Visualizing the Interrenal Steroidogenic Tissue and Its Vascular Microenvironment in Zebrafish
Summary
The interrenal gland in zebrafish is the teleostean counterpart of the mammalian adrenal gland. This protocol introduces how to perform the 3-β-hydroxysteroid dehydrogenase (Δ5-4 isomerase; 3β-Hsd) enzymatic activity assay, which detects differentiated steroidogenic cells in the developing zebrafish.
Abstract
This protocol introduces how to detect differentiated interrenal steroidogenic cells through a simple whole-mount enzymatic activity assay. Identifying differentiated steroidogenic tissues through chromogenic histochemical staining of 3-β-Hydroxysteroid dehydrogenase /Δ5-4 isomerase (3β-Hsd) activity-positive cells is critical for monitoring the morphology and differentiation of adrenocortical and interrenal tissues in mammals and teleosts, respectively. In the zebrafish model, the optical transparency and tissue permeability of the developing embryos and larvae allow for whole-mount staining of 3β-Hsd activity. This staining protocol, as performed on transgenic fluorescent reporter lines marking the developing pronephric and endothelial cells, enables the detection of the steroidogenic interrenal tissue in addition to the kidney and neighboring vasculature. In combination with vibratome sectioning, immunohistochemistry, and confocal microscopy, we can visualize and assay the vascular microenvironment of interrenal steroidogenic tissues. The 3β-Hsd activity assay is essential for studying the cell biology of the zebrafish interrenal gland because to date, no suitable antibody is available for labeling zebrafish steroidogenic cells. Furthermore, this assay is rapid and simple, thus providing a powerful tool for mutant screens targeting adrenal (interrenal) genetic disorders as well as for determining disruption effects of chemicals on steroidogenesis in pharmaceutical or toxicological studies.
Introduction
The adrenal gland, a crucial component of the hypothalamo-pituitary-adrenal axis, secretes steroids and coordinates steroid homeostasis and the bodily response to stress. The adrenal gland comprises the outer cortex, which secretes steroids in a zone-specific manner, and inner medulla, which synthesizes catecholamines. The interrenal gland in teleosts is the counterpart of the adrenal gland in mammals and is composed of steroidogenic interrenal and chromaffin cells, which are functional equivalents of the adrenal cortex and medulla, respectively1-3. Studies conducted using the zebrafish model have reported that both steroidogenic and chromaffin cell lineages are formed by molecular and cellular mechanisms highly resembling those in mammals1,2. Therefore, the zebrafish is a potentially powerful model for studying genetic disorders, neuroendocrine control, and systems biology of the hypothalamo-pituitary-adrenal (interrenal) axis.
In the adrenal gland, 3β-Hsd catalyzes the conversion of progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnelolone, and androstenedione from dehydroepiandrosterone4,5. 3β-Hsd is essential for biosynthesizing all classes of hormonal steroids, namely progesterone, glucocorticoids, mineralocorticoids, androgens, and estrogens. The two human 3β-Hsd isozymes HSD3B1 and HSD3B2 are differentially expressed6. HSD3B1 is expressed in the placenta and peripheral tissues, whereas HSD3B2 is expressed in the adrenal cortex and gonads. Human HSD3B1 and HSD3B2 are co-orthologs of zebrafish hsd3b1, which is expressed at the interrenal tissue and adult gonads; zebrafish hsd3b2 is a maternally expressed gene whose transcripts disappear before organogenesis7. The protocol of the whole-mount 3β-Hsd enzymatic activity assay for zebrafish was developed by modifying Levy's method, as described by Milano et al., on frozen sections of eight teleost species8. Because of the tissue permeability and optical transparency of the developing zebrafish, whole-mount 3β-Hsd histochemistry can be successfully used for the fixed zebrafish embryo and larvae and specifically delineate the differentiated interrenal tissues.
This sensitive and rapid assay has been applied to various mutants and morphants demonstrating different types of interrenal dysmorphogenesis. The interrenal 3β-Hsd activity is absent in the embryo where specification of the interrenal tissue is disrupted through a specific knockdown of the Ff1b transcription factor and is decreased as the interrenal differentiation is affected by a knockdown of the Ff1b coregulator Prox19,10. Notably, the 3β-Hsd activity can be detected in mutants with severe early stage defects, such as one-eyed pinhead and squint, where the 3β-Hsd histochemistry delineates how the interrenal cell migration is affected11. The differentiation of the interrenal tissue is not compromised even in the complete absence of blood and vasculature. Therefore, how endothelium-derived signals shape the developing interrenal organ can be determined12,13. Overall, this histochemical assay has been successfully used for studying specification, differentiation, and migration of steroidogenic cells in the zebrafish model. Therefore, it should be an efficient and a reliable tool for any genetic or chemical screens targeting adrenal and interrenal organ disorders.
Protocol
Ethics Statement: All experimental procedures on zebrafish were approved by the Institutional Animal Care and Use Committee of Tunghai University (IRB Approval NO. 101-12) and carried out in accordance with the approved guidelines.
1. Stock Solutions for 3β-Hsd Enzymatic Activity Staining
- Prepare trans-dehydroandrosterone [10 mg/ml in dimethyl sulfoxide (DMSO)].
- Prepare β-nicotinamide adenine dinucleotide hydrate (1.2 mg/ml in 0.1 M phosphate buffer, pH 7.2).
- Prepare nicotinamide (vitamin B3, 50 mg/ml in H2O).
- Prepare 4-nitro blue tetrazolium (50 mg/ml in 70% dimethylformamide).
- Aliquot all these components into microfuge tubes and store at -20 °C.
NOTE: In our experience, repeated freezing and thawing of the aliquots of all the aforementioned solutions do not affect the intensity of 3β-Hsd activity staining.
2. Whole-mount 3β-Hsd Activity Staining
NOTE: The 3β-Hsd enzymatic activity is detectable in zebrafish embryos as early as 28 hours post fertilization (hpf) when cultured at 28.5 °C, which is consistent with the onset of mRNA expression of hsd3b12,7. The whole-mount 3β-Hsd activity staining protocol in this study is based on a previously described method8 and was determined to be successful in the developing zebrafish up to 7 days post fertilization9. For analyzing the vascular microenvironment of the interrenal steroidogenic tissue, whole-mount 3β-Hsd activity staining must be applied to transgenic lines expressing endothelium-specific fluorescence, such as Tg(kdrl:EGFP)s843 14 and Tg(kdrl:mCherry)ci5 15. To analyze the interrenal steroidogenic tissue considering zebrafish kidney development, 3β-Hsd activity staining can be applied to the Tg(wt1b:GFP)li1 fish16, where the formation of the glomerulus and pronephric tubules is delineated.
- Treat the developing zebrafish by incubating them in 0.03% phenylthiourea in egg water (0.06 mg/L reef salt in deionized water), starting from a stage between 12 and 24 hpf, to inhibit pigment formation.
- Fix dechorionated embryos or larvae in (A) 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) supplemented with 0.1% Tween 20 (PFAT) for 1 hr at room temperature (RT) or (B) 2% PFAT overnight at 4 °C or for 2 hr at 4 °C, followed by 4 hr at RT.
Caution: PFA is toxic by inhalation and by skin contact. It is destructive to the skin, mucous membranes, eyes, and upper respiratory tract. - Wash the embryos 4x for 10 min with PBS supplemented with 0.1% Tween 20 (PBST) at RT.
NOTE: It is critical to not dry the samples while changing the washing solutions. - Freshly prepare the staining solution before the reactions (Table 1). Prepare, vortex, and centrifuge parts A and B in separate tubes.
NOTE: Adding all the components straight into one single tube would cause severe precipitation. - Add the entire solution of part A to the entire solution of part B, mix well to prepare the staining solution, and immediately distribute 1 ml into each microfuge tube containing fixed and washed embryos.
- Wrap microfuge tubes with aluminum foil to protect them from light and place on either a rotator or a rocking platform for gentle shaking at RT.
- Empirically determine the reaction time by monitoring chromogenic signals through microscopy. Slight precipitations will develop over time; however, they will not interfere with the reaction process.
NOTE: For embryos fixed at 28 hpf, staining at 25 °C for 4 hr generates a clear signal at the interrenal tissue. - Stop the reaction by washing 4x for 10 min in PBST. If no further immunohistochemical (IHC) analysis is required, post-fix the stained embryos with 4% PFAT for 60 min at RT. Otherwise, store the washed embryos at 4 °C until further use.
- For whole-mount microscopy, clear the stained, post-fixed, and washed embryos with 50% glycerol in PBS, orient them with the dorsal side facing up on a slide, and observe using an upright microscope under bright field illumination.
NOTE: To make a high-resolution image of the interrenal tissue morphology, flat-mount analysis of the 3β-Hsd activity-stained and deyolked embryo is recommended. As the interrenal steroidogenic tissue is positioned right above the yolk sac, a ventral flat-mount analysis of the interrenal tissue allows a direct and clear observation of the interrenal morphology17.
3. IHC Analysis of 3β-Hsd Activity-stained Embryos
NOTE: For analyzing the vascular microenvironment of the steroidogenic tissue, the 3β-Hsd activity-stained embryos with a transgenic endothelium-specific fluorescent reporter are subjected to transverse vibratome sectioning and subsequent IHC analysis12,18.
- Post-fixation
- Post-fix the 3β-Hsd activity-stained and washed embryos with 2% PFA supplemented with 1%Triton X-100 for 1 hr at 4 °C and wash 4x for 10 min in PBS containing 1% Triton X-100 (PBSTx) at RT.
- Embedding
- Prepare 4% low-melting agarose by dissolving the agarose in PBS at 95 °C, aliquot into microfuge tubes, and store at 4-8 °C.
- Melt an aliquot of 4% low-melting agarose at 70 °C for 10 min, and then keep the aliquot at 47 °C. Embed each embryo in molten 4% low-melting agarose in a detached cap of a 1.5 ml microfuge tube which serves as a mold, and allow to cool until solid.
- Vibratome Sectioning
- Stick a piece of paper tape to the dry platform of the vibratome.
- Remove the hardened agarose block from the mold by a razor blade. Apply superglue to the paper tape on the platform, and fix the agarose block onto the paper tape, with the anterior side of the sample facing up. Trim the agarose block to a trapezoidal prism shape, with approximately 4 mm and 8 mm on each side of the upper and lower facets, respectively.
- Rinse the sample-containing agarose block with cold PBS supplemented with 0.5% Triton X-100 (0.5% PBSTx) by using a dropper.
- Slice the agarose block into 100 µm sections according to the instruction manual of the vibratome. Constantly rinse the agarose block to prevent drying. As each slice is cut, remove it from the vibratome using a 26 G syringe needle, and transfer to a spot plate containing cold 0.5% PBSTx (500 µl /well).
- Collect the 3β-Hsd activity-positive tissue sections by checking under a dissection microscope, and transfer the sections by a pipette tip with its end cut-off (about 1 mm inner diameter at the opening) into a 96-well plate containing cold 0.5% PBSTx (150 µl/well). The tissue samples usually dissociate from the agarose block after this step.
- Permeabilization and Washing
- Wash the slices 6x for 15 min in 0.5% PBSTx at RT, 10 min in PBS, and 4x for 10 min in PBS supplemented with 1% bovine serum albumin/1% DMSO/0.1% Triton X-100 (PBDTx).
- Blocking
- Incubate the slices for 1 hr in 2% fetal calf serum (FCS) in PBDTx at RT.
- Primary Antibody Reaction
- Incubate the slices for 1.5 days in PBDTx containing a primary antibody (e.g., rabbit polyclonal anti-human fibronectin and mouse anti-human focal adhesion kinase) at 4 °C.
- Washing
- Wash the slices 6x for 10 min in PBDTx at RT.
- Blocking
- Incubate the slices for 1 hr in 2% FCS in PBDTx at RT.
- Secondary Antibody Reaction
- Incubate the slices for 1.5 days in PBDTx containing a secondary antibody (fluorophore-conjugated goat anti-rabbit or anti-mouse IgG) at 4 °C.
- Washing
- Wash the slices 6x for 10 min in PBDTx at RT and 4x for 10 min in PBST at RT.
NOTE: For confocal microscopic analysis, clear the samples with 50% glycerol in PBS.
- Wash the slices 6x for 10 min in PBDTx at RT and 4x for 10 min in PBST at RT.
4. Confocal Analysis
- Observe the whole mount embryo and the section by a confocal microscope equipped with 10X/0.5 and 20X/0.75 objective lens.
- For simultaneous detection of the fluorescence and the 3β-Hsd staining, set the multitrack mode as follows: 488 nm argon laser and 505-530 nm bandpass filter for the detection of GFP; 543 nm Helium-Neon laser and 560-615 nm bandpass filter for the detection of mCherry; and transmitted light channel for the detection of 3β-Hsd staining. The pinhole size is 1 Airey unit, and the resolution is 1,024 x 1,024 pixel.
- Assemble the z stacks (with 6 μm interval) as a 3D projection, and merge the projection and the bright field, by using the built-in confocal software.
Representative Results
To determine how the steroidogenic interrenal tissue codevelops with the pronephric kidney glomerulus and its nascent vasculature, the 3β-Hsd enzymatic activity assay was performed on the double transgenic Tg(wt1b:GFP)li1;Tg(kdrl:mCherry)ci5 embryo at 34 hpf (Figure 1). At this stage, the 3β-Hsd activity-positive steroidogenic tissue is located right to the midline and immediately caudal to the pronephric kidney glomerulus, whereas some steroidogenic cells start migrating across the midline and form a protruding edge from the dorsal view. The steroidogenic tissue is closely associated with the dorsal aorta (DA) and posterior cardinal vein (PCV).
The 3β-Hsd enzymatic activity assay on Tg(kdrl:GFP)s843 embryos allowed a clear delineation of the peri-interrenal vessels, including the DA, PCV, and interrenal vessel (IRV; Figure 2). Vibratome sections of the 3β-Hsd activity-stained embryos were subjected to IHC analysis for detecting the extracellular matrix protein Fibronectin and its downstream effector phosphorylated Focal Adhesion Kinase. The peri-DA deposition of Fibronectin is essential for supporting IRV growth18.
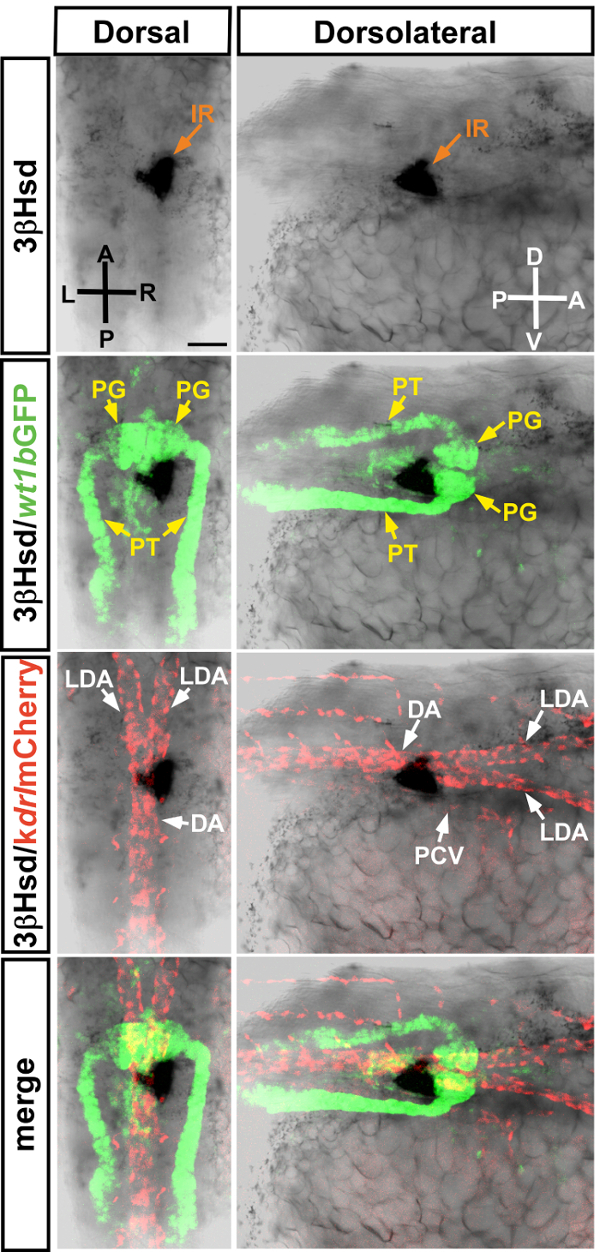
Figure 1: Whole-mount 3β-Hsd activity staining performed on zebrafish expressing fluorescent reporters. Dorsal (left panels) and dorsolateral (right panels) views of a 3β-Hsd activity-stained Tg(wt1b:GFP)li1;Tg(kdrl:mCherry)ci5 embryo at 34 hpf, which allows visualizing the relative spatial distribution of the interrenal tissue (IR), pronephric glomerulus (PG), pronephric tubules (PT), dorsal aorta (DA), lateral dorsal aorta (LDA), and posterior cardinal vein (PCV). Due to the location of the interrenal tissue at this stage, the dorsolateral views are from the right side of the embryo. D, dorsal; V, ventral; A, anterior; P, posterior; R, right; L, left. Scale bar = 50 µm. Please click here to view a larger version of this figure.
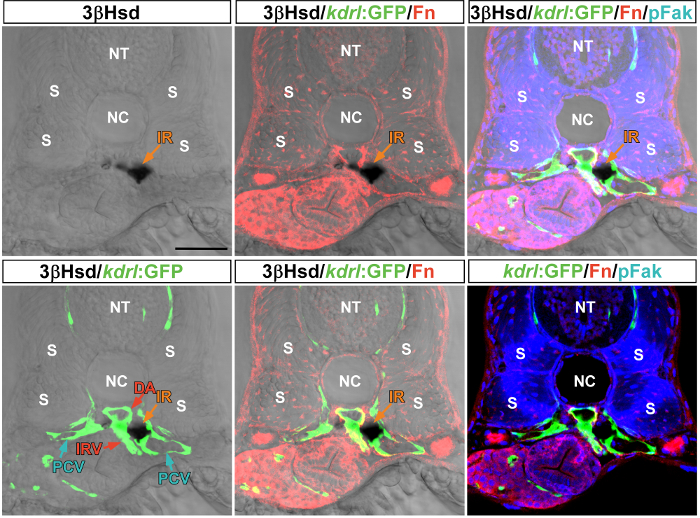
Figure 2: Vibratome sectioning and IHC analysis of 3β-Hsd activity-stained Tg(kdrl:GFP)s843 embryos. The 34-hpf embryo was subjected to a 3β-Hsd enzymatic activity assay and then vibratome sectioning. The 3β-Hsd activity-positive section was stained by double IHC with rabbit anti-human fibronectin (Fn; 1/200) and mouse anti-human phosphorylated focal adhesion kinase (pFak; pY397, 1/100). The transverse section shows the relative distribution of the interrenal tissue (IR) and its neighboring vessels, including the dorsal aorta (DA), posterior cardinal vein (IRV), and posterior cardinal vein (PCV). NT, neural tube; NC, notochord; S, somite. Scale bar = 50 µm. Please click here to view a larger version of this figure.
| Stock concentration | In 1,000 μl | Final concentration | |
| Part A. | |||
| DMSO | 40 μl | ||
| DHEA | 10 mg/ml | 10 μl | 0.1 mg/ml |
| NAD | 1.2 mg/ml | 10 μl | 12 μg/ml |
| Part B. | |||
| PBST | 936 μl | ||
| Vitamin B3 | 50 mg/ml | 2 μl | 0.1 mg/ml |
| NBT | 50 mg/ml | 2 μl | 0.1 mg/ml |
Table 1: Components of the 3β-Hsd staining reaction.
Discussion
The signal strength of the 3β-Hsd activity increased over the course of reaction. Clear signals of the 3β-Hsd activity were detected after 4 hours of reaction for stages from 28 hpf onward. However, the reaction duration requires empirical determination, depending on the purpose of the assay. In cases where the staining requires overnight processing, a slightly bluish background tends to develop on the samples. This problem can be overcome by increasing the fixation duration (e.g., 4 hr in 4% PFAT at RT) before the 3β-Hsd activity staining. By contrast, overfixation, such as fixing for overnight with 4% PFAT at RT, leads to an extremely clear background. However, longer time would be required to obtain a desirable intensity of the signal. We noticed that fixing the samples overnight with 2% rather than 4% PFAT before 3β-Hsd activity staining typically yields more desirable results in a subsequent fluorescent reporter observation or IHC analysis. However, fixing the embryos with 4% PFAT allows a rapid assay of the 3β-Hsd activity (completed within 1 day) and would thus benefit genetic or chemical screens targeting the interrenal steroidogenic tissues.
Post-fixing the embryos after 3β-Hsd activity staining is essential because trace amounts of chemical components tend to persist in the stained samples even after extensive washing and would result in high background after a long storage duration. However, if the 3β-Hsd activity-stained embryos are to be subjected to further IHC analysis, storing the 3β-Hsd activity-stained and washed embryos at 4 °C overnight does not result in a noticeable enhancement of the background.
In situ hybridization (ISH) analysis of 3β-Hsd activity-stained embryos is possible. For example, the expression of prox1 transcripts is colocalized with the 3β-Hsd activity at the steroidogenic tissue10. To perform 3β-Hsd activity staining in addition to ISH, the embryos must be fixed in 4% PFAT for 4 hr both before and after the 3β-Hsd activity assay so that cellular RNA transcripts can be preserved with desirable quality for the ISH analysis.
ISH analysis by using riboprobes of ff1b, cyp11a1, hsdb1, and star have been applied in the developmental studies of the interrenal steroidogenic tissue1,2,11. The riboprobe of ff1b detects primordial interrenal cells by 22 hpf, while those of cyp11a1 and star detect differentiating interrenal cells by 24 hpf. As compared to 3β-Hsd histochemical staining which only detects differentiated interrenal tissues from 28 hpf onwards, ISH analysis can be used to analyze a spectrum of interrenal-specific genes in both primordial and differentiated interrenal cells. Nevertheless, 3β-Hsd histochemical staining has the advantage of being simple, rapid, and reliable for analyzing the phenotype of the differentiated steroidogenic interrenal tissue, and therefore is a powerful tool for detailed developmental studies as well as large-scale genetic or chemical screens. As per our review of relevant literature, there exists no specific antibody that can reliably detect the protein expression at the steroidogenic interrenal tissue through immunohistochemistry. Therefore, the 3β-Hsd histochemical staining method is particularly helpful in delineating the tissue and cellular morphology of the steroidogenic interrenal tissue.
Although 3β-Hsd histochemical staining is useful for the visualization of the differentiated steroidogenic interrenal tissue, it does not directly report steroid synthesis capability or functionality of interrenal cells. Mutations or chemicals affecting enzymes upstream or downstream of 3β-Hsd in the steroidogenic synthesis cascade may not necessarily be detected with this described method, since they may or may not perturb either size or morphology of the interrenal tissue. Therefore, users of this protocol should bear in mind that certain enzymatic impairments affecting function but not morphology of the interrenal tissue may not be readily detected by this method.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Prof. Christoph Englert and Prof. Didier Stainier for gifting the Tg(wt1b:GFP) li1 and Tg(kdrl:EGFP)s843 strains, respectively, and the Taiwan Zebrafish Core Facility for providing Tg(kdrl:mCherry)ci5. This study was supported by grants from Taiwan's Ministry of Science and Technology (96-2628-B-029-002-MY3, 101-2313-B-029-001, 102-2628-B-029-002-MY3, 102-2321-B-400-018).
Materials
| Confocal microscope | Carl Zeiss | LSM510 |
| DMSO | Sigma | D8418 |
| Glycerol | USB | US16374 |
| Hyclone Fetal Calf Serum | GE Healthcare Life Sciences | SH30073 |
| Nicotinamide | Sigma | N0636 |
| β-Nicotinamide adenine dinucleotide hydrate | Sigma | N1636 |
| 4-Nitro blue tetrazolium | Promega | S380C |
| Nusieve GTG | Lonza | 50081 |
| Paraformaldehyde | Sigma | P6148 |
| Phenylthiourea | Sigma | P7629 |
| Phosphate buffered saline | Sigma | P4417-100Tab |
| PYREX Spot Plate | Corning | 7220-85 |
| Reef Salt | AZOO | AZ28001 |
| trans-Dehydroandrosterone | Sigma | D4000 |
| Triton X-100 | Sigma | T8787 |
| Tween 20 | Sigma | P9416 |
| Vibratome | Leica | VT1000M |
References
- Hsu, H. J., Lin, G., Chung, B. C. Parallel early development of zebrafish interrenal glands and pronephros: differential control by wt1 and ff1b. Development. 130, 2107-2116 (2003).
- To, T. T., et al. Pituitary-interrenal interaction in zebrafish interrenal organ development. Mol Endocrinol. 21, 472-485 (2007).
- Liu, Y. W. Interrenal organogenesis in the zebrafish model. Organogenesis. 3, 44-48 (2007).
- Cravioto, M. D., et al. A new inherited variant of the 3 beta-hydroxysteroid dehydrogenase-isomerase deficiency syndrome: evidence for the existence of two isoenzymes. J Clin Endocrinol Metab. 63, 360-367 (1986).
- Lachance, Y., et al. Characterization of human 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase gene and its expression in mammalian cells. J Biol Chem. 267, 3551 (1992).
- Simard, J., et al. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 26, 525-582 (2005).
- Lin, J. C., et al. Two zebrafish hsd3b genes are distinct in function, expression, and evolution. Endocrinology. 156, 2854-2862 (2015).
- Grassi Milano, E., Basari, F., Chimenti, C. Adrenocortical and adrenomedullary homologs in eight species of adult and developing teleosts: morphology, histology, and immunohistochemistry. Gen Comp Endocrinol. 108, 483-496 (1997).
- Chai, C., Liu, Y. W., Chan, W. K. Ff1b is required for the development of steroidogenic component of the zebrafish interrenal organ. Dev Biol. 260, 226-244 (2003).
- Liu, Y. W., Gao, W., Teh, H. L., Tan, J. H., Chan, W. K. Prox1 is a novel coregulator of Ff1b and is involved in the embryonic development of the zebra fish interrenal primordium. Mol Cell Biol. 23, 7243-7255 (2003).
- Chai, C., Liu, Y. W., Chan, W. K. Ff1b is required for the development of steroidogenic component of the zebrafish interrenal organ. Dev. Biol. 260, 226-244 (2003).
- Chou, C. W., Zhuo, Y. L., Jiang, Z. Y., Liu, Y. W. The hemodynamically-regulated vascular microenvironment promotes migration of the steroidogenic tissue during its interaction with chromaffin cells in the zebrafish embryo. PLoS One. 9, e107997 (2014).
- Liu, Y. W., Guo, L. Endothelium is required for the promotion of interrenal morphogenetic movement during early zebrafish development. Dev Biol. 297, 44-58 (2006).
- Jin, S. W., Beis, D., Mitchell, T., Chen, J. N., Stainier, D. Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 132, 5199-5209 (2005).
- Proulx, K., Lu, A., Sumanas, S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev Biol. 348, 34-46 (2010).
- Perner, B., Englert, C., Bollig, F. The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol. 309, 87-96 (2007).
- Chou, C. W., Chiu, C. H., Liu, Y. W. Fibronectin mediates correct positioning of the interrenal organ in zebrafish. Dev Dyn. 242, 432-443 (2013).
- Chiu, C. H., Chou, C. W., Takada, S., Liu, Y. W. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS One. 7, e43040 (2012).

