Assessment of Neuronal Viability Using Fluorescein Diacetate-Propidium Iodide Double Staining in Cerebellar Granule Neuron Culture
Summary
This protocol describes how to accurately measure neuronal viability using Fluorescein diacetate (FDA) and Propidium Iodide (PI) double staining in cultured cerebellar granule neurons, a primary neuronal culture used as an in vitro model in neuroscience and neuropharmacology research.
Abstract
Primary cultured Cerebellar Granule Neurons (CGNs) have been widely used as an in vitro model in neuroscience and neuropharmacology research. However, the co-existence of glial cells and neurons in CGN culture might lead to biases in the accurate assessment of neuronal viability. Fluorescein diacetate (FDA) and Propidium Iodide (PI) double staining has been used to measure cell viability by simultaneously evaluating the viable and dead cells. We used FDA-PI double staining to improve the sensitivities of the colorimetric assays and to evaluate neuronal viability in CGNs. Furthermore, we added blue fluorescent DNA stains (e.g., Hoechst) to improve the accuracy. This protocol describes how to improve the accuracy of assessment of neuronal viability by using these methods in CGN culture. Using this protocol, the number of glial cells can be excluded by using fluorescence microscopy. A similar strategy can be applied to distinguish the unwanted glial cells from neurons in various mixed cell cultures, such as primary cortical culture and hippocampal culture.
Introduction
Colorimetric cytotoxicity assays, such as the 3- (4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) assay, are commonly used to measure cell viability in vitro. Primary cultured Cerebellar Granule Neurons (CGNs) from rats are sensitive to various neurotoxins, including 1-methyl-4-phenylpyridinium ion, hydrogen peroxide, and glutamate1,2. Therefore, CGN cultures can be used as an in vitro model in the field of neuroscience. CGN cultures may contain a variety of cells, including neurons and glial cells, which can account for about 1% of the total cells in the CGN culture. However, glial cells respond differently to neurotoxins as compared to neurons, leading to a bias in the neuronal viability measured by colorimetric assays3.
In viable cells, Fluorescein diacetate (FDA) can be converted into fluorescein by esterase. Propidium Iodide (PI) can interact with the DNA after penetrating dead cells and can be used to indicate apoptosis within the culture. Therefore, FDA-PI double staining can simultaneously evaluate viable cells and dead cells, suggesting that the cell viability can be measured more accurately by combining both colorimetric methods. Moreover, by adding Hoechst, a blue fluorescent stain for nuclei, the accuracy of cell viability could be further improved. The protocol presented here describes FDA-PI double staining and FDA-PI-Hoechst triple staining, which can be used to accurately analyze neuronal viability in primary cultured CGNs.
This protocol takes advantage of visualizing and distinguishing CGNs and glial cells by their different sizes and shapes. After staining, the numbers of viable neurons and dead neurons are counted from representative images taken by fluorescent microscopy. The large-size glial cells are excluded by the comparison of typical CGNs taken under fluorescent mode with those taken under phase contrast mode. A similar strategy can be performed to measure neuronal viability in mixed cell cultures containing neurons and glial cells, such as primary cortical cultures and hippocampal cultures.
Protocol
All procedures followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee (IACUC) at Ningbo University (SYXK-2008-0110).
1. Preparation of Solutions and Culture Media
Note: Reagents and stocks need to be prepared under sterile conditions. Sterilize by filtering using a filter with a pore size of 0.22 µm.
- Prepare Poly-L-Lysine (PLL) stock solution by adding 5 mg of PLL to 10 mL of double-distilled water (ddH2O). Sterilize by filtering. Aliquot and store the PLL stock solution at -20 °C for several months.
- Prepare Krebs buffer by adding 7.25 g of NaCl, 0.4 g of KCl, 0.14 g of NaH2PO4, 2.6 g of D-glucose, and 5.94 g of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid to 100 mL of ddH2O. Sterilize by filtering. Store the Krebs buffer solution at 4 °C for up to one month.
- Prepare 3.82% Mg2SO4 solution by adding 3.82 g of Mg2SO4 to 100 mL of ddH2O. Sterilize by filtering. Store the Mg2SO4 solution at 4 °C for several months.
- Prepare 1.2% CaCl2 solution by adding 1.2 g of CaCl2 to 100 mL of ddH2O. Sterilize by filtering. Store the CaCl2 solution at 4 °C for several months.
- Prepare 2M KCl solution by adding 15 g of KCl to 100 mL of ddH2O. Sterilize by filtering. Store the KCl solution at 4 °C for several months.
- Prepare the D-glucose stock solution by adding 1.8 g of D-glucose to 100 mL of ddH2O. Sterilize by filtering. Aliquot and store the D-glucose solution at 4 °C for up to one month.
- Prepare Cytosine β-D-Arabinofuranoside (Ara-C) stock solution by adding 0.24 g of Ara-C to 10 mL of ddH2O. Sterilize by filtering. Aliquot and store the Ara-C stock solution at 4 °C for several months.
- Prepare 250 mL of culture medium (for 25-30 pups) using 25 mL of Fetal Bovine Serum (FBS), 2.5 mL of 100x glutamine, 2.78 mL of 2M KCl solution, and 2.5 mL of 100x antibiotics in Basal Medium Eagle (BME). Adjust the volume to 250 mL and sterilize by filtering.
- Prepare 25K medium using 2.5 mL of 100x glutamine, 2.78 mL of 2M KCl solution, and 2.5 mL of 100x antibiotics in BME. Adjust the volume to 250 mL and sterilize by filtering.
- Prepare 5K medium using 2.5 mL of 100x glutamine and 2.5 mL of 100X antibiotics in BME. Adjust the volume to 250 mL and sterilize by filtering.
NOTE: The culture medium, 25K medium, and 5K medium need to be freshly prepared - Prepare FDA stock solution by adding 5 mg of FDA to 1 mL of acetone. Store the FDA stock solution at 4 °C for long-term storage.
- Prepare PI stock solution by adding 1 mg of PI to 1 mL of ddH2O. Store the PI stock solution at 4 °C for several months.
- Prepare Hoechst stock solution by adding 5 mg of Hoechst 33342 to 1 mL of ddH2O. Store the Hoechst stock solution at 4 °C for several months.
2. Preparation of Dissection Solutions
NOTE: Do this 1 d prior to the dissection.
- Prepare the dissection solution by adding 15 mL of Krebs buffer, 1.2 mL of 3.82% Mg2SO4 solution, and 0.45 g of Bovine Serum Albumin (BSA) to 150 mL of ddH2O. Adjust the pH to 7.4 using NaOH.
- Label 5 x 50 mL tubes as follows: 1, 2, 3, 4, and 5.
- Place 40 mL of dissection solution into a 50 mL syringe with a filter. Filter 30 mL of dissection solution into tube 1, and 2 mL each to several 35 mm cell culture dishes, which will be used to process tissues.
- To tube 2, add 12.5 mg of trypsin in 50 mL of dissection solution. Mix completely by vortexing. Sterilize by filtering.
- To tube 3, add 1.2 mg of DNAse I, 7.8 mg of soybean trypsin inhibitor, and 150 µL of 3.82% Mg2SO4 solution in 15 mL of dissection solution. Mix completely by vortexing. Sterilize by filtering.
- To tube 4, add 5 mL of the solution from tube 3. Add 10.5 mL of dissection solution. Sterilize by filtering.
- To tube 5, add 100 µL of 3.82% Mg2SO4 solution and 15 µL of 1.2% CaCl2 solution in 12.5 mL of dissection solution. Mix completely by vortexing. Sterilize by filtering.
- Store all tubes at 4 °C before use.
3. Coating the Cell Culture Plates
NOTE: Do this 1 d prior to the dissection.
- Add 1 mL of PLL stock solution to 100 mL of ddH2O (final concentration: 5 µg/mL). Coat plastic 6-well or 12-well cell culture plates (2 mL per well for 6-well cell culture plates or 1 mL per well for 12-well cell culture plates) by adding 5 µg/mL of poly-L-lysine solution.
- Incubate at room temperature for 1 day (or at 37 °C for 2 h). 1-2 h before the dissection, remove the solution using a pipette. Wash once with ddH2O and dry in the cell culture hood.
4. Processing Tissues for 8 day old Sprague-Dawley Rats.
- Put a 100 mm dish on ice. Decapitate the rat pups into the dish using a pair of sterilized scissors.
- Insert the scissors into the foramen magnum and cut the two sides of skull, from the ears to the eyes. Lift the skull using a pair of forceps. Ensure that the whole brain is in the skull. Isolate the cerebellum and put it into the abovementioned 35 mm dish using a pair of forceps.
- Work under a dissecting microscope. Remove the meninges and blood vessels with two pairs of forceps.
- Chop the tissues with a blade. Put the tissues into tube 1. Centrifuge for 5 min at RT and 1,500 x g.
- Aspirate the supernatant. Save the pellet.
- Add the solution in tube 2 to the pellet. Resuspend by shaking gently.
- Place the tube in a 37 °C water bath for 15 min. Gently shake every 3 min.
- Add the solution in tube 4 to this solution. Shake and centrifuge for 5 min at RT and 1,500 x g.
- Aspirate the supernatant. Save the pellet.
- Add the solution in tube 3 to resuspend the pellet.
- Prepare two 15 mL tubes. Place half of the cells in each tube. Pipette up and down 60-70x using a cotton-plugged Pasteur pipette to homogenize the cells.
- Add 3 mL of the solution in tube 5 to each 15-mL tube. Let them sit at RT for 10 min and then carefully remove the supernatant with a cotton-plugged Pasteur pipette. Place the supernatant into new 15 mL tubes and centrifuge for 5 min at RT and 1,500 x g.
- Aspirate the supernatant. Save the pellet.
- Add culture medium into the pellet to get a cell density of around 1.5 x 106 cells/mL (about 100 mL for 10 pups).
- Put the cells into culture plates and culture them in the incubator at 37 °C and 5% CO2.
5. Addition of Ara-C and D-glucose during Culturing
- After 24 h, add Ara-C stock solution (10 µL/well for 12-well cell culture plates or 20 µL/well for 6-well cell culture plates; final concentration: 1 mM) to inhibit the growth of the glial cells.
- On day 7, add 50 µL of D-glucose stock solution per well for 12-well cell culture plates or 100 µL per well for 6-well cell culture plates so that final concentration is 1 mM.
6. FDA-PI Double Staining and FDA-PI-Hoechst Triple Staining in CGN Culture
- Prepare FDA-PI working solution by adding 20 µL of FDA stock solution (final concentration: 10 µg/mL) and 50 µL of PI stock solution (final concentration: 50 µg/mL) in 10 mL of PBS. Mix by vortexing and place this on ice.
- For the FDA-PI-Hoechst working solution, add 20 µL of FDA stock solution (final concentration: 10 µg/mL), 50 µL of PI stock solution (final concentration: 50 µg/mL), and 10 µL of Hoechst stock solution (final concentration: 5 µg/mL) in 10 mL of PBS. Mix by vortexing and place this on ice.
NOTE: Freshly prepare FDA-PI working solution and FDA-PI-Hoechst working solution just before use.
- For the FDA-PI-Hoechst working solution, add 20 µL of FDA stock solution (final concentration: 10 µg/mL), 50 µL of PI stock solution (final concentration: 50 µg/mL), and 10 µL of Hoechst stock solution (final concentration: 5 µg/mL) in 10 mL of PBS. Mix by vortexing and place this on ice.
- Take the cell culture plate out of the incubator. Put it on ice.
- Aspirate the culture medium and replace it with cold PBS.
NOTE: The change of solution must be done slowly and carefully. Avoid touching the cells with pipette tips. - Aspirate the cold PBS and replace it with cold FDA-PI or FDA-PI-Hoechst working solution (500 µL/well for 12-well cell culture plates or 1 mL/well for 6-well cell culture plates). Leave on ice for 5 min.
- Aspirate the FDA-PI or FDA-PI-Hoechst working solution and add cold PBS (100 µL/well for 12-well cell culture plates or 50 µL/well for 6-well cell culture plates).
NOTE: Cells should not be allowed to dry when taking images. - Take images using fluorescent microscopy. Use an excitation filter with a pass band of 450 – 490 nm. Detect the fluorescence emissions for FDA, PI, and Hoechst at 520, 620, and 460 nm, respectively. Under the same conditions, take an image under normal light using the phase contrast mode of fluorescent microscopy.
NOTE: Take images within 15 min after FDA-PI or FDA-PI-Hoechst staining. The exposure time is 100-300 ms and the analog gain is 2.8x.
7. Assessment of Neuronal Viability
- For FDA-PI double staining, overlay the images of the cells detected after filtering by different fluorescence filters by dragging the FDA-positive layer on the PI-positive layer in a graphics editor software.
- Adjust the opacity of the FDA-positive layer by entering "50%" in the "Opacity" field of the "Layers" panel. Merge two layers by clicking on the "Merge Visible" button in the "Layer" menu. Set the contrast of the overlay images by entering "50" in the "Contrast" field under "Image"| "Adjustments" | "Brightness/Contrast." Make sure that there is no FDA and PI double-positive cell in the overlay images.
- For FDA-PI-Hoechst triple staining, overlay the images of the cells detected after filtering different fluorescence filters by dragging the FDA-positive layer on the PI-positive layer in a graphics editor software. Adjust the opacity of the FDA-positive layer by entering "50%" in the "Opacity" field of the "Layers" panel.
- Merge two layers by clicking on the "Merge Visible" button in the "Layer" menu. Drag the Hoechst-positive layer on the merged layer. Adjust the opacity of the Hoechst-positive layer by entering "50%" in the "Opacity" field of the "Layers" panel. Merge the two layers by clicking on the "Merge Visible" button in the "Layer" menu.
- Visually distinguish large, irregular glial cells from CGNs by comparing the images taken under fluorescent mode with those taken under phase contrast mode—cells with diameters three times larger than CGNs are considered glial cells and should be excluded.
- Count the number of viable neurons and dead neurons, respectively, by using a cell counter plugin in an image processing program (e.g., ImageJ)4.
- For FDA-PI double staining, manually mark small FDA-positive cells as viable neurons. Manually mark PI-positive cells as dead neurons. For FDA-PI-Hoechst triple staining, manually mark small FDA- and Hoechst-double-positive cells as viable neurons. Manually mark PI- and Hoechst-double-positive cells as dead neurons.
- Calculate the percentage of neuronal viability by using the following equation: % of neuronal viability = [number of viable neurons / (number of dead neurons + number of viable neurons)] x 100%. For each well, choose five random fields and average the percentage of neuronal viability.
Representative Results
The dual immunostaining of GAP43 (red) and GFAP (green) was used to analyze the shape of neurons and glial cells, respectively2,5. Both neurons and glial cells are present in CGN culture. The GFAP-positive glial cells are large and irregular in shape, as indicated by the arrows in the images (Figure 1). Traditional assays for cell vitality cannot distinguish glial cells from neurons when used to measure neuronal viability. Therefore, FDA-PI and FDA-PI-Hoechst staining, which can distinguish glial cells from neurons, are advantageous for the accurate evaluation of neuronal viability in CGN culture.
The low-potassium challenge was used to induce neuronal death in a CGN culture. The representative images show the CGN culture challenged with a low-potassium medium (5K), normal medium (25K), or original medium, as analyzed by FDA-PI double staining (Figure 2A) or FDA-PI-Hoechst triple staining (Figure 2B). Neuronal viability measured by various methods is presented in Figure 3 (mean ±SEM). The cell viabilities measured by FDA-PI double staining in the control, 25K, and 5K groups were 99.8 ±4.2%, 98.2 ±2.9%, and 43.9 ±8.6%, respectively (Figure 3A). The cell viabilities measured by FDA-PI-Hoechst triple staining in the control, 25K, and 5K groups were 99.8 ±1.6%, 96.7 ±4.4%, and 48.3 ±4.4%, respectively (Figure 3B). The cell viabilities measured by MTT assay in the control, 25K, and 5K groups were 102.1 ±3.9%, 96.5 ±1.7%, and 57.5 ±5.7%, respectively (Figure 3C). The percentages of lactic dehydrogenase (LDH) release in the control, 25K, and 5K groups were 100.0 ±5.5%, 94.5 ±11.2%, and 202.1 ±15.3%, respectively (Figure 3D). Neuronal viability could not be directly calculated by the LDH assay2. The MTT assay assesses the activity of NADPH-dependent cellular oxidoreductase, which reflects the number of viable cells2. However, this method could not distinguish glial cells from neurons when used to measure neuronal viability in CGN culture. By using FDA-PI and FDA-PI-Hoechst staining, the number of glial cells could be excluded, and neuronal viability could be accurately measured. Moreover, the neuronal viability in 5K medium-treated CGNs measured by FDA-PI or FDA-PI-Hoechst staining was slightly smaller than that measured by MTT assay. This might be because most glial cells that were not sensitive to 5K medium-induced neurotoxicity were excluded by FDA-PI or FDA-PI-Hoechst staining, but not by the MTT assay.
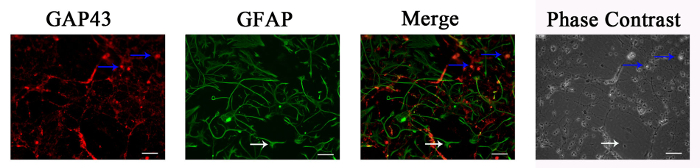
Figure 1: Both Small Neurons and Large, Irregular Glial Cells are Present in CGN Culture. At day 8 in vitro, CGNs were dual immunostained with GAP43 (red) and GFAP (green) for neurons and glial cells, respectively. The phase contrast images show the morphology of the cells. Traditional assays for cell vitality cannot distinguish glial cells from neurons when used to measure neuronal viability. Therefore, FDA-PI and FDA-PI-Hoechst staining, which can distinguish glial cells from neurons, are advantageous for the accurate evaluation of neuronal viability in CGN culture. Scale bar: 100 µm. Blue arrow: typical neurons; white arrow: typical glial cells. Please click here to view a larger version of this figure.
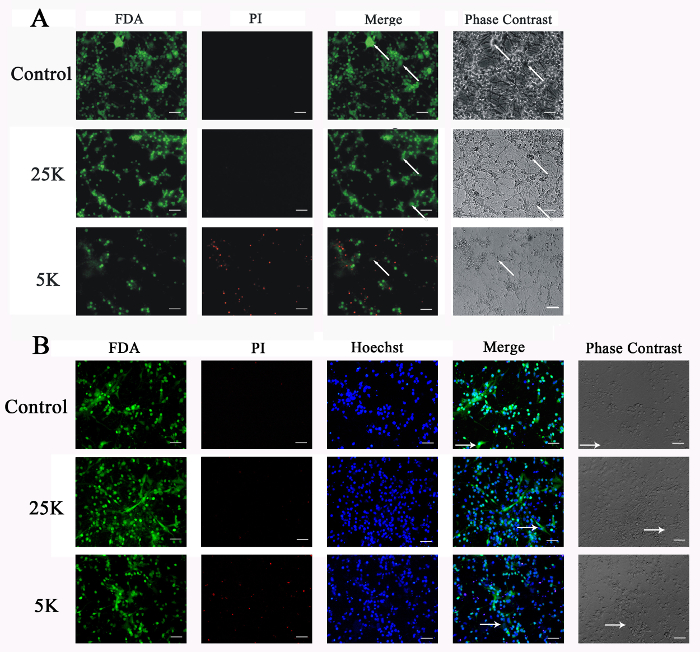
Figure 2: FDA-PI Double Staining and FDA-PI-Hoechst Triple Staining Demonstrate Low-potassium-induced Neuronal Death in CGN Culture. At day 8 in vitro, CGN cultures were switched to 5K or 25K medium. The medium in the control cultures was not changed. After 24 h of challenge, the CGN cultures were assayed by (A) FDA-PI double staining or (B) FDA-PI-Hoechst triple staining. Scale bar = 100 µm. Arrow: typical glial cells. Please click here to view a larger version of this figure.
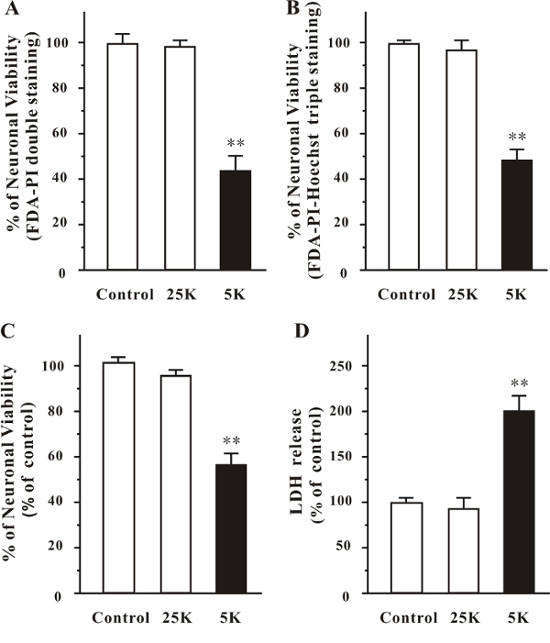
Figure 3: Quantification of Neuronal Viability in CGN Culture. At day 8 in vitro, CGN cultures were switched to 5K or 25K medium. The medium in the control cultures was not changed. After 24 h of challenge, cell viability was analyzed by (A) FDA-PI double staining, (B) FDA-PI-Hoechst triple staining, and (C) MTT assay. (D) The LDH release was analyzed by LDH assay. The data are expressed as the means ±SEM. **p <0.01 versus control (ANOVA, Tukey's test). Please click here to view a larger version of this figure.
Discussion
This protocol was modified from procedures that have been described previously6,7. Researchers have spent time trying to reduce non-neuronal cell growth by improving the conditions when culturing primary neurons in vitro8. However, even with improved culture conditions, some glial cells remain. Moreover, non-neuronal cells are necessary in primary neuronal cultures because they help with neuronal growth and maturation9. In this study, Ara-C was used to minimize the growth of glial cells, although there was still about a 1 – 5% presence of glial cells in the CGN culture. This problem is also presented in other groups' studies10. In CGN culture, the sizes and shapes of glial cells are largely different from neurons. The diameter of CGNs is about 10 µm, and mature CGNs have rich processes that connect the neurons. However, the diameter of glial cells in culture is relative large, about 30-100 µm, so it is easy to distinguish glial cells from neurons in images. Therefore, one advantage of FDA-PI and FDA-PI-Hoechst staining is to accurately measure neuronal viability in primary neuronal cultures in which non-neuronal cell growth is not prevented by the culture techniques.
Low-potassium-induced apoptosis in CGN cultures, with 25K medium-treated cells as the control, represents an excellent model to study neuronal apoptosis. Many groups, including our lab, have used this classic model study the underlying apoptotic mechanisms of neurons2,11. Therefore, this model was used to induce neuronal death. The concentration of dyes and the duration of staining used in this protocol have been optimized. Low concentrations of dyes and short durations of staining can cause insufficient staining, leading to the inaccurate counting of dead neurons. However, high concentrations of dyes and prolonged durations of staining can further induce neuronal death and cause a bias in the estimation of cell viability. Therefore, the images should be taken as soon as possible after the dyes are added. In the FDA-PI double staining images, small FDA-positive cells are marked as viable neurons. However, cell bodies tend to group up in CGN culture. Therefore, it can be hard to separate viable cells by FDA staining. In this study, Hoechst, a nuclear stain, was used to improve the accuracy. Small cells with FDA-positive cell bodies and Hoechst-positive nuclei can be regarded as viable neurons in FDA-PI-Hoechst triple staining images.
Compared to colorimetric cytotoxicity assays, FDA-PI and FDA-PI-Hoechst staining require more time for the evaluation of neuronal viability in CGN culture. Therefore, the current protocol might not be suitable to screen neuroprotective drugs. However, this limitation can be resolved by using a high-content imaging system. Another limitation of this technique is that CGNs and glial cannot be discriminated by PI. However, because neurons are more susceptible to neurotoxins than glial cells, PI-positive dead cells are mainly neurons12.
Besides FDA, PI, and Hoechst, other dyes, such as MTT, SYTO13, and SYBR14, are also used to measure cell viability13,14. For the MTT assay, glial cells that are not sensitive to neurotoxins can convert MTT to formazan, leading to the over-estimation of neuronal viability. SYTO13 is cell-permeable and has a high green fluorescent yield when bound to DNA or RNA. A previous study suggested that SYTO13 staining mainly indicates apoptotic cells in CGN culture but does not distinguishes glial cells from neurons13. SYBR14 is a membrane-permanent nucleic acid dye. SYBR14-PI double staining is used to measure cell viability because both dyes label DNA, thereby circumventing the ambiguity14. However, SYBR14-PI double staining cannot effectively distinguish glial cells from neurons in CGN culture. Therefore, FDA-PI and FDA-PI-Hoechst staining, which can distinguish glial cells from neurons, are advantageous for the accurate evaluation of neuronal viability in CGN culture.
Finally, FDA-PI and FDA-PI-Hoechst staining are not limited to the analysis of neuronal viability in CGN cultures. Many mixed cell cultures (e.g., primary cortical cultures and primary hippocampal cultures) also contain neurons and glial cells. Therefore, a similar strategy can be applied to those mixed cell cultures to accurately analyze neuronal viability.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (LY15H310007), the Applied Research Project on Nonprofit Technology of Zhejiang Province (2016C37110), the National Natural Science Foundation of China (U1503223, 81673407), the Ningbo International Science and Technology Cooperation Project (2014D10019), the Ningbo Municipal Innovation Team of Life Science and Health (2015C110026), the Guangdong Provincial International Cooperation Project of Science and Technology (No. 2013B051000038), the Shenzhen Basic Research Program (JCYJ20160331141459373), the Guangdong-Hong Kong Technology Cooperation Funding Scheme (GHP/012/16GD), the Research Grants Council of Hong Kong (15101014), Hong Kong Polytechnic University (G-YBGQ), and the K. C. Wong Magna Fund at Ningbo University.
Materials
| Poly-L-lysine | Sigma | P2636 | |
| D-glucose | Sigma | G8270 | |
| Cytosine β-D-Arabinofuranoside | Sigma | C1768 | |
| Fetal bovine serum | Gibco | 10099141 | high quality FBS is essential for culture |
| 100× glutamine | Gibco | 25030081 | |
| 100× anti-biotic | Gibco | 15240062 | |
| BME medium | Gibco | 21010046 | |
| Fluorescein diacetate | Sigma | F7378 | |
| Propidium iodide | Sigma | P4170 | |
| Hoechst 33342 | Yesen | 40731ES10 | |
| rabbit Anti-GAP43 antibody | Abcam | ab75810 | |
| mouse Anti-GFAP antibody | Cellsignaling | 3670 | |
| Bovine serum albumin | Sangon Biotech | A602440 | |
| Trypsin | Sigma | T4665 | |
| DNAse | Sigma | D5025 | |
| Soybean trypsin inhibitor | Sigma | T9003 | |
| Pasteur pipette | Volac | Z310727 | burn the tip round before use |
| 12-well cell culture plates | TPP | Z707783 | |
| 6-well cell culture plates | TPP | Z707759 | high quality cell culture plate is essential for culture |
| Filter | Millipore | SLGP033RB | |
| Pipet 5 ml | Excell Bio | CS017-0003 | |
| Pipet 10 ml | Excell Bio | CS017-0004 | |
| Dissect microscope | Shanghai Caikang | XTL2400 | |
| CO2 Incubator | Thermo Scientific | 311 | |
| Fluorescence microscope | Nikon | TI-S | |
| Fluorescence filter and emmision cubes | Nikon | B-2A, G-2A, UV-2A | |
| Photo software | Nikon | NIS-Elements | |
| Graphics editor softeware | Adobe | Photoshop CS | |
| Image process softeware | NIH | ImageJ |
References
- Yu, J., et al. Indirubin-3-oxime prevents H2O2-induced neuronal apoptosis via concurrently inhibiting GSK3beta and the ERK pathway. Cell Mol Neurobiol. , (2016).
- Cui, W., et al. The anti-cancer agent SU4312 unexpectedly protects against MPP(+) -induced neurotoxicity via selective and direct inhibition of neuronal NOS. Br J Pharmacol. 168 (5), 1201-1214 (2013).
- Jebelli, J., Piers, T., Pocock, J. Selective Depletion of Microglia from Cerebellar Granule Cell Cultures Using L-leucine Methyl Ester. J Vis Exp. (101), e52983 (2015).
- Labno, C. . Two way to count cells with ImageJ. , (2008).
- Haag, D., et al. Nos2 Inactivation promotes the development of medulloblastoma in Ptch1(+/-) mice by deregulation of Gap43-dependent granule cell precursor migration. Plos Genet. 8 (3), e1002572 (2012).
- Lee, H. Y., Greene, L. A., Mason, C. A., Manzini, M. C. Isolation and culture of post-natal mouse cerebellar granule neuron progenitor cells and neurons. J Vis Exp. (23), e990 (2009).
- Messer, A. The maintenance and identification of mouse cerebellar granule cells in monolayer culture. Brain Res. 130 (1), 1-12 (1977).
- Li, W., et al. Novel dimeric acetylcholinesterase inhibitor bis7-tacrine, but not donepezil, prevents glutamate-induced neuronal apoptosis by blocking N-methyl-D-aspartate receptors. J Biol Chem. 280 (18), 18179-18188 (2005).
- Kaech, S., Banker, G. Culturing hippocampal neurons. Nat Protoc. 1 (5), 2406-2415 (2006).
- Cheung, G., Cousin, M. Quantitative analysis of synaptic vesicle pool replenishment in cultured cerebellar granule neurons using FM Dyes. J Vis Exp. (57), e3143 (2011).
- Atlante, A., et al. Genistein and daidzein prevent low potassium-dependent apoptosis of cerebellar granule cells. Biochem Pharmacol. 79 (5), 758-767 (2010).
- Silva, R., Rodrigues, C., Brites, D. Rat cultured neuronal and glial cells respond differently to toxicity of unconjugated bilirubin. Pediatr Res. 51 (4), 535-541 (2002).
- Jekabsons, M. B., Nicholls, D. G. Bioenergetic analysis of cerebellar granule neurons undergoing apoptosis by potassium/serum deprivation. Cell Death Differ. 13 (9), 1595-1610 (2006).
- Garner, D. L., Johnson, L. A. Viability Assessment of Mammalian Sperm Using Sybr-14 and Propidium Iodide. Biol Reprod. 53 (2), 276-284 (1995).

