorhabditis elegans behaviour. Pharmacogenomics J. 7, 411-417 (2007).
- Vidal-Gadea, A., et al. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc. Natl. Acad. Sci. U S A. 108, 17504-17509 (2011).
- Bettinger, J. C., Leung, K., Bolling, M. H., Goldsmith, A. D., Davies, A. G. Lipid environment modulates the development of acute tolerance to ethanol in Caenorhabditis elegans. PLoS ONE. 7, e35129 (2012).
- Davies, A. G., et al. Different genes influence toluene- and ethanol-induced locomotor impairment in C. elegans. Drug Alcohol Depend. 122, 47-54 (2012).
- Newlin, D., Thomson, J. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol. Bull. 108, 383-402 (1990).
- Ponomarev, I., Crabbe, J. C. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J. Pharmacol. Exp. Ther. 302, 257-263 (2002).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9, 671-675 (2012).
- Husson, S. J., Costa, W. S., Schmitt, C., Gottschalk, A. Keeping track of worm trackers. WormBook. , (2012).

