Human Primary Trophoblast Cell Culture Model to Study the Protective Effects of Melatonin Against Hypoxia/reoxygenation-induced Disruption
Summary
This manuscript presents a unique in vitro model of immunopurified human villous cytotrophoblast cells cultured under hypoxia/reoxygenation. This model is suitable to study the protective effects of promising treatments, such as melatonin, on pregnancy complications associated with increased oxidative stress and altered placental function.
Abstract
This protocol describes how villous cytotrophoblast cells are isolated from placentas at term by successive enzymatic digestions, followed by density centrifugation, media gradient isolation and immunomagnetic purification. As observed in vivo, mononucleated villous cytotrophoblast cells in primary culture differentiate into multinucleated syncytiotrophoblast cells after 72 hr. Compared to normoxia (8% O2), villous cytotrophoblast cells that undergo hypoxia/reoxygenation (0.5% / 8% O2) undergo increased oxidative stress and intrinsic apoptosis, similar to that observed in vivo in pregnancy complications such as preeclampsia, preterm birth, and intrauterine growth restriction. In this context, primary villous trophoblasts cultured under hypoxia/reoxygenation conditions represent a unique experimental system to better understand the mechanisms and signalling pathways that are altered in human placenta and facilitate the search for effective drugs that protect against certain pregnancy disorders. Human villous trophoblasts produce melatonin and express its synthesizing enzymes and receptors. Melatonin has been suggested as a treatment for preeclampsia and intrauterine growth restriction because of its protective antioxidant effects. In the primary villous cytotrophoblast cell model described in this paper, melatonin has no effect on trophoblast cells in normoxic state but restores the redox balance of syncytiotrophoblast cells disrupted by hypoxia/reoxygenation. Thus, human villous trophoblast cells in primary culture are an excellent approach to study the mechanisms behind the protective effects of melatonin on placental function during hypoxia/reoxygenation.
Introduction
Throughout human pregnancy, the placental cytotrophoblast cells, which are mononucleated stem cells, rapidly proliferate and differentiate into either villous or extravillous cytotrophoblast cells. Extravillous cytotrophoblasts invade and remodel the spiral arteries of the uterine wall. Villous cytotrophoblasts, on the other hand, continue to proliferate, differentiate and fuse to form multinucleated syncytiotrophoblast (the syncytium)1. The maintenance of villous trophoblast homeostasis is essential for fetal well-being and healthy pregnancy. In fact, villous trophoblasts allow maternal-fetal exchange of oxygen and nutrients, and produce essential hormones for pregnancy. Moreover, the syncytiotrophoblast is the only cell-type in direct contact with the maternal blood circulation and provides an essential physical and immunological barrier. Therefore, the syncytiotrophoblast must undergo apoptosis and replacement for homeostatic maintenance and to avoid placental pathologies2-5.
The technique developed by Kliman et al.6 in 1986 to isolate primary villous cytotrophoblasts from human placentas caused a revolution in placental research by allowing the study of the molecular mechanisms involved in villous trophoblast differentiation. This classical technique, based on sequential enzymatic digestions with trypsin and DNase, followed by isolation in density centrifugation media (colloidal silica particles coated by polyvinylpyrrolidone, or Percoll) is now recognized as the gold standard for isolating villous cytotrophoblast cells. The technique can be optimized by magnetic immunopurification, a procedure that separates villous cytotrophoblasts from non-trophoblastic cells based on the differential expression of specific antigens on the surfaces of these cells. We chose the human leukocyte antigen ABC (HLA-ABC) due to the absence of its expression on the trophoblastic cell membrane7,8.
The placenta is an organ that undergoes dramatic variations in oxygen levels during pregnancy. In the first trimester, the oxygenation ratio is physiologically very low (2% O2) but increases to mild levels of oxygenation (8% O2) in the second and third trimester. Tuuli et al.9 described that the in vitro reproduction of the trophoblast environment inside the placental villi is a challenge and variations in oxygenation levels may even lead to phenotypical changes. It is, therefore, suggested to adopt 8% oxygen as normoxia to mimic the oxygen tension found in placental villi during the third trimester of gestation8,9. Chen et al.10 extensively studied several variables related to oxygen tension in trophoblast cell culture and demonstrated the importance of determining oxygen levels in a pericellular environment. The levels of oxygen in the villi tend to increase due to vasculogenesis. The blood flow in placental villi increases constantly and the level of hydrogen peroxide (an abundant reactive oxygen species) is an important signal that controls vasculogenesis11,12. In pregnancy complications, a lack of vasculogenesis generates hypoxia, and more importantly, intermittent variations of oxygenation (called hypoxia/reoxygenation). These conditions lead to an abnormal increase in oxidative stress, which compromises placental and fetal viability13,14. The alterations that trophoblast cells undergo in vivo during episodes of hypoxia/reoxygenation can be mimicked in vitro as follows: villous cytotrophoblasts are maintained under normoxic conditions (8% O2) until they differentiate into syncytiotrophoblast. They are then subjected to hypoxic conditions (0.5% O2) for 4 hr, followed by an additional 18 hr of normoxia (reoxygenation). Using this hypoxia/reoxygenation approach, trophoblasts exhibit deregulated redox status and increased levels of intrinsic apoptosis8, as has been observed in certain pregnancy complications. Hence, this is a useful in vitro model to evaluate new preventive and therapeutic approaches to combat pregnancy complications associated with placental hypoxia/reoxygenation.
Placental cells produce melatonin, which has several important functions, such as an ability to obviate oxidative stress and placental dysfunction15. Here, we present the experimental approach and cell models used to demonstrate the protective effects of melatonin in placental trophoblast cells at the molecular, cellular and functional level8.
Protocol
Placentas were obtained immediately after spontaneous vaginal deliveries from uncomplicated pregnancies at the CHUM-St-Luc Hospital, Montreal, QC, Canada, with informed patient consent and approval of ethical committees (CHUM-St-Luc Hospital and INRS-Institut Armand-Frappier, Laval, QC, Canada).
1. Isolation and Purification of Villous Cytotrophoblast Cells
- Solutions and media
- Prepare transport media by supplementing Dulbecco's Modified Eagle's Medium High-Glucose (DMEM-HG) with 1% vol/vol antibiotic (10,000 units/ml penicillin G, 100 mg/ml streptomycin sulphate) and store at 4 °C.
- Prepare primary culture media by supplementing DMEM-HG with 10% vol/vol fetal bovine serum (FBS), 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1% vol/vol antibiotic (10,000 units/ml penicillin G, 100 mg/ml streptomycin sulphate) and store at 4 °C. Warm media to 37 °C before use.
- Prepare 4 L of saline solution (0.9% weight/vol sodium chloride).
- Prepare modified Hank's Balanced Salt Solution (HBSS) by adding 25 mM HEPES to 1x HBSS (pH 7.4).
- Prepare fresh, four bottles of digestion solution with modified HBSS (prepared in 1.1.4), magnesium sulfate (MgSO4), calcium chloride (CaCl2), trypsin, deoxyribonuclease IV (DNase IV), and 1% vol/vol antibiotic (10,000 units/ml penicillin G, 100 mg/ml streptomycin sulfate) as shown in Table 1.
- Prepare density centrifugation media gradient
- Prepare density centrifugation media solution by supplementing density centrifugation media with 10% vol/vol HBSS 10x.
- Prepare 14 assay tubes with density centrifugation media solution and modified HBSS, as described in Table 2.
- Mix each density solution (step 1.1.6.2) vigorously before adding its content to the gradient. Gently add the density solutions to a 50 ml glass centrifuge tube with a peristaltic pump (1 ml/min), beginning with the highest concentration (70%). Avoid droplets by draining the solutions against the tube wall to maintain the proper separation of each layer of the gradient.
- In absence of a peristaltic pump, apply the layers very gently with Pasteur pipettes.
- Prepare final running buffer by supplementing the running buffer (see Table of Materials) with 2% vol/vol antibiotic/antimycotic (10,000 units/ml penicillin G, 100 mg/ml streptomycin sulfate). Store at 4 °C.
- Villous cytotrophoblast isolation
Note: Use sterile surgical equipment, glassware, pipettes, flasks, etc.- On the day of villous cytotrophoblast isolation, in a 37 °C water bath, warm the digestions solutions (from step 1.1.5) and 70 ml of FBS. Note: Use 50 ml of FBS to interrupt the digestions and the remaining 20 ml for the freezing step (1.2.22) which can be placed on ice once thawed.
- After delivery, bring the placenta to the laboratory in ice-cold transport medium (from step 1.1.1) as quickly as possible (less than 1 hr).
- Discard transport medium and placental blood in liquid dustbin. Weigh the placenta and immerse in cold saline solution.
- Measure and analyze the following features: umbilical cord length; umbilical cord localization; placental length, width, shape (oval, discoid); membrane color; cotyledon structure pathologies. Note: Data are presented in the results section.
- Cut the umbilical cord alongside its placental insertion (i.e., at its base with an additional 1 cm radius circle around the cord). Immerse in 300 ml of histological tissue fixative solution (formalin 10%) for later histological analysis.
- Cut the entire placenta into cubes of 5 x 5 x 5 cm. Wash thoroughly (4 times x ~ 1 min) in saline solution (0.9%) to remove blood cells until saline solution is clear. Discard rinsing liquid.
- In a watch glass, remove placental membranes and mince tissues to remove blood vessels and calcifications. Hold blood vessels firmly with forceps and remove tissues using the back of Metzenbaum scissors.
- Place minced placenta in a Büchner funnel. Rinse with approximately 100 ml of saline buffer. Continue mincing until 30 – 35 g of minced tissue is obtained (use plastic weighing boat and scale). If needed, mince the remainder of the placenta to obtain up to three additional 30 – 35 g preparations. During this time, put minced tissue in a weighing boat on ice.
Note: This step will take around 45 min to 1 hr.
- Place minced placenta in a Büchner funnel. Rinse with approximately 100 ml of saline buffer. Continue mincing until 30 – 35 g of minced tissue is obtained (use plastic weighing boat and scale). If needed, mince the remainder of the placenta to obtain up to three additional 30 – 35 g preparations. During this time, put minced tissue in a weighing boat on ice.
- Add the 30 – 35 g minced placental tissue to a trypsinizing flask. Transfer 150 ml of the prepared digestion solution 1 (Table 1) to the trypsinizing flask and mix well.
- Place the trypsinizing flask in a shaking water bath for 30 min at a speed of no more than 50 cycles/min and manually mix the trypsinizing flask every 5 min for homogenous digestion.
- At the end of the first digestion, remove the trypsinizing flask from the water bath and tilt it (45°) for 1 min to sediment the placental tissue. With a 10 ml sterile pipette, remove and discard approximately 80 ml of supernatant. Avoid aspirating the tissue.
- Transfer 100 ml of digestion solution 2 (Table 1) to the trypsinizing flask; mix well and repeat step 1.2.9.
- At the end of the second digestion, remove the trypsinizing flask from the water bath and tilt it 45° for 1 min. With a 10 ml sterile pipette, remove 80 ml supernatant and gently transfer to a centrifuge tube with a cell strainer (100 µm mesh). Transfer the filtered supernatant to a beaker containing 2 ml of FBS every time the centrifuge tube is full.
- Perform the third digestion as described for the second digestion (1.2.11 and 1.2.12) using 75 ml digestion solution 3. In parallel, perform steps 1.2.15 to 1.2.16.1 for digestion 2.
- Perform the fourth digestion exactly as the third digestion using the digestion solution 4 (75 ml), but collect a maximum amount of supernatant. Perform steps 1.2.15 to 1.2.16.1 in parallel for digestion 3, and finally for digestion 4.
- Aliquot the supernatant (from digestions 2, 3 and 4) into 13.5 ml parts, each part into one 15 ml centrifuge tube. With a 22.8 cm long glass Pasteur pipette, very gently and slowly place 1.5 ml of FBS at the bottom of each tube in order to create a separate layer. Do not mix FBS and supernatant. Centrifuge the tubes without brake for 20 min at 1,250 x g at room temperature.
Note: After centrifugation, 4 layers are visible in the tube, as shown in Figure 1. The separation of trypsin and trophoblast cells avoids excessive cellular digestion. - With a vacuum pump, aspirate and discard the supernatant (digestion solution) and FBS layers, including the whitish film between the two layers. Resuspend the pellet (the trophoblasts and red blood cell layers) with 1 ml of warm cell culture medium (step 1.1.2; with no FBS and no HEPES).
- Collect resuspended cells from all tubes and combine them in 1 tube. Let the tube stand at room temperature until the end of all digestions. Note: After all digestions, the usual yield is 3 tubes of resuspended cells (one per digestion).
- Make up the volume to 15 ml with warm cell culture medium. Centrifuge at 1,250 x g for 10 min at room temperature. Remove the supernatant with a vacuum pump. Avoid aspirating the pellet.
- Gently resuspend the pellet obtained from the 3 tubes with 1 ml of warm cell culture medium. Pool their content in 1 tube. To obtain 8 ml, complete the volume with warm cell culture medium.
- Very gently layer the cell suspension on a separation gradient with a Pasteur pipette. Centrifuge without brake for 30 min at 507 x g at room temperature.
- After centrifugation, identify the different layers of cells in the gradient with back-lighting. Locate the layers containing trophoblast and contaminating cells between 40 – 50% of density centrifugation medium. With a vacuum pump, remove upper layers (> 50%).
- Collect cells located in the layers of interest with a Pasteur pipette and transfer them to a 50 ml centrifuge tube. Make up the volume to 50 ml with cell culture medium. Centrifuge for 10 min at 1,250 x g at room temperature.
- Under sterile conditions, discard the supernatant, resuspend the pellet with 20 ml of FBS and count the number of cells using a hemocytometer. On ice, add 2.22 ml of sterile dimethyl sulfoxide (DMSO) and mix gently by flipping. Aliquot 1.5 ml of cell suspension into cryogenic vials, freeze overnight at -80 °C and transfer to a liquid nitrogen tank.
- Trophoblast purification
- Install the rinsing and running buffers and a new filter column on the magnetic purification instrument according to manufacturer's instructions. Perform the "clean program" to clean negative 1, positive 1 and positive 2 ports, and then introduce the 50 ml tubes under each port according to manufacturer's instructions.
- Thaw the cells that were frozen in step 1.2.22 quickly in a 37 °C water bath. Transfer cells to a 50 ml tube and resuspend cells gently with 20 ml of cold running buffer solution. Centrifuge the tube for 5 min at 450 x g and 4 °C.
- Discard the supernatant. Repeat the wash step with cold running buffer. Count cells using a hemocytometer to determine viability. Repeat the centrifugation (for 5 min, 450 x g at 4 °C). Carefully remove the supernatant. Add 1 ml of cold running buffer containing 1% vol/vol of mouse anti-HLA-ABC antibodies. Incubate at 4 °C for 30 min, mixing gently every 5 min.
- Add 6 ml of cold running buffer. Centrifuge for 5 min at 450 x g and 4 °C. Discard the supernatant and repeat this step. Resuspend cells in 1 ml of cold running buffer containing 10% vol/vol of anti-mouse secondary antibody-coupled magnetic beads. Incubate at 4 °C for 15 min, mixing gently every 5 min.
- Add 6 ml of cold running buffer. Centrifuge for 5 min at 450 x g and 4 °C. Discard the supernatant and resuspend in 5 ml cold running buffer.
- Separate the trophoblast cells using the magnetic purification instrument. Collect cells at the negative port and add 20 ml of cold running buffer.
Note: Trophoblast cells do not contain the complex HLA-ABC, and are thus separated from other cell types and directed towards the negative 1 port. - Centrifuge for 5 min at 450 x g and 4 °C. Discard the supernatant and gently resuspend the cells in 20 ml warm primary culture medium. Count the cells using a hemocytometer to determine viability.
- Plate the cells at the following densities: 0.15 x 106 cells/well in 96-well plates, 1.6 x 106 cells/well in 24-well plates and 4.5 x 106 cells/well in 6-well plates. Incubate plates at 37 °C and 5% CO2.
- Confirm the purity of the trophoblast cells by flow cytometry16,17 and/or by immunocytochemistry18.
Note: The purity of the immunopurified cells was determined using FITC-conjugated monoclonal antibodies against cytokeratin-7 and vimentin17-19. This protocol is well detailed in Lanoix et al., 20087. - After at least 4 hr, rinse the cells twice with warm culture medium to remove unattached cells and then transfer the plates to the normoxia chamber, which is composed of 8% O2 (see Figure 2 and section 2).
2. In Vitro Induction of Normoxia and Hypoxia/Reoxygenation
- Incubator chamber operation (see Figure 2A for set-up).
- In a laminar flow hood, place a Petri dish containing sterile water at the bottom of the incubator chamber to avoid dryness; then place the previously prepared cell culture plates or flasks (step 1.3.10) on the superior shelves of the chamber.
- Outside the hood, attach the chamber (inlet port) to the gas hose (Figure 2A: 5a, b and c) to reach the tube of gas (8% O2 or 0.5% O2) (Figure 2A: 7). Open both inlet and outlet ports of the chamber. At this moment, the gas regulator (Figure 2A: 4) should remain closed.
- Carefully open the gas regulator valve (Figure 2A: 4). Flush for 4 min with an air flow of 25 L/min to completely replace the air inside the chamber.
- After flushing the chamber, close the gas regulator then the inlet and outlet ports of the chamber.
- Unplug the flow meter outlet hose (Figure 2A: 5c) from the inlet port of the chamber and place the chamber in a cell culture incubator at 37 °C.
- Replace the air currently present in the plates, flasks and dissolved in the culture medium by filling the chamber with gas 1 hr after step 2.1.3.
- Repeat steps 2.1.1 to 2.1.6 for the other gas compositions (e.g., 2% O2 for first trimester trophoblast culture9).
- Confirm the oxygen percentage (Figure 2B-C)
- To confirm the concentration of oxygen in the cell culture medium (without cells) inside the chamber, use an oxygen electrode connected to an oxygen adapter. Connect the oxygen electrode to a voltmeter.
- Create a calibration curve in the same solution (i.e., cell culture medium) by exposing the solution to gases with known oxygen contents (e.g., 0% and 21% oxygen). After the readings are stabilized for each concentration, introduce the electrode into the medium in the chamber.
Note: Take all measurement at the same depth in order to avoid any bias in oxygen concentration10.
- Induction of normoxia and hypoxia/reoxygenation in trophoblasts.
- After adding primary trophoblast cells to the appropriate cell culture flasks, plates or Petri dishes, perform treatments as necessary.
- In parallel, inside the chamber, expose cells to the desired gas mixture to reproduce a specific condition every 24 hr (Figure 3).
Representative Results
Isolation and immunopurification of villous cytotrophoblast cells from a normal term placenta obtained by vaginal delivery yielded 1 x 108 viable cells. The placenta weighed 350 g, was 19 cm in diameter, 4 cm tall with discoid shape and transparent membranes. No cotyledon malformation was detected. The umbilical cord had paracentral localization and a length of 56 cm. The purity was evaluated by flow cytometry using vimentin and cytokeratin-7 markers. More than 98% of the cells were negative for vimentin and positive for cytokeratin-7, confirming the purity of villous trophoblasts cells obtained from the immunopurification. Villous cytotrophoblast cells were added to 96-well culture plates under normoxic conditions in the presence or absence of 1 mM melatonin. The biochemical differentiation of villous cytotrophoblasts was monitored by determining levels of β-human chorionic gonadotropin (β-hCG) secretion as described previously1,7,20,21. The morphological differentiation and apoptosis were assessed by immunofluorescence using anti-desmoplakin and anti-caspase-cleaved cytokeratin 18 intermediate filaments7,22. Cell culture media from day 1 (mainly villous cytotrophoblasts) to day 4 (mainly syncytiotrophoblasts) were collected, centrifuged and β-hCG levels were measured in the supernatants. Production of β-hCG, which is exclusive to the syncytiotrophoblast, increased with culture time (Figure 4). Not only hypoxia/reoxygenation, but hyperoxia (> 20% O2) also activated apoptosis23. Thus, adoption of an 8% O2 concentration was representative of the quantity of oxygen to which a villous trophoblast cell would be exposed during the third trimester of pregnancy10. The peak of β-hCG levels observed at 72 hr confirmed the capacity of villous cytotrophoblasts to differentiate under these conditions. Melatonin did not alter β-hCG secretion under these study conditions. The decrease of β-hCG levels at 96 hr was likely caused by apoptosis of trophoblast cells, which increases after prolonged periods in primary culture5,7,22,24,25 (Figure 4). DMSO (0.1% vol/vol) was selected because it did not affect β-hCG levels26,27. The protective role of melatonin was strongly related to its antioxidant properties. Hypoxia/reoxygenation after 72h of culture induced oxidative stress in villous trophoblast cells. The protective effect of melatonin was assessed with Reactive Oxygen Species (ROS) Detection Reagent (Figure 5A). After 96 hr of culture, trophoblast cells were incubated for 45 min with 10 µM of 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) to detect the total amount of ROS produced8. Villous trophoblast cells that underwent hypoxia/reoxygenation had significantly increased ROS levels (54%) compared to those under normoxia. This increase was reversed by treatment with 1 mM melatonin. Moreover, under normoxia melatonin did not modulate ROS levels (homeostasis), which was similar to non-treated villous trophoblast cells (Figure 5A). Figure 4 and 5A show that under normoxia melatonin did not alter levels of oxidative stress or β-hCG secretion in the trophoblast cells, which corroborates previous studies showing no modulation of cell homeostasis under normal conditions28,29.
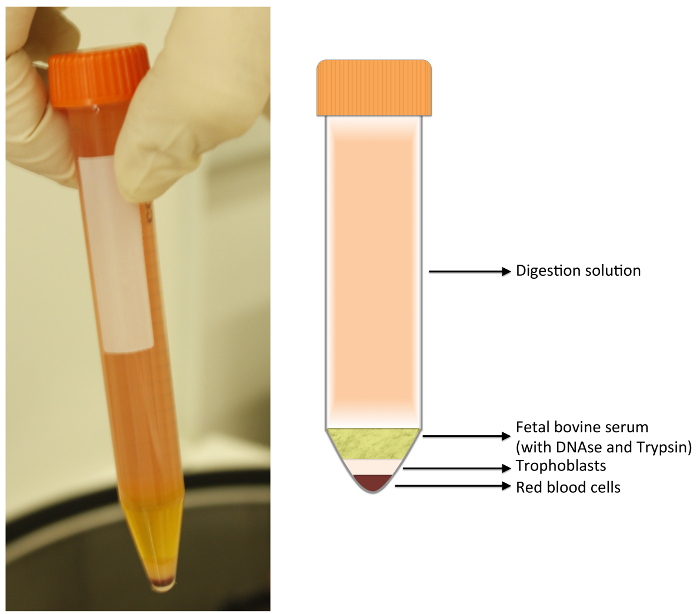
Figure 1: Digestion Tube. After centrifugation, 4 layers are formed. The upper layer is composed of digestion solution; just below, the fetal bovine serum (FBS). Both layers should be discarded with a vacuum pump. The lower layers are composed as follow: a white layer containing fibroblasts, leukocytes, macrophages, and trophoblasts; and a bottom layer composed of red blood cells. Please click here to view a larger version of this figure.
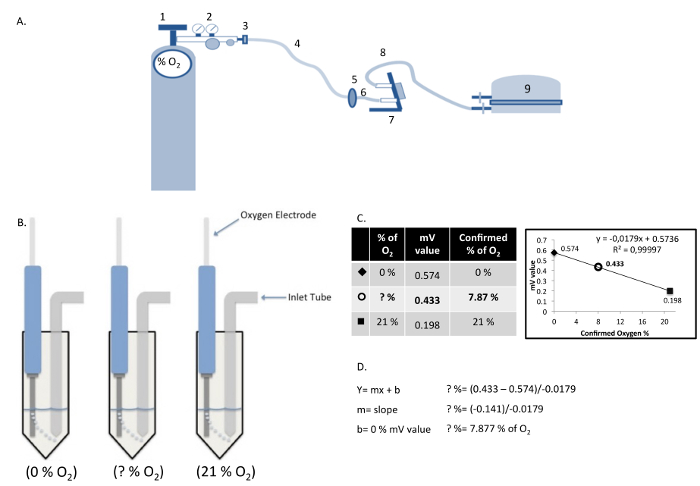
Figure 2: Components of the Hypoxia Chamber and Measurement/Calculation of Dissolved Oxygen Concentration. (A) Hypoxia chamber and gas cylinder assembly: (1) Gas cylinder; (2) Gas regulator; (3) Gas hose clamp; (4) Cylinder gas hose; (5) Inlet filter; (6) Inlet hose; (7) Flow meter; (8) Outlet hose; (9) Modular incubator chamber. (B) Calculation of actual oxygen concentration in cell culture medium using a standard curve produced with known oxygen concentrations. (C and D) The relative values obtained in the solutions "0% O2" and "21% O2", are plotted graphically as a linear function to determine the oxygen concentration in the cell culture medium "?% O2". Please click here to view a larger version of this figure.
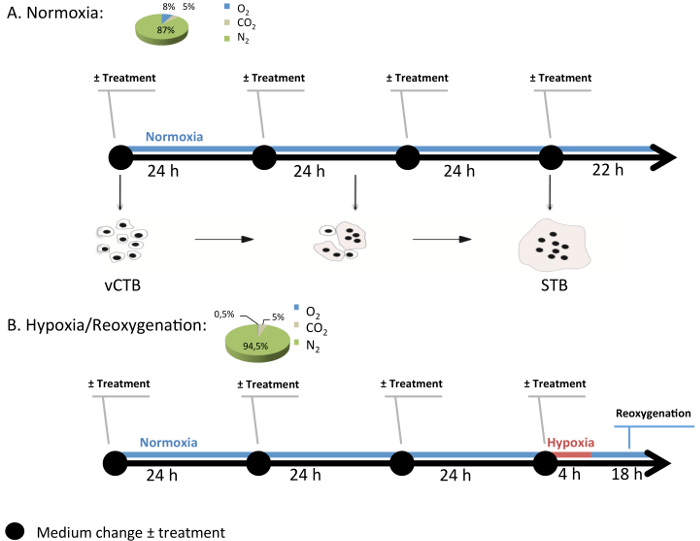
Figure 3: Generic Experimental Design of Cell Culture in the Modular Incubation Chamber. Normoxia (8% O2; 5% CO2; 87% N2) and hypoxia/reoxygenation (H/R) (0.5% O2; 5% CO2; 94.5% N2) are conditions used to study pathological conditions in villous cytotrophoblast (vCTB) and syncytiotrophoblast (STB) cells. Every 24 hr, medium with or without melatonin (1 mM) is changed and the gas mixture is renewed. Under H/R, STB cells undergo hypoxia (0.5% O2) for 4 hr and then return to normoxia (8% O2). Please click here to view a larger version of this figure.
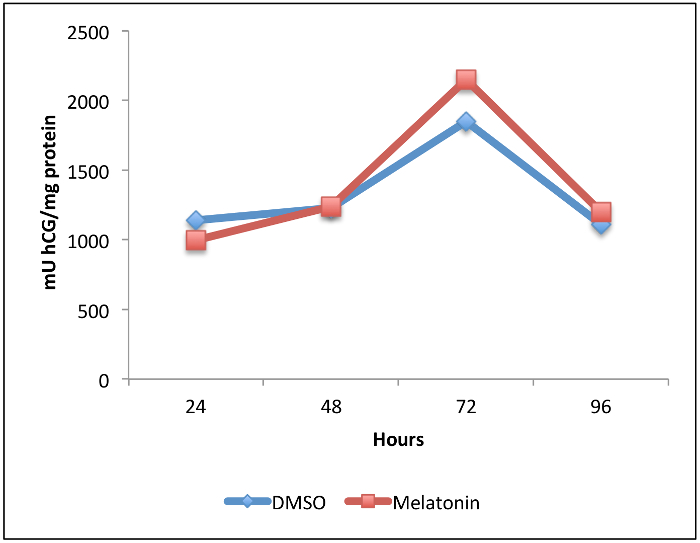
Figure 4: Effect of Melatonin on beta-human Chorionic Gonadotropin (β-hCG) Secretion during Villous Trophoblast Differentiation. Villous cytotrophoblast cells were isolated and purified from human healthy term placentas. Cells were treated for 96 hr with 1 mM melatonin or dimethyl sulfoxide (DMSO 0.1%: vehicle control) under normoxic conditions (8% O2; 5% CO2; 87% N2). β-hCG levels in culture medium were measured by enzyme-linked immunosorbent assay (ELISA) after 24, 48, 72 and 96 hr of primary culture. Levels were normalized to the protein content of the whole-cell lysate from each corresponding well. Please click here to view a larger version of this figure.
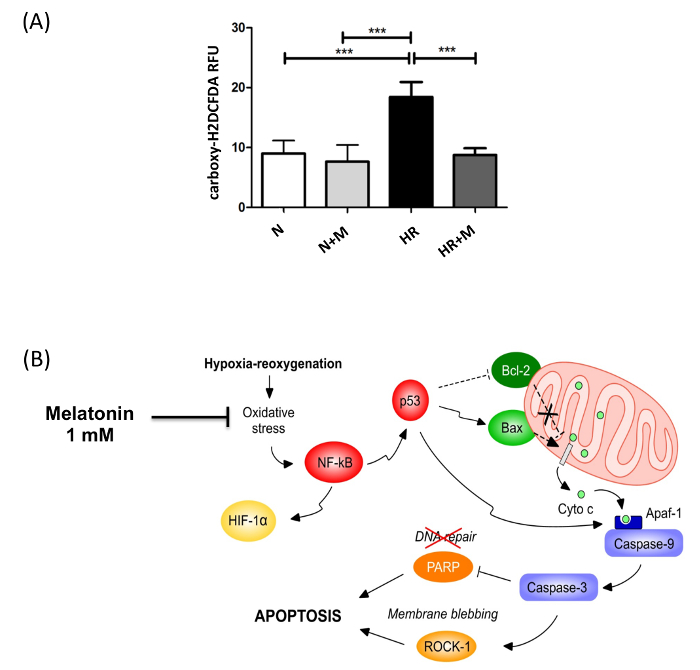
Figure 5: Anti-oxidant Effect of Melatonin in Syncytiotrophoblast Exposed to Hypoxia/Reoxygenation. (A) The effect of melatonin (M; 1 mM) on intracellular reactive oxygen species (ROS) levels in syncytiotrophoblast cells under normoxia (N) or hypoxia/reoxygenation (HR), induced after 72 hr of culture, was assessed by 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) fluorescence. Results are expressed as the mean ± SD of 3 different placentas; *** P < 0.001 (Lanoix, et al.8). (B) The cellular pathways involved in the putative protection of melatonin against hypoxia/reoxygenation-induced apoptosis. Primary villous cytotrophoblast cells were cultured for 72 hr under normoxia (8% O2) to allow differentiation into syncytiotrophoblast. Cells were exposed to 1 mM of melatonin or vehicle control and then subjected to hypoxia (0.5% O2) for 4 hr followed by an 18 hr reoxygenation period (8% O2). Hypoxia/reoxygenation-induced oxidative stress activates redox sensitive transcription factors such as nuclear factor kappa B (NF-κB) and hypoxia inducible factor 1 (HIF-1). NF-κB induces p53, which triggers the Bax/Bcl-2 pathway of mitochondrial apoptosis involving the cleavage and activation of caspases 9 and 3. Caspase 3 activates Rho-associated, coiled coil-containing protein kinase 1 (ROCK-1), the cleavage of poly(ADP-ribose) polymerase (PARP) and the impairment of DNA repair. Melatonin prevents the induction of mitochondrial apoptosis by acting as a powerful antioxidant to reduce the oxidative stress caused by hypoxia/reoxygenation. This figure has been modified from Lanoix et al., 20138. Please click here to view a larger version of this figure.
| Digestion 1 | Digestion 2 | Digestion 3 | Digestion 4 | |
| Modified HBSS (ml) | 150 | 100 | 75 | 75 |
| DNAse (µl) | 300 | 200 | 150 | 150 |
| (0.1 mg/µl) | ||||
| MgSO4 (µl) | 150 | 100 | 75 | 75 |
| (800 mM) | ||||
| CaCl2 (µl) | 150 | 100 | 75 | 75 |
| (100 mM) | ||||
| Trypsin (U) | 1,824,000 | 1,200,000 | 960,000 | 960,000 |
| P/S (ml) | 1 | 0 | 0 | 0 |
Table 1: Quantities of Ingredients for the Digestion Solution. Penicillin and streptomycin (P/S); magnesium sulfate (MgSO4); calcium chloride (CaCl2); deoxyribonuclease IV (DNase IV).
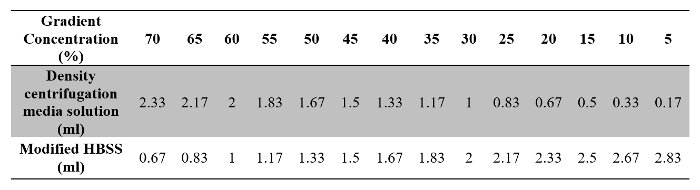
Table 2: Volumes of Density Centrifugation Media Solution and Modified HBSS Required for Preparation of the Gradient Solution.
Discussion
In mammals, fetal development is directly dependent on adequate placental function. The developmental origins of health disorders are based on the hypothesis that the cause of diseases manifested later in life can be traced back to early development and that the placenta has a mechanistic role in fetal programming30-32. The placenta is the key mediator of fetal growth and development: it regulates nutrient transfer, protects against harmful exposures, and has major endocrine functions. The development by Kliman et al. of a reproducible technique to isolate viable primary cytotrophoblasts is a milestone in the study of normal and abnormal placental functions6. Many researchers have adapted this technique to reproduce specific conditions in vitro to understand placental physiology18,20,33,34. As described by Petroff et al., many steps are important to guarantee a purified and robust yield of isolated cytotrophoblast cells18. For example, the digestion steps have undergone several modifications since the development of the technique in 1988, such as an increased number and length of the digestions, as well as to the composition and quantity of digestion enzymes, resulting in greater numbers of viable isolated cytotrophoblasts18. This current protocol has three main advantages: cryopreservation, which allows for the possibility to continue the protocol later immunomagnetic purification, which increases cytotrophoblast purity and the use of pre-coated microplates improving cell attachment7,34-36. The existing literature contains several examples of diverse modifications of the isolation technique for cytotrophoblast cells, but the characteristics of the density centrifugation media has remained virtually unmodified37. The presented technique of isolation/immunopurification has several critical steps. Hence, it is important to evaluate the purity of the villous cytotrophoblast cells at the end of each immunopurification. This can be done by flow cytometry using appropriate antibodies as markers: cytokeratin-7 (trophoblastic marker), cluster of differentiation 45 (CD45) and vimentin (non-trophoblast cells markers)18,38,39. Other critical aspects are the quality of the obtained placentas, the FBS and density centrifugation media gradient, as well as centrifugation speed, which can all influence the yield and quality of the cells20,40.
Although widely used, the present technique has unavoidable limitations. Firstly, the amount of viable cytotrophoblasts cells obtained after immunopurification is relatively low and is the main limiting factor in the number of possible conditions/treatments that can be tested. Secondly, the life-span of primary trophoblast cells is short and in vitro differentiation into syncytiotrophoblast is closely followed by a reduction of cell viability and an increase of apoptosis. The short length of trophoblast cell viability which does not proliferate in vitro, limits the evaluation of longer term treatments, because apoptosis is irreversibly triggered after about 4 days of culture7,41. Thirdly, interplacental variability is large, so a relatively large number of placentas is required to obtain statistically interpretable results. On the other hand, primary trophoblast culture has unique advantages, such as the capacity of the cells to differentiate into syncytium, which allows for the study of conditions and treatments in different cell phenotypes according to the various stages of differentiation. The oxygenation method presented in this protocol is highly adaptable and its configuration can be tailored for other situations, such as the culture of primary trophoblast cells from first trimester pregnancy, which should be exposed to a lower oxygen tension for normoxia9,42,43.
There are other approaches to study human placental function in vitro. Snap-freezing placental tissues allows for multi-omics analyses, but requires placental multisite sampling, to avoid for example metabolic variations due to the oxygenation gradient, which decreases from the central villi to the periphery44. However, live trophoblast cell biology and behavior cannot be studied using this approach45. Villous explants have the advantage of maintaining the whole villous structure with the constituent cell types and their communication, but responses to treatments are not specific to trophoblast cells23. Commercially available trophoblast-like choriocarcinoma cell lines, such as BeWo, Jeg-3 and JAR can be used to study placental functions, such as fusion, differentiation, and transplacental transport. However, recent studies show that gene expression in primary cytotrophoblasts and BeWo tumor cells are poorly correlated46-48. Thus, primary villous trophoblast cell culture, despite its limitations, possess the unique advantage of mimicking the in vivo environment of the normal or abnormal placenta.
Studies using villous trophoblast cell in primary culture and villous explants show that hypoxia and hypoxia/reoxygenation systematically decrease trophoblast cell viability, concomitant with increased levels of oxidative stress, inflammation, autophagy and apoptosis43,49-52. This hypoxia/reoxygenation cell culture model, specifically, has allowed us to demonstrate the antioxidant and anti-apoptotic effects of melatonin in villous trophoblast cells. In primary villous trophoblast cells exposed to hypoxia/reoxygenation, melatonin prevents the following: induction of oxidative stress, decreased antioxidant enzyme activities, increased activity of redox-sensitive signaling pathways, and induction of mitochondrial apoptosis (Figure 5B)8. The hypoxia/reoxygenation model is a unique tool to ascertain the preventive role of melatonin in oxidative stress-induced damage and its possible protective role in pregnancy complications such as preeclampsia, where placental melatonin synthesis is reduced53. Melatonin is a powerful antioxidant with a wide range of targets54. Also, the safety of melatonin as a treatment has been largely established. The beneficial results with melatonin have been reproduced by several researchers and melatonin is currently in clinical trials as a potential preventive or therapeutic treatment in pregnancies complicated by preeclampsia or intrauterine growth restriction55,56.
In conclusion, the isolation, purification and primary culture of high quality cytotrophoblast cells, together with the technique of hypoxia/reoxygenation enable a wide range of promising experimental approaches to better understand pregnancy complications related to oxidative stress and improve placental health.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) (no. 262011-2009) to CV and March of Dimes Social and Behavioral Sciences Research grant (#12-FY12-179) to CV and JTS; by studentships to LSF from the Ministère de l’éducation, de l’Enseignement supérieurs et de la recherche (MEESR)-Fonds de recherche du Québec (FRQ)-Nature et technologies (NT) and the Fondation Universitaire Armand-Frappier INRS, to HC from the Réseau Québécois en Reproduction-NSERC-CREATE, to AAHT from the Canadian Institutes of Health Research (CIHR) and FRQ-Santé, and to JBP from NSERC; by a fellowship to EMAS from the Conselho Nacional de Desenvolvimento Cientìfico e Tecnològico (CNPq) and the Programme de bourses d’excellence pour étudiants étrangers MEESR-FRQNT.
Materials
| Curved Metzenbaum Scissors | Shandon | 9212 | surgical equipment (cell isolation) (2 units) |
| Splinter Forceps Fine 41/2in | Fisherbrand | 13-812-42 | surgical equipment (cell isolation) (2 units) |
| Scissors 4.5 Str Dissection | Fisherbrand | 08-940 | surgical equipment (cell isolation) (2 units) |
| Gauze Sponge 10cm X 10cm | Cardinal Health | 361020733 | |
| Oblong Glass Baking Dish | Pyrex | 1105397 | Glassware (2.8L) |
| Funnel Buchner | Coorstek Inc | 10-356E | Glassware (114MM DIAMeter) |
| Watch Glass | pyrex | 9985100EMD | Glassware |
| Formalin solution, neutral buffered, 10% | Sigma-Aldrich | HT501128-4L | histological tissue fixative solution |
| Trypsinizing Flasks | Wheaton | 355395 | Glassware (1 unit) |
| Disposable Culture Tubes | Kimble | 73750-13100 | Glassware |
| Borosilicate Glass Pasteur Pipet (22.8 Cm) | Fisherbrand | K63B1367820C | Glassware |
| 250 Ml Glass Beakers | Fisherbrand | KFS14005250 | Glassware |
| Glass Media Bottles With Cap | Fisherbrand | KFS14395250 | Glassware (8 units) |
| 50 Ml Corex Tube | Corning | 8422-A | (1 unit) |
| 15 Ml Polystyrene Centrifuge Tube | Corning | 430791 | |
| 50 Ml Polystyrene Centrifuge Tube | Corning | 430829 | |
| 10ml Serological Pipet | Corning | 11415038 | |
| Cell Strainer 100μm Nylon | Corning | 431752 | |
| Absorbant Liner | Scienceware | 1199918 | |
| 500 Ml Bottles Top Filter | Corning | Pore: 0,22 µm / medium and HBSS preparation | |
| 2 Ml Criogenic Vials | Corning | 430488 | |
| Freezing Container, Nalgene Mr. Frosty | Sigma-Aldrich | C1562-1EA | |
| Peristaltic Pump | Pharmacia Fine Chemicals | P3 model | |
| Shaking Water Bath | Fisher | Model 127 | |
| Vacuum Pump | ABM | 4EKFS6CX-4 | |
| Sodium Chloride | Fisherbrand | EC231-598-3 | Saline solution 0.9% |
| Hank’s Buffered Salt Solution (Hbss) | Sigma-Aldrich | H2387 | Quantity: 9.25 (one vial) for 1L of digestion solution |
| Hydroxypiperazineethansulphonic Acid (Hepes) | Life Technologies | 15630-080 | 25mL (1M) for 1L of digestion solution |
| Trypsin Type I | Sigma-Aldrich | T8003 | 9,888U |
| Deoxyribonuclease Type Iv | Roche | 10-104-159-001 | 402,000U |
| Calcium Chloride | Sigma-Aldrich | C4901 | 100mM |
| Magnesium Sulfate | Baker | 2500-01 | 800mM |
| Dulbecco’s Modified Eagle Medium High Glucose (Dmem) | Life Technologies | 10564-045 | |
| Penicillin/Streptomycin Sulphate | Hyclone | SV30010 | |
| Fetal Bovine Serum | Corning | 35-010-CV | |
| Percoll | Sigma-Aldrich | P1644 | Density centrifugation media gradient. Volume: 36mL |
| Isopropanol | Acros | 42383-0010 | 50mL |
| Dimethyl Sulfoxide | Sigma-Aldrich | 472301 | |
| Automacs Magnetic Separator | Miltenyi Biotec | Model 003 | |
| Automacs Columns | Miltenyi Biotec | 130-021-101 | |
| Automacs Running Buffer | Miltenyi Biotec | 130-091-221 | http://www.miltenyibiotec.com/~/media/Images/Products/Import/0001100/IM0001131.ashx?force=1 |
| Automacs Rinsing Solution | Miltenyi Biotec | 130-091-222 | http://www.miltenyibiotec.com/en/products-and-services/macs-cell-separation/cell-separation-buffers/automacs-rinsing-solution.aspx |
| Anti-Human Hla Abc Purified Clone W6/32 | Affymetrix eBioscience | 14-9983-82 | anti-mouse antibody |
| Anti Mouse Igg Microbeads | Miltenyi Biotec | 130048401 | |
| Multiple Well Plate - 6 Well With Lid | Corning | 3335 | Cell Bind surface |
| Multiple Well Plate - 24 Well With Lid | Corning | 3337 | Cell Bind surface |
| Multiple Well Plate - 96 Well With Lid | Corning | 3300 | Cell Bind surface |
| Modular Incubator Chamber | Billups-Rothenberg | MIC-101 | A set of two is necessary for simultaneous to generate normoxia and hypoxia/reoxygenation conditions |
| Single Flow Meter | Billups-Rothenberg | SFM3001 | |
| 50 Mm In-Line Filter | Whatman | 6721-5010 | PTFE, pore: 1.0 µm |
| Gas Regulator | Pro Star | PRS301233 | A set of two is necessary for simultaneous to generate normoxia and hypoxia/reoxygenation conditions |
| Gas Hose Class Vi Clear 5/16 | Parker | 100-05070102 | 3 pieces with ~ 0.5 m |
| 17 Mm Adjustable Gas Hose Clamp | Tiewraps | THCSS-16 | |
| Normoxia Gas Cylinder | Praxair | NI CDOXR1U-K | Size K (3rd trimester‘s composition: 5% CO2, 8% O2, Bal. N2) |
| Normoxia Gas Cylinder | Praxair | NI CDOXR1U-K | Size K (3rd trimester‘s composition: 5% CO2, 0.5% O2, Bal. N2) |
| Oxygen Microelectrode Mi-730 | Microelectrodes INC | 84477 | |
| Oxygen Adapter | Microelectrodes INC | 3572 | |
| ROS Detection Reagent: CM-H2DCFDA | Invitrogen | C-400 | |
| β-hCG ELISA kit | DRG internatinal | EIA-4115 | |
| Anti-Vimentin ourified antibody | eBioscience | 14-9897 | Host: mouse |
| Anti-Cytokeratin 7 (FITC) antibody | Abcam | ab119697 | Host: mouse |
| Alexa Fluor 488 Goat Anti-mousse IgG H&L antibody | Life Technologies | A-11029 |
References
- Vaillancourt, C., Lanoix, D., Le Bellego, F., Daoud, G., Lafond, J. Involvement of MAPK signalling in human villous trophoblast differentiation. Mini Rev Med Chem. 9 (8), 962-973 (2009).
- Gauster, M., Moser, G., Orendi, K., Huppertz, B. Factors involved in regulating trophoblast fusion: potential role in the development of preeclampsia. Placenta. 30, 49-54 (2009).
- Huppertz, B., Kadyrov, M., Kingdom, J. C. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 195 (1), 29-39 (2006).
- Lanoix, D., Lacasse, A. A., Reiter, R. J., Vaillancourt, C. Melatonin: the smart killer: the human trophoblast as a model. Mol Cell Endocrinol. 348 (1), 1-11 (2012).
- Huppertz, B., Frank, H. G., Reister, F., Kingdom, J., Korr, H., Kaufmann, P. Apoptosis cascade progresses during turnover of human trophoblast: analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab Invest. 79 (12), 1687-1702 (1999).
- Kliman, H. J., Nestler, J. E., Sermasi, E., Sanger, J. M., Strauss, J. F., 3rd, Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 118 (4), 1567-1582 (1986).
- Lanoix, D., Beghdadi, H., Lafond, J., Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J Pineal Res. 45 (1), 50-60 (2008).
- Lanoix, D., Lacasse, A. A., Reiter, R. J., Vaillancourt, C. Melatonin: The watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol Cell Endocrinol. 381 (1-2), 35-45 (2013).
- Tuuli, M. G., Longtine, M. S., Nelson, D. M. Review: Oxygen and trophoblast biology–a source of controversy. Placenta. 32, 109-118 (2011).
- Chen, B., Longtine, M. S., Nelson, D. M. Pericellular oxygen concentration of cultured primary human trophoblasts. Placenta. 34 (2), 106-109 (2013).
- Roberts, J. M., Hubel, C. A. Is oxidative stress the link in the two-stage model of pre-eclampsia. Lancet. 354 (9181), 788-789 (1999).
- Burton, G. J., Jauniaux, E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 25 (3), 287-299 (2011).
- Ji, L., Brkic, J., Liu, M., Fu, G., Peng, C., Wang, Y. L. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia. Mol Aspects Med. 34 (5), 981-1023 (2013).
- Redman, C. W., Sargent, I. L. Placental stress and pre-eclampsia: a revised view. Placenta. 30, 38-42 (2009).
- Sagrillo-Fagundes, L., Soliman, A., Vaillancourt, C. Maternal and placental melatonin: actions and implication for successful pregnancies. Minerva Ginecol. 66 (3), 251-266 (2014).
- Blaschitz, A., Weiss, U., Dohr, G., Desoye, G. Antibody reaction patterns in first trimester placenta: implications for trophoblast isolation and purity screening. Placenta. 21 (7), 733-741 (2000).
- Potgens, A. J., Gaus, G., Frank, H. G., Kaufmann, P. Characterization of trophoblast cell isolations by a modified flow cytometry assay. Placenta. 22 (2-3), 251-255 (2001).
- Petroff, M. G., Phillips, T. A., Ka, H., Pace, J. L., Hunt, J. S. Isolation and culture of term human trophoblast cells. Methods Mol Med. 121, 203-217 (2006).
- Maldonado-Estrada, J., Menu, E., Roques, P., Barre-Sinoussi, F., Chaouat, G. Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J Immunol Methods. 286 (1-2), 21-34 (2004).
- Le Bellego, F., Vaillancourt, C., Lafond, J. Isolation and culture of term human cytotrophoblast cells and in vitro methods for studying human cytotrophoblast cells’ calcium uptake. Methods Mol Biol. 550, 73-87 (2009).
- Mounier, C., Barbeau, B., Vaillancourt, C., Lafond, J. Endocrinology and cell signaling in human villous trophoblast. Methods Mol Biol. 550, 89-102 (2009).
- Chen, B., et al. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab. 302 (9), 1142-1152 (2012).
- Reti, N. G., et al. Effect of high oxygen on placental function in short-term explant cultures. Cell Tissue Res. 328 (3), 607-616 (2007).
- Pidoux, G., et al. Biochemical characterization and modulation of LH/CG-receptor during human trophoblast differentiation. J Cell Physiol. 212 (1), 26-35 (2007).
- Pidoux, G., et al. ZO-1 is involved in trophoblastic cell differentiation in human placenta. Am J Physiol Cell Physiol. 298 (6), 1517-1526 (2010).
- Williams, J. L., Fyfe, G. K., Sibley, C. P., Baker, P. N., Greenwood, S. L. K+ channel inhibition modulates the biochemical and morphological differentiation of human placental cytotrophoblast cells in vitro. Am J Physiol Regul Integr Comp Physiol. 295 (4), 1204-1213 (2008).
- Schild, R. L., Schaiff, W. T., Carlson, M. G., Cronbach, E. J., Nelson, D. M., Sadovsky, Y. The activity of PPAR gamma in primary human trophoblasts is enhanced by oxidized lipids. J Clin Endocrinol Metab. 87 (3), 1105-1110 (2002).
- Menendez-Pelaez, A., Reiter, R. J. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res. 15 (2), 59-69 (1993).
- Perrone, S., Stazzoni, G., Tataranno, M. L., Buonocore, G. New pharmacologic and therapeutic approaches for hypoxic-ischemic encephalopathy in the newborn. J Matern Fetal Neonatal Med. 25, 83-88 (2012).
- Nelissen, E. C., van Montfoort, A. P., Dumoulin, J. C., Evers, J. L. Epigenetics and the placenta. Hum Reprod Update. 17 (3), 397-417 (2011).
- Barker, D. J. Intrauterine programming of adult disease. Mol Med Today. 1 (9), 418-423 (1995).
- Barker, J. R., Thomas, C. F., Behan, M. Serotonergic projections from the caudal raphe nuclei to the hypoglossal nucleus in male and female rats. Respir Physiol Neurobiol. 165 (2-3), 175-184 (2009).
- Yui, J., et al. Functional, long-term cultures of human term trophoblasts purified by column-elimination of CD9 expressing cells. Placenta. 15 (3), 231-246 (1994).
- Kilani, R. T., Chang, L. J., Garcia-Lloret, M. I., Hemmings, D., Winkler-Lowen, B., Guilbert, L. J. Placental trophoblasts resist infection by multiple human immunodeficiency virus (HIV) type 1 variants even with cytomegalovirus coinfection but support HIV replication after provirus transfection. J Virol. 71 (9), 6359-6372 (1997).
- Knofler, M., Stenzel, M., Husslein, P. Shedding of tumour necrosis factor receptors from purified villous term trophoblasts and cytotrophoblastic BeWo cells. Hum Reprod. 13 (8), 2308-2316 (1998).
- Douglas, G. C., King, B. F. Isolation of pure villous cytotrophoblast from term human placenta using immunomagnetic microspheres. J Immunol Methods. 119 (2), 259-268 (1989).
- Li, L., Schust, D. J. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod Biol Endocrinol. 13, 71 (2015).
- Stenqvist, A. C., et al. An efficient optimized method for isolation of villous trophoblast cells from human early pregnancy placenta suitable for functional and molecular studies. Am J Reprod Immunol. 60 (1), 33-42 (2008).
- Potgens, A. J., Kataoka, H., Ferstl, S., Frank, H. G., Kaufmann, P. A positive immunoselection method to isolate villous cytotrophoblast cells from first trimester and term placenta to high purity. Placenta. 24 (4), 412-423 (2003).
- Lanoix, D., Vaillancourt, C. Cell culture media formulation and supplementation affect villous trophoblast HCG release. Placenta. 31 (6), 558-559 (2010).
- Vaillancourt, C., Lafond, J. Human embryogenesis: overview. Methods Mol Biol. 550, 3-7 (2009).
- Armant, D. R., et al. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development. 133 (4), 751-759 (2006).
- McCaig, D., Lyall, F. Hypoxia upregulates GCM1 in human placenta explants. Hypertens Pregnancy. 28 (4), 457-472 (2009).
- Burton, G. J., et al. Optimising sample collection for placental research. Placenta. 35 (1), 9-22 (2014).
- Lanoix, D., et al. Quantitative PCR pitfalls: the case of the human placenta. Mol Biotechnol. 52 (3), 234-243 (2012).
- Bilban, M., et al. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 31 (11), 989-996 (2010).
- Novakovic, B., et al. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: implications and opportunities for understanding trophoblast function. Mol Hum Reprod. 17 (6), 344-353 (2011).
- Burleigh, D. W., et al. Microarray analysis of BeWo and JEG3 trophoblast cell lines: identification of differentially expressed transcripts. Placenta. 28 (5-6), 383-389 (2007).
- Hung, T. H., Skepper, J. N., Charnock-Jones, D. S., Burton, G. J. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 90 (12), 1274-1281 (2002).
- Heazell, A. E., Moll, S. J., Jones, C. J., Baker, P. N., Crocker, I. P. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 28, 33-40 (2007).
- Heazell, A. E., Lacey, H. A., Jones, C. J., Huppertz, B., Baker, P. N., Crocker, I. P. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta. 29 (2), 175-186 (2008).
- Chen, B., Longtine, M. S., Nelson, D. M. Hypoxia induces autophagy in primary human trophoblasts. Endocrinology. 153 (10), 4946-4954 (2012).
- Lanoix, D., Guerin, P., Vaillancourt, C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: new insights into the role of this hormone in pregnancy. J Pineal Res. 53 (4), 417-425 (2012).
- Galano, A., Tan, D. X., Reiter, R. J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 51 (1), 1-16 (2011).
- Alers, N. O., Jenkin, G., Miller, S. L., Wallace, E. M. Antenatal melatonin as an antioxidant in human pregnancies complicated by fetal growth restriction–a phase I pilot clinical trial: study protocol. BMJ Open. 3 (12), 004141 (2013).
- Hobson, S. R., Lim, R., Gardiner, E. E., Alers, N. O., Wallace, E. M. Phase I pilot clinical trial of antenatal maternally administered melatonin to decrease the level of oxidative stress in human pregnancies affected by pre-eclampsia (PAMPR): study protocol. BMJ Open. 3 (9), 003788 (2013).

