The C. elegans Excretory Canal as a Model for Intracellular Lumen Morphogenesis and In Vivo Polarized Membrane Biogenesis in a Single Cell: labeling by GFP-fusions, RNAi Interaction Screen and Imaging
Summary
The C. elegans excretory canal is a unique single-cell model for the visual in vivo analysis of de novo polarized membrane biogenesis. This protocol describes a combination of standard genetic/RNAi and imaging approaches, adaptable for the identification and characterization of molecules directing unicellular tubulogenesis, and apical membrane and lumen biogenesis.
Abstract
The four C. elegans excretory canals are narrow tubes extended through the length of the animal from a single cell, with almost equally far extended intracellular endotubes that build and stabilize the lumen with a membrane and submembraneous cytoskeleton of apical character. The excretory cell expands its length approximately 2,000 times to generate these canals, making this model unique for the in vivo assessment of de novo polarized membrane biogenesis, intracellular lumen morphogenesis and unicellular tubulogenesis. The protocol presented here shows how to combine standard labeling, gain- and loss-of-function genetic or RNA interference (RNAi)-, and microscopic approaches to use this model to visually dissect and functionally analyze these processes on a molecular level. As an example of a labeling approach, the protocol outlines the generation of transgenic animals with fluorescent fusion proteins for live analysis of tubulogenesis. As an example of a genetic approach, it highlights key points of a visual RNAi-based interaction screen designed to modify a gain-of-function cystic canal phenotype. The specific methods described are how to: label and visualize the canals by expressing fluorescent proteins; construct a targeted RNAi library and strategize RNAi screening for the molecular analysis of canal morphogenesis; visually assess modifications of canal phenotypes; score them by dissecting fluorescence microscopy; characterize subcellular canal components at higher resolution by confocal microscopy; and quantify visual parameters. The approach is useful for the investigator who is interested in taking advantage of the C. elegans excretory canal for identifying and characterizing genes involved in the phylogenetically conserved processes of intracellular lumen and unicellular tube morphogenesis.
Introduction
All internal organs are composed of tubes, crucial for their many different functions, such as the transport and exchange of gases, liquids and nutrients and the excretion of metabolic waste. Their polarized character, with distinct apical and lumenal membranes, is adapted to these specific functions, and defects in the biogenesis of their endo- and plasma membrane systems are a frequent cause of human disease1,2. The majority of tubes of the vasculature and of internal organs are multicellular and form a lumen intercellularly; however, unicellular tubes, which form the lumen intracellularly, can, for example, represent as much as 30–50% of human capillary beds2. The polarized membranes of multi- and unicellular tubes are similar in composition, although their microdomains may differ based on the tube’s specific function (e.g., excretory canal canaliculi versus intestinal microvilli in Caenorhabditis elegans; see accompanying paper on C. elegans intestinal tubulogenesis)3. The principles of polarized membrane biogenesis and tubulogenesis are conserved among metazoans, and a similar molecular machinery directs them1,2,4.
The C. elegans excretory system consists of five cells: the excretory cell (EC), duct cell (DC), pore cell (PC) and two gland cells. Ablation of the EC, DC or PC causes fluid accumulation in the body cavity and the animals die at an early larval stage5. Intriguingly, these three unicellular tubes create their lumens in three different ways: by cell hollowing (EC); cell wrapping coupled with autocellular junction formation (PC); and by cell wrapping coupled with autofusion (DC); different mechanisms of lumen morphogenesis that are all phylogenetically conserved6,7. The EC, located at the left lateral side of the posterior pharyngeal bulb, sends out two lateral extensions from which the four canals branch out to extend anteriorly and posteriorly (on both the right and left side) to the tip of the worm’s nose and tail, respectively (Figure 1)5,6,8. The EC extends from approximately 1 µm to 2 x 1,000 µm, making it the largest cell in the animal. On a subcellular level, the excretory canal is a simple tube, generated from a basal membrane directed towards the pseudocoelom, and tunneled by a lumenal membrane (endotube). The canal lumenal membrane connects to the duct lumenal membrane at its only intercellular junction; the canals are otherwise junctionless along their lengths (Figure 1). The excretory canal lumenal membrane and its submembraneous cytoskeleton are apical, defined by their molecular composition that resembles the composition of the apical membrane and submembraneous cytoskeleton of multicellular tubes, such as the intestine, and of other (e.g., flat) epithelia. Cytoplasmic organelles, including endosomal vesicular and other (e.g., Golgi) endomembranes are distributed along the length of the canal. In addition, multiple canalicular vesicles – either connected to the lumenal membrane, and/or interconnected, or isolated – are threaded through the canal cytoplasm7,8,9,10. This dynamic plasma-membrane/canalicular connection further expands the canal’s membrane system and contributes to both lumen morphogenesis and osmoregulation10. The excretory canal thus consists almost entirely of endo- and plasma membranes, providing an excellent model for the analysis of polarized membrane biogenesis and the regulation of the endo- to plasma membrane interface. The dramatic expansion of the apical membrane during canal morphogenesis – in this single-cell system coincident with lumen extension – also allows to analyze the architectural problems arising by the need to stabilize and center an intracellular lumenal membrane. This protocol focuses on the analysis of the canal tube’s and lumen’s structural morphogenesis and the intracellular membrane dynamics required for this process rather than on the signals that direct the cell movements that generate the EC’s position in the excretory system and construct its intricate connections to other cellular elements (reviewed in6).
A further advantage of the C. elegans single-cell canal system for the analysis of polarized membrane and intracellular lumen biogenesis is its ability to separate, through developmental time, the generation of different components of its membranes and junctions. The EC is born at the time of ventral closure and settles ventro-laterally of the pharynx during mid embryogenesis5,6,8, during which time lateral canal extension and branching occur. This is followed by anterior-posterior canal extension during late embryogenesis, a process that continues into the L1-larval stage (Figure 1). In a newly hatched larva, the posterior canal tip reaches approximately the middle of the worm, fully extending to the tail at the end of the L1 stage, after which time the canal elongates along with the worm8. Active canal growth at a speed exceeding that of the animal’s growth thus ends at the first larval stage, however, further growth occurs in parallel with the growth of the whole animal during the additional larval stages (L2–4). This setting provides the opportunity to analyze different steps of de novo polarized membrane biogenesis independent of polarized cell division or migration. Moreover, it permits the separation of this process from the assembly of junctions (which occur in the embryo before lumen initiation); their exact requirement in membrane polarization is still an open question in the polarity field. Finally, it uniquely separates apical from basal membrane expansion, the latter process preceding the former in the excretory canals10. The C. elegans excretory canal model is therefore a particularly informative complement to the intestinal model which shares a number of these advantages for the analysis of polarized membrane biogenesis but executes it in a multicellular setting (see the accompanying paper on intestinal tubulogenesis3).
Although wild-type canals are ultrathin tubules in this tiny worm, their lumens can be visualized directly by Nomarski optics in this transparent animal. In fact, mutant cystic canal morphologies can be characterized in unlabeled animals using low magnification dissecting microscopy, which has been used to great effect in forward genetic screens to identify genes involved in tubulogenesis11. Improved visualization of the morphology of canals and distinction of their polarized membranes, cytoskeletal components, different intracellular organelles and other subcellular structures, however, requires labeling and higher power fluorescent dissecting and confocal microscopy. Although the canals’ fine structure poses a number of difficulties for labeling and microscopy, membranes and subcellular components can be distinguished via the specific molecules unique to each compartment, and animals can be safely mounted for microscopy if certain precautions are taken to avoid introducing artifacts (see Protocol and Discussion). Labeling can be done by immunohistochemistry in fixed specimens, or by generating transgenic worms expressing fluorescent fusion proteins under the control of their own or excretory canal-specific promoters for in vivo imaging. This protocol describes the latter labeling technique (see the accompanying paper on intestinal tubulogenesis for antibody staining3).
The ability to combine in vivo loss- or gain-of-function studies with in vivo imaging analysis at the single cell level throughout development makes the C. elegans excretory canal a particularly strong model for the molecular and cellular analysis of unicellular tubulogenesis. Forward or reverse genetic screens can be performed starting with a wild-type or labeled transgenic animal to identify canal morphogenesis phenotypes (for instance, cysts) and their underlying gene defects. Alternatively, such screens can start with a mutant phenotype (e.g., a cystic canal) and identify suppressors or enhancers of this phenotype to identify genes that functionally interact with the gene causing the mutant phenotype. The genetic defect causing the mutant phenotype can induce a loss (e.g., via gene deletion) or a gain (e.g., via an activating mutation or via the introduction of excess gene copies) of the investigated function. Forward mutagenesis or systematic RNAi screens are without preconceptions on gene function and permit the unbiased identification of genes involved in the function of interest. Given the availability of genome-wide RNAi feeding libraries, almost every gene can be easily knocked down by RNAi in C. elegans, such that any single gene of interest or any group of genes (e.g., in targeted screens) can also be rapidly probed for their effect in a reverse genetics approach. To demonstrate a possible combination of approaches, we here describe a targeted RNAi interaction screen, starting with a gain-of-function cystic excretory canal mutant, labeled with cytoplasmic canal green fluorescent protein (GFP). The mutant phenotype was generated by overexpression of erm-1, a highly conserved C. elegans ortholog of the membrane-actin linker family Ezrin-Radixin-Moesin (ERM), which has been implicated in lumen morphogenesis and membrane organization in many species12. C. elegans ERM-1 localizes to lumenal membranes of internal organs, such as the excretory canal and intestine, and is required for lumen formation in both13. ERM-1 overexpression recruits excess actin and vesicles to the canal lumenal membrane, increasing flux into the lumen and generating a short cystic canal and a crimped lumenal membrane with thickened actin undercoat9. The protocol describes how to generate transgenic strains with excretory-canal-expressed labeled fusion proteins (or other proteins); how to perform targeted RNAi screens starting with such strains, to identify modifiers of a canal phenotype; and how to visually analyze the results of such screens by fluorescence dissecting and confocal microscopy, including simple ways to quantify informative tubulogenesis phenotypes. Alternative labeling techniques and the details of RNAi, adjusted to the often lethal tubulogenesis genes, can be found in the accompanying paper on intestinal tubulogenesis3. All methods can be used in various combinations for the investigation of other questions on canal tubulogenesis.
Protocol
1. Labeling the C. elegans Excretory Canal by Fluorescent Fusion Proteins14
Note: See the accompanying paper on intestinal tubulogenesis3 for labeling by in situ antibody staining procedures adaptable to the excretory canal. See Table 1 for examples of molecules proven useful for visualizing C. elegans excretory canal endo- and plasma membranes, Table 2 for examples of promoters driving expression to the excretory canal, and Table 3 for resources for more comprehensive collections of markers and promoters, including references discussing the selection of different fluorophores.
- Construction of tissue specific fluorescent marker plasmids by restriction enzyme based cloning15
Note: See the Discussion for alternative techniques of constructing fluorescent fusion proteins.- Identify the sequence of the promoter (for transcriptional fusions) or of the entire gene of interest with its promoter (for translational fusions) in WormBase44.
Note: For promoters, about 1–3 kilobase (kb) is sufficient for most C. elegans genes. Translational fusion proteins can also be constructed by placing a gene of interest under an excretory canal specific promoter (see Table 2). - Design forward and reverse primers for amplification of the promoter (for transcriptional fusions) and/or a full gene with promoter (for translational fusions). Add restriction enzyme linkers at the 5’ and 3’ ends of the primers.
Note: Choose restriction enzymes that are present in the vector plasmid (e.g., pPD95.79)16. For translational fusions, the 3’ linker should be designed so that after restriction enzyme digestion and ligation with the vector, the insert codon frame will be continuous with the codons of the fluorophore, e.g., GFP. One may need to add 1 or 2 more bases to the typical restriction enzyme linkers14; take care not to create a stop codon. - Perform polymerase chain reaction (PCR) to amplify the promoter or full-length gene using live worms or wild-type genomic DNA or cDNA as template15.
Note: When using worms as template, first lyse worms in lysis buffer (PCR buffer plus Proteinase K)17. Mixed stage worms can be used as template. Starved worms can be used to avoid contamination with bacterial DNA. - Perform agarose (1%) gel electrophoresis on PCR products to identify the correct size of amplified product.
Note: If band size is correct, proceed with next step. If multiple bands are generated, improve amplification conditions to produce single band. If this does not work, cut the correct band from the gel and purify DNA by standard methods15 and then proceed with next step. - Perform restriction digest on the PCR product and the vector plasmid that contains the fluorophore (e.g., pPD95.79) in separate tubes by standard methods15.
- Separate the digested DNAs by gel electrophoresis and elute the PCR product and vector DNA bands in separate tubes.
- Purify the DNAs from gel slices by standard methods15. Measure DNA concentration by spectrophotometer.
- Ligate the PCR product and vector DNA by standard methods and transform the recombinant DNAs into competent cells by standard methods15.
- Spread 10 μL, 50 μL and 100 μL of the transformed cells on three individual Luria Broth (LB) plates supplemented with 50 μg/mL ampicillin.
Note: Spread different amounts of cells onto different plates for a spectrum of transformation efficiencies. For instance, plating too densely may not permit the isolation of colonies if transformation is efficient. - Incubate the plates at 37 °C overnight. Next morning, take out the plates from the incubator.
Note: If the colonies are very small, incubate for several more hours. - Prepare plasmid DNAs from single colonies by standard methods15. Mix template DNA and primers and send out for sequencing (typically performed in a core service center).
- Read the sequences and verify the integrity of the fusion construct.
Note: CRITICAL: Confirm the correct codon frame between inserted gene and fluorescent marker gene for translation fusions. Ideally, sequence the whole gene to confirm that no mutation was introduced during PCR and ligation procedures. - Generate more plasmid DNA (using step 1.1.11) for injection for step 1.2.
- Identify the sequence of the promoter (for transcriptional fusions) or of the entire gene of interest with its promoter (for translational fusions) in WormBase44.
- Generation of transgenic animals by microinjection of DNA for germline transformation18
Note: See the discussion for alternative techniques for introducing transgenes. The outlined procedures can be used to generate transgenic animals that carry a fluorescence fusion protein or any other protein of interest. For instance, an exogenous protein can be newly introduced (e.g., a heterologous ortholog) or an endogenous protein can be reintroduced (e.g., into its corresponding germline mutant for rescue) or overexpressed to generate a phenotype (e.g., injection of erm-1 was used to generate the overexpression cystic canal phenotype that serves as the target for modification by the RNAi interaction screen described below).- Mix construct DNA (1–50 ng/μL) with marker plasmid DNA (typically 100 ng/μL), for instance the dominant marker rol-6 (su1006) (see 1.2.3 for marker options).
Note: CRITICAL: Concentration of injected DNA must be empirically determined to avoid introduction of artefactual phenotypes (cysts, extension defects, lethality) when expressing genes in the excretory canals that are particularly sensitive to the expression of transgenes. One can, for instance, make several mixtures of plasmids at concentrations of 1 ng/μL, 10 ng/μL, 50 ng/μL, and 100 ng/μL with 100 ng/μL rol-6(su1006) to test a range of concentrations for the generation of a viable strain with the desired expression or phenotype (high concentrations are likely to be non-specifically toxic for canal morphogenesis and may be lethal). - Filter the DNA mixture through a 0.22-μm (micrometer) pore size spin-x centrifuge tube filter.
Note: Do not leave the lid of the tube open to avoid dust that can block the microinjection needles. - Microinject recombinant plasmids into the gonad of wild-type or mutant worms by standard methods for germline transformation (see reference18 for procedure details).
Note: Standard marker plasmids are, for instance: rol-6(su1006), dpy-20, unc-119, pha-1. Dominant transgenes like rol-6(su1006) are introduced into wild type worms, whereas rescuing transgenes are introduced into their respective mutants. Marker plasmids are co-injected for easy maintenance of the transgenic lines since extrachromosomal transgenes are randomly lost during cell division (see 1.2.8). When injecting genes encoding for fluorophore fusions, one can also use the fluorophore, GFP, itself as marker. rol-6 induces worms to roll around themselves which is often advantageous for the evaluation of morphogenesis phenotypes. - Transfer injected worms onto Escherichia coli seeded Nematode Growth Medium (NGM) plates (e.g., 5 worms/plate) (see reference19 for standard C. elegans culture and maintenance procedures and Table of Materials).
- Incubate the plates and let progeny develop at 20 °C for about 3 d.
- Examine the F1 progeny under the dissecting microscope for Rol (rolling) worms (or any other specific injection marker, e.g., GFP) and pick rollers to individual plates.
- Select plates with rolling F2 animals and confirm presence of fluorescence under a dissecting fluorescence microscope (usually all roller animals are GFP positive).
Note: F2 rollers indicate the successful generation of a transgenic line. Individual lines may be different, e.g., with regard to transgene transmission rate. It is therefore useful to maintain and store several lines. - Maintain the transgenic lines by enriching new plates for marker-positive animals.
Note: The injected DNA is incorporated into the germline as an extrachromosomal array. Transmission rates for extrachromosomal arrays are variable but generally around ~50%. To not lose the strain, it is therefore critical to manually enrich lines with dominant markers (e.g., lines not secured by negative selection). - Freeze transgenic lines by standard freezing techniques for long term storage at -80 °C19.
Note: Transgenes on extrachromosomal arrays can also be integrated into the germline by UV irradiation in an additional step to yield homogenous lines18. For example, erm-1 was integrated into the germline by UV irradiation to obtain the ERM-1[++] strain fgIs2[erm-1p::erm-1;rol-6p::rol-6(su1006)] where every animal carries the transgene, a requirement for its use in the RNAi-based interaction screen described below. This strain was additionally labeled by cytoplasmic excretory canal GFP via crossing in a strain containing a vha-1p::GFP transgene (generated by the same procedures as outlined above; see reference20 for basic genetic procedures such as crosses) and is referred to as ERM-1[++]; vha-1p::GFP strain below.
- Mix construct DNA (1–50 ng/μL) with marker plasmid DNA (typically 100 ng/μL), for instance the dominant marker rol-6 (su1006) (see 1.2.3 for marker options).
2. Construction of a Targeted RNAi Library and Design of an RNAi Interaction Screen to Modify a Canal Phenotype
Note: A targeted RNAi-based genetic interaction screen is described that uses an overexpression cystic canal phenotype to search for interacting excretory canal morphogenesis genes. The ERM-1[++] strain (see step 1.2.9) serves as example9. This approach presents only one of many possible approaches for the genetic analysis of excretory canal lumen morphogenesis (see Introduction and Discussion for other genetic approaches). See the accompanying paper on intestinal tubulogenesis3 and references17,21,22 for background on RNAi, details of RNAi procedures, modulation of RNAi strength (adjusted to the often lethal tubulogenesis genes) and discussion of technical problems connected to RNAi. See reference19 for standard worm culture and maintenance and Table of materials.
- Search for ERM-1 (or other gene of interest) interacting molecules in databases and published articles.
Note: Potential ERM-1 interactors would include all molecules that were experimentally shown to functionally, genetically, or physically interact with ERM proteins in any species and/or were predicted to do so by any in silico, high throughput or systems biology approach (see Table 3 for examples of databases and resources). - Generate a list of all genes and find C. elegans homologs where required.
Note: Consider expanding the list of identified genes to gene classes, which takes into account the function of the gene of interest and widens the net for identifying interactors (e.g., for the membrane-actin linker ERM-1, select all actins and actin-related molecules). - Identify the corresponding RNAi bacterial feeding clones in commercially available genome-wide bacterial RNAi libraries for all genes (e.g., Ahringer genomic C. elegans RNAi feeding library21; Table of Materials)
- Generate a spreadsheet for all genes and their corresponding RNAi plate and well number.
- Pick and streak ~50 RNAi clones on LB/ampicillin/tetracycline plates (choice of antibiotic is determined by library construction) per day and continue until targeted RNAi library is generated.
Note: Depending on library size and projected workflow, omit generating a full library and proceed directly with batch analysis. Plates can be stored at 4 °C, for no longer than approximately 2 weeks (if needed, re-streak onto new plates after that). Conversely, one can generate larger frozen libraries in replicate 96-well or 384-well formats for long term storage at -80 °C. - Incubate the plates at 37 °C overnight. Next morning, remove plates from incubator and store at 4 °C.
- Pick RNAi bacteria from a plate by a sterile toothpick, mix bacteria with 600 μL LB/ampicillin (50 ng/μL) broth in 1.5 mL microtube, incubate the tubes at 37 °C, shake for 6 h.
Note: Inoculate RNAi bacteria into the broth by rubbing the pick (toothpick or micropipette tip) along the side of the microtube. - Seed 70 μL cultured bacteria into each well of 6-well RNAi plates in duplicate or triplicate sets. Incubate the RNAi plates at 22 °C overnight.
Note: RNAi plates are generated by standard procedures (Table of Materials and references3,17,21) and here used in a 6-well tissue culture plate format for a higher throughput approach that still allows for the microscopic evaluation of canal morphogenesis in live animals on the plates. - Next morning, pick 3 L4 stage ERM-1[++]; vha-1p::GFP worms onto each well of the RNAi plates.
- To avoid contamination with OP50 bacteria (see reference19) that interfere with RNAi first seed the worms onto a NGM plate without bacteria and let the animals crawl for about 10 min. Only use non-starved healthy animals.
- Incubate the plates at 22 °C for 3 d to allow animals to produce progeny.
- Examine canal phenotypes in F1 progeny under the dissecting fluorescence microscope.
3. In Vivo Imaging of the C. elegans Excretory Canal by Fluorescence Dissecting Microscopy and Scoring of Tubulogenesis Phenotypes
- Prepare a phenotype scoring sheet (example shown in Table 4 and Figure 5).
- Place the agar plate with worms directly under the fluorescence dissecting microscope, open lid of the plate for evaluation, use lower magnification to focus.
Note: This protocol describes the use of a scope with 1.5X and 10X objectives and a zoom range from 3.5 to 45 (Table of Materials). - Evaluate animals by focusing on each well separately, starting with well 1, and work down the plate.
Note: Always start with the evaluation of controls. For instance, a mock (empty vector) negative control (HT115 RNAi bacteria (see references17,21) without or with an unrelated gene insert) and appropriate positive controls, e.g., in this interaction screen, erm-1 RNAi (suppresses the ERM-1[++] phenotype) and sma-1/spectrin RNAi (enhances the ERM-1[++] canal phenotype). - First, examine general phenotypes visible under bright light (for example: Let/lethal, Clr/clear, Emb/embryonic lethal, Ste/sterile, Unc/uncoordinated, Dpy/dumpy, etc.), quantify the phenotype by counting total number of animals and number of animals with the phenotype, record the numbers (see Table 4).
Note: For knockdowns of genes causing defects that may affect evaluation of canal phenotypes (e.g., Emb, Ste), consider repeating the experiment with conditional, post embryonic RNAi (see accompanying paper for procedures3). - Second, examine excretory canal phenotypes under fluorescent light, score quantifiable phenotypes (e.g., length of canal, width of the lumen, cysts), record the numbers and describe phenotypes on a scoring sheet (see Table 4, Figure 5).
Note: Higher magnification with zoom range is required to evaluate more subtle canal phenotypes. Move back and forth between low and high magnification to carefully evaluate the canal’s length and width and any other canal morphogenesis phenotype. For quantification or semi-quantification of simple phenotypes, count 100 animals (e.g., L4s in this targeted RNAi screen; phenotypes: canal length of 1/4, 1/2, 3/4 and full extension of posterior canals, and lumen diameter of posterior canals, small cysts (< 1/3 of animal width), large cysts (> 1/3 of animal width); see Table 4). - Acquire images of the predominant phenotypes from at least 3 different animals by a microscope mounted digital charge-coupled device (CCD) camera and corresponding imaging software (see Table of Materials)
- To acquire images, first turn on camera, turn on attached computer, double click the image capture software icon, focus an area of the worm plate manually under low magnification, and open the camera shutter.
- Click on the “live preview” icon of image capture software on the computer screen to visualize the worms on the computer screen, adjust the focus manually to clearly visualize the worms on the screen, then click the “snap” icon, then click the “save” icon.
Note: Animals will move faster under fluorescent light, therefore keep one hand on computer mouse ready while moving the plate into the area of interest with the other hand. Then promptly click the “snap” icon to acquire the image. It is usually possible to acquire a good image with several tries. - Save the acquired images with a proper file name (include strain name, RNAi clone name and date).
Note: Faster moving wild-type worms with thin and long canals are more difficult to image than mutants. Mutants with cystic canals and/or other phenotypes are likely to move slowly, thus facilitating imaging. Marker plasmids such as Rol can be useful for imaging by keeping animals “on the spot” rather than moving forward, and may also provide an improved view on the phenotype with the animal rolling around itself.
4. Imaging of the C. elegans Excretory Canal at High Resolution by Laser Scanning Confocal Microscopy
- Mounting live animals
- Place a tiny amount of grease or petroleum jelly at the tip of a cotton swab or at the tip of the index finger, and spread the grease to generate an ultrathin circle with a diameter of ~ 6–8 mm in the middle of a clean glass slide.
- Place 6 μL 5% lidocaine solution (anesthetic) into the circle by a micropipette.
Note: A lidocaine stock solution can be made by dissolving lidocaine powder in water. Dilute to 5% with M9 buffer19 (Table of Materials). It is critical for the analysis of the excretory canal to avoid the common immobilization solutions (such as sodium azide) that cause cysts to rupture and that induce canal phenotypes. - Pick several animals from an RNAi plate, and place them into the lidocaine solution by immerging the worm pick19 into the solution.
Note: Preferably pick stage-specific animals that will facilitate even-mounting by uniform thickness. Animals can be preselected on the dissecting fluorescence microscope. - Place a 22 mm x 22 mm coverslip onto the glass slide; let it gently settle onto the grease circle.
Note: Do not apply any physical pressure on the coverslip which may damage canal morphology, especially in mutants or RNAi treated worms with canal and possibly other phenotypes. It is important therefore to avoid a thick grease circle; ideally animals are gently sandwiched between glass slide and cover slip. - Write the name of the sample on the frosted side of the glass slide. Immediately take the slide to the confocal microscope for analysis of canal phenotype and to acquire images.
Note: Delays may result in damage of canal cysts or change in canal lumen morphology.
- Acquiring confocal images
- Place the slide on the sample stage of the confocal microscope, focus the worms at low magnification (10X).
- View and select animals under 60X and/or 100X objectives, examine the excretory canal’s cellular and subcellular phenotypes, e.g., lumen shape and diameter, size and shape of cysts; or the subcellular components labeled for analysis, e.g., apical/lumenal membrane, basal membrane, cytoplasm, endosomal versus canalicular vesicles, other organelles (see Discussion, Figure 2, and Figure 4).
- Acquire images of the specific phenotype of interest.
Note: This protocol describes the use of a laser scanning confocal microscope (Table of Materials). To resolve subcellular components in the thin C. elegans excretory canals, higher magnification objectives (60X to 100X) are required. A spinning-disk confocal microscope can be used to acquire time lapse images but provides less confocality (see Discussion).- To acquire confocal images, turn on the computer, double click on the confocal microscope software, and select the laser by clicking on a specific laser icon.
- Click the “scan” icon to visualize the focused worm on the computer screen, adjust laser intensity through the software, and click again on “scan” icon to stop scanning, then click on “capture” icon to capture an image, then click on “save” icon.
- Save images with proper file name and include RNAi clone name, strain name and date.
Note: Images can be acquired as single and multiple sections (e.g., 10–15 sections along the z-axis). Sectioning allows 3D visualization. Acquire projection images and save separately if required (depending on microscope). For optimal resolution, use laser settings with low gain, do not open pinhole too much, and add several averaging per image (see references23,24 for general discussion of confocal imaging). Take care to acquire images at a brightness below saturation level to allow for modification by imaging software if required (preferably use unmodified images). - Acquire images of double- or multiply labeled canals (e.g. green, red, and blue) in the same fashion, by clicking on multiple laser icons, but use sequential scanning to avoid bleed-through between channels (critical for co-localization studies).
Note: One may need to take into consideration the time required for sequential scanning (which also results in a corresponding increase in photo bleaching) and modify scanner settings. Do not scan animals from one slide for more than 30 min maximum to avoid unspecific effects on canal morphology. Mount new slide if longer scanning is required. - Acquire corresponding differential interference optics (DIC)/Nomarski images, particularly if quantifying canal length and lumen diameter in relation to the worm’s body length and diameter. Overlay fluorescence and Nomarski images by clicking on “overlay” icon to demonstrate landmarks (Figure 1D and Figure 4A–D).
- For quantification, measure fluorescence intensity of a labeled component of interest by ImageJ software25 (Figure 5C).
Representative Results
This protocol describes how to use the C. elegans excretory canals to visually and molecularly analyze unicellular tubulogenesis and intracellular lumen morphogenesis in a single cell. During their extension from the time of mid-embryogenesis to adulthood, the four excretory canals continue to expand their basolateral and apical/lumenal membranes together with their canalicular and endosomal endomembrane system, providing a unique model for the in vivo analysis of de novo polarized membrane biogenesis (Figure 1). Subcellular components such as apical membranes, the cytoplasm, and endosomal versus canalicular vesicles can be visualized by expressing specific fluorescent fusion proteins (described in protocol section 1), and they can be distinguished from each other by double or triple labeling in a single transgenic animal (Figure 2). A unique excretory canal phenotype (Figure 3A–B) generated by overexpressing an apical membrane associated molecule (ERM-1), is used to demonstrate how to perform a targeted RNAi screen to identify genes functioning in apical membrane and lumen biogenesis; this serves as an example of a molecular and visual analysis of polarized membrane biogenesis in this model (described in protocol section 2). This ERM-1[++] canal phenotype is used to demonstrate how to visually evaluate and score suppression (Figure 3C–D) and enhancement (Figure 3E–F) by dissecting fluorescence microscopy (described in protocol section 3). Canal phenotypes induced by modulation of ERM-1 levels serve to show how to resolve defects at the subcellular level by confocal microscopy (Figure 4) (described in protocol section 4), and how to quantify simple canal phenotypes (canal length and cyst size) and apical membrane biogenesis defects (Figure 5).
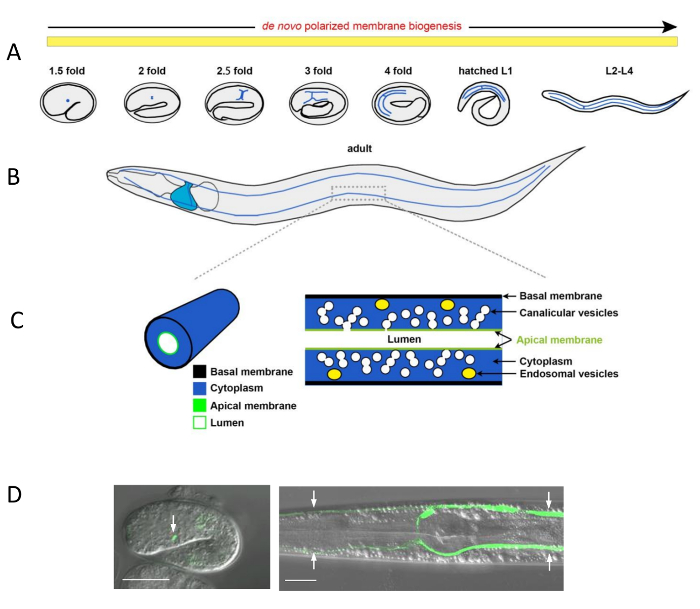
Figure 1: Morphogenesis of the C. elegans Excretory Canal and Subcellular Canal Structures
(A) Schematic representation of excretory canal extension (blue lines) during development of the embryo and larva. The excretory cell reaches its final location at the left ventro-lateral side of the posterior pharyngeal bulb at the comma stage of the embryo (not shown). As the embryo elongates, it first extends two arms laterally towards the left and right sides; then each arm bifurcates into an anterior and posterior branch. These anterior and posterior branches extend further throughout elongation of the animal during four larval stages, first catching up with the animal’s growth at the L2 stage, then accompanying its further growth, until adulthood. De novo polarized membrane biogenesis supports this extension up to adulthood. (B) In the adult animal, the anterior canal branches reach the nose tip and the posterior branches, the tail (blue lines). The excretory cell body is shown near the posterior pharyngeal bulb (blue). Polarized membrane domains are maintained during adulthood. (C) Enlarged views of a canal arm section. Left: 3D view of the canal shows: basal membrane (black), cytoplasm (blue), lumenal membrane (green) and the lumen (white). Right: inside view of the canal and its membranes: basal membrane (black), cytoplasm (blue), endosomes (yellow ovals), canalicular vesicles (white spheres, connected to each other, connected to the lumen, or isolated single vesicles), apical membrane (green) and the lumen (white). (D) Confocal/DIC overlay micrographs of the excretory cell in an embryo and the excretory canals in a larva, labeled by lumenal versus cytoplasmic GFP, respectively. Left image: excretory cell (green, arrow) in a 1.5-fold embryo, with canal lumen visualized by expressing apical ERM-1::GFP under the erm-1 promoter. Right image: four excretory canal branches (green, arrow) in L3 larvae, visualized by cytoplasmic vha-1p::GFP. Scale bars = 20 μm. Please click here to view a larger version of this figure.
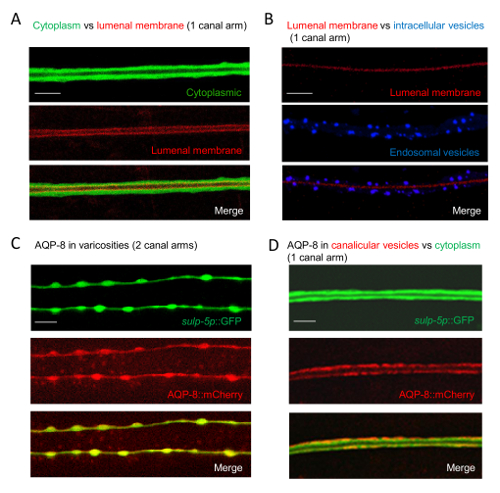
Figure 2: Visualizing Subcellular Components in Wild-type Excretory Canal Arms by Double Labeling with Fluorescent Fusion Proteins
(A) Distinguishing the cytoplasm from the apical membrane. Expression of GFP under the sulp-5 promoter visualizes the canal cytoplasm (green, top); expression of ERM-1::mCherry under its own promoter visualizes the apical membrane (red, middle); merging these images (bottom) distinguishes between cytoplasm and the apical membrane that would otherwise be indistinguishable by single labeling even at high magnification. Scale Bar = 5μm. (B) Determining the spatial relationship of endosomal vesicles to the lumen. Visualization of the lumen by an apical membrane-associated GFP fusion protein (pseudo colored to red, top); visualization of endosomal vesicles by expressing mCherry::RAB-7 (pseudo colored to blue, middle); merging these images (bottom) shows the relative spatial positions of endosomal vesicles to the lumen. Scale Bar = 5μm. (C and D) Resolving canal tube shapes versus subcellular canal components at different magnifications. The cytoplasm of L1 larvae is labeled by expressing GFP under the sulp-5 promoter and cytoplasmic canalicular vesicles by expressing the water channel AQP-8::mCherry under the aqp-8 promoter. (C) Images acquired at lower magnification resolve a “beads-on-a-string” pattern corresponding to stage-specific varicosities (varicosities are reservoirs abundant in canalicular vesicles and other components required for canal growth), but do not resolve cytoplasmic AQP-8 puncta. (D) Images acquired at higher magnification resolve AQP-8 puncta in the canal cytoplasm, corresponding to canalicular vesicles. Scale Bars: 20 μm in C and 5 μm in D. All panels show confocal projections of 10–15 sections each. Please click here to view a larger version of this figure.
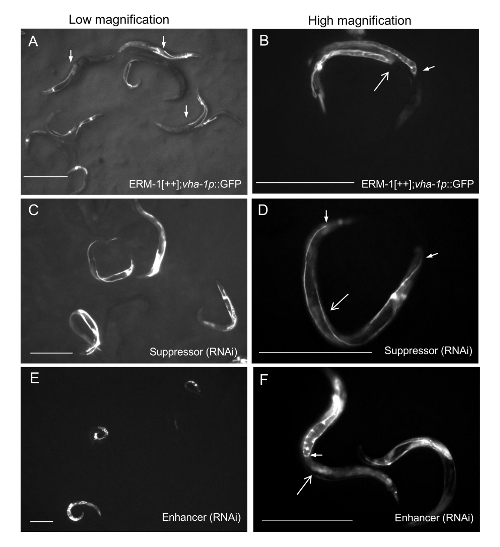
Figure 3: Scoring Enhancers and Suppressors of a Cystic Canal Phenotype (ERM-1[++]) by Dissecting Microscopy
(A) ERM-1[++]; rol-6(su1006) excretory canals are visualized by expressing GFP under a vha-1 promoter. Posterior canals extend up to the vulva or mid body of the animal (arrows; considered as 1/2 extension) at lower magnification (1.5X objective and low zoom). (B) At higher magnification, the signature ERM-1[++] small-cystic crimped canal can be resolved, with the tip of the posterior canals (one arm indicated by small arrow) extending up to the vulva or slightly beyond (vulva indicated by large arrow; canal lumen in panel D can be considered as wild type for comparison). (C) Suppression, low magnification: elongated and thin canals in RNAi treated ERM-1[++] animals. (D) At higher magnification, posterior canals can be seen to almost fully extend to the tail (small arrows; compare to that of the parent animals, shown in panel B); large arrow indicates position of the vulva. (E) Enhancement: further shortened canals with larger cysts in RNAi treated ERM-1[++] animals; low magnification, no zoom. (F) At higher magnification, posterior canal extension can be determined as less than 1/2 (small arrow) or not extended up to the vulva (indicated by large arrow) and cyst size as exceeding 1/3 of the animal’s width (wider than that of the parent animal shown in B). Scale bars= 400 µm. Please click here to view a larger version of this figure.
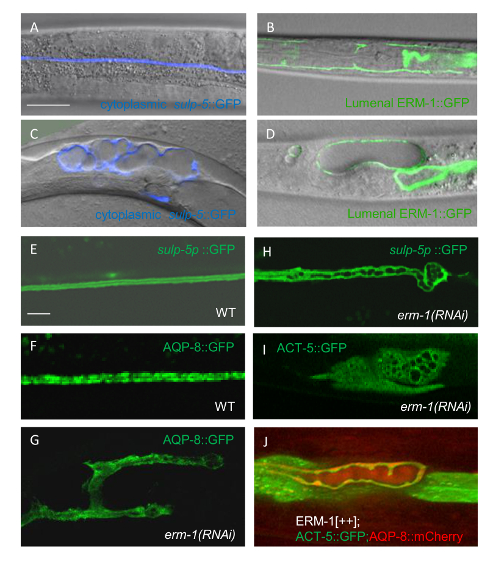
Figure 4: Visual Analysis of Excretory Canal Morphology and Subcellular Canal Components in Mutant/RNAi Animals by Confocal Microscopy
(A–D) Distinguishing a vacuolar from a cystic canal phenotype (confocal/DIC overlay micrographs are shown). (A) The cytoplasm of a wild-type canal is visualized by expressing GFP under the sulp-5 promoter (pseudo-colored to blue in distinction from apical membrane labeling, shown in green in panel B). (B) The apical membrane of a wild-type canal is visualized by expressing ERM-1::GFP; note that lumen is not visible at this magnification and that single labeling cannot distinguish cytoplasmic from membrane labeling. (C) Excretory canal phenotype induced by RNAi: cytoplasmic labeling cannot distinguish cytoplasmic vacuoles from intralumenal cysts. (D) Excretory canal phenotype induced by RNAi: Apical ERM-1::GFP labeling detects fluid accumulation inside the lumen, identifying (lumenal) cysts rather than (cytoplasmic) vacuoles (compare Figure 4I for cytoplasmic vacuoles). Scale Bar = 40 μm for A–D, shown in A. (E–J) Analyzing loss- and gain-of-function effects on subcellular canal components (confocal images of single canal arms are shown). (E-G) Effect of erm-1 RNAi on the subcellular localization of canaliculi. (E) Wild-type canal cytoplasm; note GFP exclusion indicating lumen. (F) Wild-type cytoplasmic AQP-8::GFP puncta, corresponding to canalicular vesicles. (G) Cytoplasmic and basal displacement of AQP-8::GFP puncta in erm-1(RNAi) animal. (H) Discontinuous lumen and detail of lumen tip pathology (curling and loss of lumen centering) in erm-1(mildRNAi) animal (see3 for modulating RNAi strength); note that canal cytoplasm extends beyond the tip of the lumen, here indicated by small cysts. (I) Cytoplasmic vacuoles in excretory canal body without any canal extension in strongly affected erm-1(RNAi) animal3; note that ACT-5::GFP is not recruited to the lumen (except several small specks around some vacuoles). (J) Recruitment of excess ACT-5::GFP and AQP-8::mCherry to the lumen in triple transgenic animal overexpressing ERM-1 (note thick belt of ACT-5::GFP and overlapping AQP-8::mCherry clumps around the cystic lumen). Scale Bar = 5 μm for E–J, shown in E. Please click here to view a larger version of this figure.
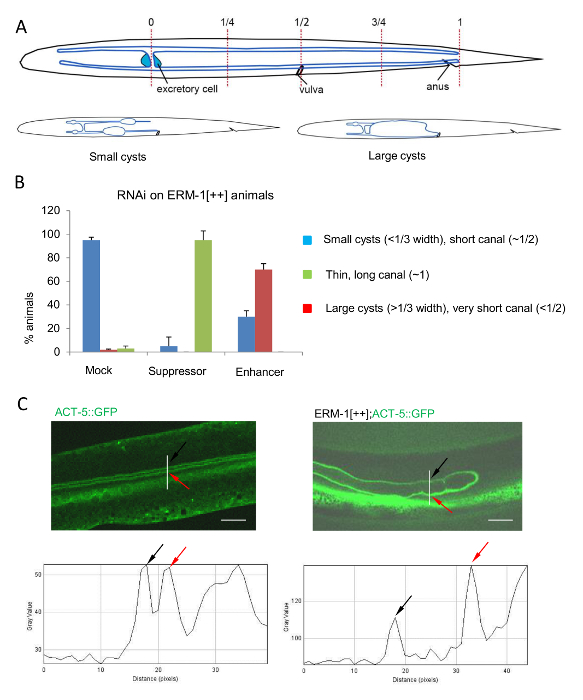
Figure 5: Examples of Quantification of Canal Defects by Dissecting and Confocal Microscopy
(A) Schematic representation of wild type (upper panel) and mutant cystic (lower panels) excretory canals for quantification of canal length and cyst size. Posterior canal length is quantified as 0, 1/4, 1/2, 3/4, and 1. Canal length ‘0’ indicates no extension and ‘1’ indicates full-length extension. Cyst size is quantified as < 1/3 (small) and > 1/3 (large) of body diameter. (B) Quantification of gross canal morphology in control and ERM-1[++](RNAi) animals by fluorescent dissecting microscopy. In mock(RNAi) ERM-1[++] animals, the posterior canal length is about 1/2 extended in 95% animals (reference image, Figure 3A–B). In suppressor ERM-1[++](RNAi) animals, the posterior canal’s length is almost fully extended in 80–90% animals (reference image, Figure 3C–D). In enhancer ERM-1[++](RNAi) animals, approximately 60-70% canals have large cysts and shorter length (< 1/2) (reference image, Figure 3E–F). Data presented as mean ± SD (n > 3). (C) Quantification of fluorescence intensity of the apical/lumenal canal cytoskeleton by ImageJ from confocal images. The canal’s apical membrane is labeled by ACT-5::GFP, wild-type canal arm is shown to the left, ERM-1[++] canal arm, to the right. Left panels: Intensity of ACT-5::GFP in wild type membrane sleeve is about 50 (gray value/membrane; indicated by black and red arrow), plot shown underneath image. Right panels: Intensity of ACT-5::GFP in ERM-1[++] is above 100 (gray value of dorsal membrane is about 110 (black arrow), and of ventral membrane is about 140 (red arrow)), plot shown beneath image. Scale bars = 5 µm. Please click here to view a larger version of this figure.
| Table 1: Examples of Markers for the C. elegans Excretory Canal Membrane System | ||||
| Protein name | Subcellular localization | Function | Examples of available strains | References |
| ERM-1 | apical domain | membrane–cytoskeleton linker | VJ402 (fgEx13[erm-1p::erm-1::gfp, rol-6p::rol-6(su1006)]) | ref (13) |
| ACT-5 | apical domain | apically localized actin | VJ268 (fgEx12[(act-5p::act-5::gfp); unc-119(+);unc-119(ed3)III]) | ref (13) |
| ABTS-2 | Basolateral | Anion/bicarbonate transporter | ABTS-2::GFP | ref (42) |
| SULP-8 | Basolateral | Sulfatepermease | SULP-8::GFP | ref (42) |
| VHA-1 | canalicular vesicles | V-ATPase | VJ535 (fgEx35(vha-1p::vha-1::gfp; rol-6p::rol-6(su1006))) | ref (9) |
| VHA-5 | canalicular vesicles | V-ATPase | ML846 (vha-5(mc38) IV;mcEx337[vha-5(+)::GFP + rol-6(su1006)] ) | ref (10) |
| AQP-8 | canalicular vesicles | aquaporin, water channel | VJ533 (fgEx33(aqp-8p::aqp-8::gfp)) | ref (9) |
| RAB-5 | endosomal vesicles | trafficking | BK209 (qpIs99 (exc9p::mCherry::rab-5)) | ref (36) |
| RAB-7 | endosomal vesicles | trafficking | BK210 (qpIs100 [Pexc-9::mCherry::rab-7]) | ref (36) |
| RAB-11 | endosomal vesicles | trafficking | BK205 (qpIs97 (exc9p::mCherry::rab-11) ) | ref (36) |
| 1Examples are selected from resources listed in Table 3. | ||||
| Table 2: Examples of C. elegans Excretory Canal-specific Promoters | ||
| promoters | Expression stage | |
| erm-1 | comma stage embryo | |
| sulp-5 | 3-fold embryo | |
| vha-1 | 2-fold embryo | |
| vha-5 | 2-fold embryo | |
| aqp-8 | 2-fold embryo | |
| glt-3 | late embryo | |
| 1Examples are selected from resources listed in Table 3. | ||
| Table 3: Resources | |||||
| Resources to identify C. elegans excretory canal-specific molecules, labeling reagents/strains and antibodies | |||||
| 1. Caenorhabditis Genetics Center (CGC)43 for available reagents and strains | |||||
| 2. Wormbase44 for information about excretory canal-specific molecules, strains and antibodies | |||||
| 3. Information about excretory canal-specific molecules: see reference6 | |||||
| 4. Transgeneome website45 for translational GFP fusion constructs | |||||
| 5. C.elegans expression pattern46 for transcriptional GFP fusion constructs | |||||
| 6. National BioResource Project (NBRP)::C.elegans47 for information on C. elegans mutants and promoters | |||||
| 7. Fluorophores: see reference48,49 | |||||
| Web-based resources for assembling molecules for a targeted RNAi library | |||||
| 1. GeneMANIA50 | |||||
| 2. AceView51 | |||||
| 3. iHOP52 | |||||
| 4. Wormbase44 | |||||
| 5. Saccharomyces Genome Database53 | |||||
| 6. Flybase54 | |||||
| 7. Mouse Genome Database55 | |||||
| 8. Human Genome Database56 | |||||
| 9. BLAST57 |
| Table 4: Example of Simple Phenotype Scoring Sheet | |||||||
| RNAi library | Strain | General Phenotype (% total) | Canal phenotype | ||||
| Canal length < 1/2 (%L4) | Canal length >1/2 (% L4) | Other Canal phenotype | Total number of animals counted | ||||
| Plate No | Well number | ||||||
| I-1 | A1 | ERM-1[++]; vha-1p::GFP | Clr (30) | 80 | 20 | 100 | |
| X-5 | B12 | same | none | 30 | 70 | 100 | |
| III-10 | C5 | same | Unc (54) | 70 | 30 | large cysts | 100 |
Discussion
C. elegans’ genetic versatility, transparency, simple body plan and invariant cell lineage all make it an excellent model for the analysis of morphogenesis. This protocol describes how to combine standard genetic manipulations and imaging studies to take advantage of the 2 micron thin C. elegans excretory canals to study polarized membrane and intracellular lumen biogenesis in a single cell tube.
Labeling
The C. elegans excretory canals can be labeled by the expression of fluorescent fusion proteins, permitting live analysis (described here), or by antibody or chemical staining (see accompanying paper on intestinal tubulogenesis for antibody staining3). The expression of two or more different fluorophores, or the combination of these different labeling techniques, allows for a high resolution visual dissection of these thin tubules (Figure 2). Fluorescent fusion proteins directed to the canals by a canal specific promoter are the first choice for excretory canal labeling for several reasons, among them: (1) the low expression levels of many canal proteins, and (2) the canal’s fine structure that is easily overwhelmed by staining outside of the canals where the protein of interest may also be expressed. On the other hand, canals are sensitive to exogenous proteins and easily develop unspecific phenotypes (e.g., extension defects and cysts, or even lethality) when such proteins are strongly expressed. This should weigh into the decision when choosing between different ways of generating transgenic animals. In principle, transgenes can be introduced as extrachromosomal arrays (e.g., by direct injection of the plasmid DNA into the gonad for germline transformation, as described here), which typically generates high-copy number arrays (often with high expression), or integrated into the genome, typically at low or single copy number (e.g., by bombardment26 or via Mos1-mediated single copy insertion [MosSCI]27). Extrachromsomal arrays can also be integrated into the genome in a second step (still, however, at a high copy number)18. On the other hand, when injected into the germline at low concentration (e.g., 1–2 ng/µL DNA, combined with a higher concentration of marker DNA), low (even single) copy number arrays can be easily generated28. This scenario has the advantage that DNA concentrations can be varied, which may help find the best expression level for canal labeling, neither too low nor too high (toxic). The relation between injected DNA concentration and expression level is dependent on multiple factors (e.g., the promoter), and thus must be empirically determined. Alternatively, the gene of interest can be directly labeled with a fluorophore in the germline by Clustered Regularly Interspersed Short Palindromic Repeats (CRISPR) based labeling procedures29,30. Although this approach places the fluorophore into its most physiological genomic context, this is not always necessary or even desirable (e.g., when the protein is expressed at very low levels). It may, however, be the best option, for instance if the gene of interest is very large.
Canal visualization can be accomplished by directing transcriptional constructs into the canal that will highlight the canal cytoplasm by a fluorophore. In contrast, labeling subcellular canal components requires a translational fusion where the full-length gene is connected in frame to the fluorophore encoding gene. When using a canal specific promoter for expression (rather than the gene’s own promoter), its choice should be based on the promoter’s strength and, for the developmental analysis, the time of its expression. Many excretory canal specific genes do not express before the larval stage when the canal’s osmotic function becomes critical; too late for the analysis of its active extension phase (erm-1 and pros-1 are early expressed genes)10,13. Examples for markers of the excretory canal endo- and plasma membranes and for canal specific promoters are given in Tables 1 and 2, respectively, and resources for finding additional canal-specific molecules in Table 3. These resources can also be used for finding an already-made suitable fluorescent fusion protein, which may be available through the Caenorhabditis Genetics Center (CGC; see also Table 1). To construct recombinant plasmids for the expression of such protein fusions, one can use various cloning methods, each with specific pros and cons (discussed elsewhere14). These include conventional restriction enzyme based cloning approaches, described here, modular cloning using the multisite Gateway system31, Gibson Assembly32, or reporter gene construction by the “PCR stitching” method14,33. These different approaches can be adapted to the method selected for their introduction into the germline and/or to other specific needs (for example, the versatile Gateway system facilitates shuffling of promoters and cDNAs for larger scale labeling approaches). Care needs to be taken to choose the correct insertion site for the fluorophore (linking it at the N- versus C-terminus of the protein), taking into consideration the function of the protein of interest.
Interference with gene function
As one of many possible ways to perturb gene function in C. elegans, this protocol describes a targeted RNAi interaction screen designed to modify a cystic canal lumen phenotype, induced by overexpressing a lumenal membrane component. In this specific case, overexpressing an apical/lumenal membrane associated marker such as ERM-1 has the advantage of directly guiding the inquiry to the desired target: the analysis of intracellular apical/lumenal membrane biogenesis. A gain-of-function phenotype generated by overexpression also provides certain advantages when combined with a loss-of-function (RNAi) interaction screen as described here (see34 for the discussion of loss- and gain-of-function analyses and the design of genetic screens). Moreover, ERM-1 itself is a well-investigated “lumen morphogenesis” and apical membrane identity molecule12. Therefore, in this case, a targeted (rather than unbiased) screen is likely to be directly informative for lumen morphogenesis while concomitantly further characterizing ERM-1’s specific function in this process. However, an unbiased non-targeted screen could be conducted in a similar fashion, starting either with a wild type animal, a transgenic animal with fluorescently labeled excretory canals, or with any canal mutant. The general advantages and disadvantages of RNAi (e.g., versus mutagenesis) for the generation of loss-of-function phenotypes, and the conduction and discussion of different types of genetic screens in C. elegans are discussed elsewhere34. RNAi produces a spectrum of phenotypes, including milder phenotypes, a specific advantage for the analysis of the often lethal tubulogenesis genes. This is described and discussed in the accompanying paper on intestinal tubulogenesis3. Different approaches to generate gain and loss-of-function mutants and classical genetic procedures such as crosses, are detailed and discussed in the general genetics methods literature20.
Microscopy and evaluation of tubulogenesis phenotypes
Visually evaluating and scoring the modification of a well-defined canal phenotype under a fluorescent dissecting microscope by using simple scoring parameters (such as canal length and lumen diameter), as described here, can be achieved in a short period of time even when processing larger numbers of animals in a targeted or systematic genetic or RNAi screen. In contrast, a similar screen searching for novel canal morphogenesis phenotypes in a strain that is wild-type (except for its canal-labeling transgene), is more time consuming, but can, in principle, be performed in the same fashion (we have carried out such a screen on a genome-wide basis in several months). Such screens will identify multiple different canal phenotypes (not shown here) and therefore must develop correspondingly different scoring parameters, e.g., a qualitative classification scheme (see 9,11,35,36,37,38 for different ways to score canal phenotypes by dissecting microscopy). When interpreting any canal phenotype, it is crucial to keep in mind that cyst formation can be an unspecific effect of many different insults to this thin tubular structure, and it is often a less informative terminal phenotype. Cyst size and location, on the other hand, may be informative, e.g., a cyst close to the canal cell (at the start of canal extension), cysts along the canal’s length, or cysts at canal tips, provide clues to the underlying mechanism of the defects. Canal cysts (here defined as intra-lumenal fluid-filled spheroids surrounded by an apical/lumenal membrane) should be distinguished from vesicles (small cytoplasmic membrane-bound fluid filled spheroids) or vacuoles (enlarged cytoplasmic membrane-bound fluid filled spheroids), not surrounded by a lumenal membrane, that can all look superficially identical (Figure 4A–D). Cytoplasmic vacuoles can likewise arise secondarily, e.g., via toxic effects of mounting solutions (sodium azide). Both, large cysts and cytoplasmic vacuoles can take up almost the size of the animal, and need to be further distinguished from the classical “clear (Clr)” canal phenotype that is caused by accumulation of fluid in the body cavity. Finally, canal length is almost always secondarily compromised in cystic canals, therefore a true canal extension defect needs to be demonstrated in a cyst free setting.
Despite their tiny diameters, subcellular components of excretory canals, such as apical versus basal membranes, intracellular endosomal versus canalicular vesicles, intracellular organelles and cytoskeletal components, can be visualized by higher magnification, e.g., confocal microscopy (Figure 2, Figure 4). By confocal imaging, a GFP positive canal cytoplasm alone is sufficient to distinguish the lumen from the canal cytoplasm via a non-fluorescent line (Figure 2A, Figure 4E). The identity of markers contributes to resolving canalicular from endosomal vesicles (also strikingly different in size), although definitive allocation will require immunoelectronmicroscopy39. Double and triple labeling can determine the subcellular localization of a molecule of interest and the relation of subcellular components to each other (Figure 2). Single labeled lumenal canal membranes produce the same double line as the cytoplasm or the basal membrane, but can be distinguished via double or triple labeling. For confocal analysis, proper mounting is important, given the fragility of the canal structure, its sensitivity to osmotic changes, and the vulnerability of its most frequent cystic phenotype. Cysts burst easily with osmotic changes or physical pressure, are sensitive to membrane toxins such as sodium azide, and are also sensitive to some anesthetics used for immobilization (that may also produce cytoplasmic vacuoles). In our hands, 5% lidocaine in M9 buffer works best for most excretory canal assays. Imaging procedures and all handling should be gentle, as described. To avoid artifacts that mimic phenotypes (e.g., cysts and vacuoles), it is best to immediately acquire images after mounting. If that is not possible, cyst images should be acquired first, before imaging other phenotypes. This protocol discusses standard scanning confocal microscopy that provides superior confocality, as compared to spinning disk confocal microscopy which, in contrast, reduces phototoxicity and is the microscopy of choice for the analysis of dynamic changes of subcellular components over time. Such questions on subcellular dynamics can also be addressed by using photobleaching techniques or labeling fusion proteins with photo-switchable fluorophores that change color40. Finally, the range of resolution can be further increased by adding transmission electron microscopy (TEM, providing the highest structural but not molecular resolution), super-resolution microscopy (providing molecular resolution in the nanometer range); or immunoelectronmicroscopy (providing both)39,41. 3D tomography can be combined with TEM serial sections and is particularly useful for the analysis of the canalicular-plasma-membrane interface of excretory canals9,10. Improved techniques for these different types of microscopy and combinations of these different imaging approaches continue to be developed.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank M. Buechner (University of Kansas, Kansas, USA), K. Nehrke (University of Rochester Medical Center, Rochester, New York, USA), and the Caenorhabditis Genetics Center, funded by National Institutes of Health, Office of Research Infrastructure Programs (P40 OD010440). This work was supported by grants NIH GM078653, MGH IS 224570 and SAA 223809 to V.G.
Materials
| Cloning | |||||
| Plasmid pPD95.75 | Addgene | Cat. No. 37464 | |||
| PCR Kit | Qiagen | Cat. No. 27106 | |||
| Ligation kit | New England Biolabs | Cat. No. E2611L | |||
| DNA marker | Thermo Scientific | Cat. No. SM1331 | |||
| Agarose DNA grade | Fisher Scientific | Cat. No. BP164-100 | |||
| Competent cells | New England Biolabs | Cat. No. C2987H | |||
| Tris | Fisher Scientific | Cat. No. BP154-1 | |||
| EDTA | Sigma | Cat. No. ED-1KG | |||
| Acetic acid | Fisher Scientific | Cat. No. A38S-500 | |||
| Ethidium bromide | Fisher Scientific | Cat. No. BP1302-10 | |||
| Equipments | |||||
| PCR machine | MJ Research | Cat. No. PTG-200 | |||
| Centrifuge | Eppendorf | Cat. No. 5415C | |||
| Water Bath | Precision Scientific | Cat. No. 666A3 | |||
| Gel running instrument | Fisher Scientific | Cat. No. 09-528-165 | |||
| Gel running power supply | Fisher Scientific | Cat. No. 45-000-465 | |||
| Molecular Imager Gel Doc XR System | Bio-Rad | Cat. No. 1708195EDU | |||
| Nanodrop Spectrophotometer | Thermo Scientific | Cat. No. ND1000 | |||
| C. elegans related1 | 1see reference27 for standard C. elegans culture and maintenance procedures. | ||||
| LB Medium and plates2 | 2see reference24 for protocols. | ||||
| Tryptone | Acros Organics | Cat. no. 611845000 | |||
| Yeast Extract | BD Biosciences | Cat. no. 212750 | |||
| NaCl | Sigma | Cat. no. S7653 | |||
| Bacto Agar | BD Biosciences | Cat. no. 214040 | |||
| Ampicillin | Sigma | Cat. no. A0116 | |||
| Tetracycline | Fisher Scientific | Cat. no. BP912 | |||
| M9 Medium2 | 2see reference24 for protocols. | ||||
| NaCl | Sigma | Cat. no. S7653 | |||
| KH2PO4 | Sigma | Cat. no. P0662 | |||
| Na2HPO4 | Sigma | Cat. no. S7907 | |||
| MgSO4 | Sigma | Cat. no. M2773 | |||
| NGM plates 2 | 2see reference24 for protocols. | ||||
| NaCl | Sigma | Cat. no. S7653 | |||
| Peptone | BD Biosciences | Cat. no. 211677 | |||
| Tryptone | Acros Organics | Cat. no. 611845000 | |||
| Bacto Agar | BD Biosciences | Cat. no. 214040 | |||
| MgSO4 | Sigma | Cat. no. M2773 | |||
| CaCl2 | Sigma | Cat. no. C3881 | |||
| Cholesterol | Sigma | Cat. no. C8667 | |||
| K2HPO4 | Sigma | Cat. no. P3786 | |||
| KH2PO4 | Sigma | Cat. no. P0662 | |||
| RNAi plates3 | 3see reference60 for protocols. | ||||
| NaCl | Sigma | Cat. no. S7653 | |||
| Peptone | BD Biosciences | Cat. no. 211677 | |||
| Tryptone | Acros Organics | Cat. no. 611845000 | |||
| Bacto Agar | BD Biosciences | Cat. no. 214040 | |||
| MgSO4 | Sigma | Cat. no. M2773 | |||
| CaCl2 | Sigma | Cat. no. C3881 | |||
| Cholesterol | Sigma | Cat. no. C8667 | |||
| K2HPO4 | Sigma | Cat. no. P3786 | |||
| KH2PO4 | Sigma | Cat. no. P0662 | |||
| IPTG | US Biological | Cat. no. I8500 | |||
| Carbenicillin | Fisher Scientific | Cat. no. BP2648 | |||
| NaOH | Fisher Scientific | Cat. no. SS266-1 | |||
| Sodium hypochlorite | Fisher Scientific | Cat. no. 50371500 | |||
| Bacteria | |||||
| OP50 bacteria | CGC | ||||
| HT115 bacteria | CGC | ||||
| Genome-wide RNAi libraries | |||||
| Ahringer genome-wide RNAi feeding library (ref29,49) | Source BioScience | ||||
| C. elegans ORF-RNAi feeding library (ref50) | Source BioScience | ||||
| Imaging related | |||||
| Lidocaine | MP Biomedicals,LLG | Cat. no. 193917 | |||
| Materials | |||||
| Vacuum Grease Silicone | Beckman | Cat. no. 335148 | |||
| Microscope slides | Fisher Scientific | Cat. no. 4448 | |||
| Microscope coverslips (22×22-1) | Fisher Scientific | Cat. no. 12-542-B | |||
| Tissue culture plate, 6 well | Corning Inc. | Cat. no. 08-772-33 | |||
| Equipment | |||||
| SMZ-U dissecting microscope (Nikon) | |||||
| SZX12 dissecting microscope (Olympus), equipped with a high-power stereo fluorescence attachment (Kramer Scientific), CCD camera with Q capture software and X-Cite fluorescent lamp (Photonic Solutions). | |||||
| TCS SL Laser-scanning confocal microscope (Leica Microsystem) | |||||
| C2 laser-scanning confocal mounted on an ECLIPSE Ti-E inverted microscope (Nikon) | |||||
References
- Lubarsky, B., Krasnow, M. A. Tube morphogenesis: making and shaping biological tubes. Cell. 112 (1), (2003).
- Sundaram, M. V., Cohen, J. D. Time to make the doughnuts: Building and shaping seamless tubes. Semin. Cell Dev. Biol. S1084-9521, 30130-30136 (2016).
- Zhang, N. The C. elegans intestine as a model for intercellular lumen morphogenesis and in vivo polarized membrane biogenesis at the single-cell level. JoVE. , (2017).
- Andrew, D. J., Ewald, A. J. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elongation. Dev. Biol. 341 (1), 34-55 (2010).
- Nelson, F. K., Albert, P. S., Riddle, D. L. Fine structure of the Caenorhabditis elegans secretory-excretory system. J. Ultrastruct. Res. 82 (2), 156-171 (1983).
- Sundaram, M. V., Buechner, M. The Caenorhabditis elegans Excretory System: A Model for Tubulogenesis, Cell Fate Specification, and Plasticity. Genetics. 203 (1), 35 (2016).
- Altun, Z. F., Hall, D. H. Excretory system. WormAtlas. , (2009).
- Buechner, M. Tubes and the single C. elegans excretory cell. Trends Cell Biol. 12 (10), 479-484 (2002).
- Khan, L. A. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat. Cell Biol. 15 (2), 143-156 (2013).
- Kolotuev, I., Hyenne, V., Schwab, Y., Rodriguez, D., Labouesse, M. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat. Cell Biol. 15 (2), 157-168 (2013).
- Buechner, M., Hall, D. H., Bhatt, H., Hedgecock, E. M. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev. Biol. 214 (1), 227-241 (1999).
- Fehon, R. G., McClatchey, A. I., Bretscher, A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11 (4), 276-287 (2010).
- Gobel, V., Barrett, P. L., Hall, D. H., Fleming, J. T. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell. 6 (6), 865-873 (2004).
- Sambrook, J., Sambrook, J., DW, R. u. s. s. e. l. l. . Molecular cloning: a laboratory manual. , (2006).
- Fire, A., Harrison, S. W., Dixon, D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 93 (2), 189-198 (1990).
- Kamath, R. S., Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 30 (4), 313-321 (2003).
- Timmons, L., Court, D. L., Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 263 (1-2), 103-112 (2001).
- Pawley, J. B. . Handbook of Biological Confocal Microscopy. , (2006).
- Hibbs, A. R. . Confocal Microscopy for Biologists. , (2004).
- Bankhead, P. . Analyzing fluorescence microscopy images with ImageJ. , (2014).
- Praitis, V., Casey, E., Collar, D., Austin, J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 157 (3), 1217-1226 (2001).
- Frøkjaer-Jensen, C. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40 (11), 1375-1383 (2008).
- Mello, C. C., Kramer, J. M., Stinchcomb, D., Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10 (12), 3959-3970 (1991).
- Barrangou, R. Cas9 Targeting and the CRISPR Revolution. Science. 344 (6185), 707-708 (2014).
- Dickinson, D. J., Goldstein, B. CRISPR-Based Methods for Caenorhabditis elegans Genome Engineering. Genetics. 202 (3), 885-901 (2016).
- Esposito, D., Garvey, L. A., Chakiath, C. S. Gateway cloning for protein expression. Methods Mol. Biol. 498, 31-54 (2009).
- Gibson, D. G. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 6 (5), 343-345 (2009).
- Hobert, O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 32 (4), 728-730 (2002).
- Jorgensen, E. M., Mango, S. E. The art and design of genetic screens: caenorhabditis elegans. Nat. Rev. Genet. 3 (5), 356-369 (2002).
- Berry, K. L., Bulow, H. E., Hall, D. H., Hobert, O. A. C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 302 (5653), 2134-2137 (2003).
- Mattingly, B. C., Buechner, M. The FGD homologue EXC-5 regulates apical trafficking in C. elegans tubules. Dev. Biol. 359 (1), 59-72 (2011).
- Shaye, D. D., Greenwald, I. The disease-associated formin INF2/EXC-6 organizes lumen and cell outgrowth during tubulogenesis by regulating F-actin and microtubule cytoskeletons. Dev. Cell. 32 (6), 743-755 (2015).
- Lant, B. CCM-3/STRIPAK promotes seamless tube extension through endocytic recycling. Nat. Commun. 6 (6), 6449 (2015).
- Paupard, M. C., Miller, A., Grant, B., Hirsh, D., Hall, D. H. Immuno-EM localization of GFP-tagged yolk proteins in C. elegans using microwave fixation. J. Histochem. Cytochem. 49 (8), 949-956 (2001).
- Lukyanov, K. A., Chudakov, D. M., Lukyanov, S., Verkhusha, V. V. Photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 6 (11), 885-891 (2005).
- Bates, M., Huang, B., Dempsey, G. T., Zhuang, X. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science. 317 (5845), 1749-1753 (2007).
- Sherman, T. The abts and sulp families of anion transporters from Caenorhabditis elegans. Am. J. Physiol. Cell Physiol. 289 (2), C341-C351 (2005).
- . Transgeneome website Available from: https://transgeneome.mpi-cbg.de/transgeneomics/index.html (2017)
- . National BioResource Project (NBRP)::C. elegans Available from: https://shigen.nig.ac.jp/c.elegans/ (2017)
- Heppert, J. K. Comparative assessment of fluorescent proteins for in vivo imaging in an animal model system. Mol. Biol. Cell. 27 (22), 3385-3394 (2016).
- Shaner, N. C., Steinbach, P. A., Tsien, R. Y. A guide to choosing fluorescent proteins. Nat Methods. 2 (12), 905-909 (2005).
- . BLAST Available from: https://blast.ncbi.nlm.nih.gov/Blast.cgi (2017)
- Kamath, R. S., Martinezcampos, M., Zipperlen, P., Fraser, A. G., Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2 (1), RESEARCH0002 (2001).
- Kamath, R. S. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 421 (6920), 231-237 (2003).
- Rual, J. F. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome research. 14 (10B), 2162-2168 (2004).

