A Rat Model of Orthotopic Liver Transplantation Using a Novel Magnetic Anastomosis Technique for Suprahepatic Vena Cava Reconstruction
Summary
The reconstruction of the suprahepatic vena cava (SHVC) remains a difficult step in rat orthotopic liver transplantation. In this article, we show a step-by-step protocol for SHVC reconstruction in rats using a novel magnetic anastomosis technique.
Abstract
The rat model of orthotopic liver transplantation (OLT) is essential for transplant research. It is a very sophisticated animal model and requires a steep learning curve. The introduction of the cuff technique for anastomosis of the portal vein (PV) and infrahepatic vena cava (IHVC) has significantly simplified the transplant procedure in rats. However, due to the short anterior wall of the recipients’ suprahepatic vena cava (SHVC), the cuff technique is very difficult to use for the reconstruction of the SHVC. Most researchers in this field still use the hand-suture technique for SHVC reconstruction, which makes it the bottleneck step in rat orthotopic liver transplantation. The magnetic anastomosis technique (i.e., magnamosis) is a method of connecting two vessels using the attractive force between two magnets. Our recent study has shown that the magnetic anastomosis technique is superior to the hand-suture technique for SHVC reconstruction in rats. In this article, we show a step-by-step protocol for SHVC reconstruction in rats using the novel magnetic anastomosis technique. In this model, the reconstruction of the PV and IHVC was performed by the standard cuff technique, while the reconstruction of the bile duct (BD) was performed by a stent technique. The hepatic re-arterialization was not performed. The magnetic anastomosis technique made SHVC reconstruction much easier and significantly shortened the anphepatic phase. After a reasonable learning curve, even researchers without advanced microsurgical skills can produce reliable and reproducible results using this rat model of OLT.
Introduction
The rat model of orthotopic liver transplantation (OLT) is essential for transplant research1,2. The first rat OLT was described by Lee et al. in 19733. In that model, all the vessels were reconstructed by the hand-suture technique. The hand-suture technique requires advanced microsurgical skills, which significantly limits its utilization. Since then, various modifications to the original protocol of rat OLT have been reported. Among them, the cuff technique for anastomosis of the portal vein (PV) and infrahepatic vena cava (IHVC) reported by Kamada et al. in 1979 is considered a major improvement to this model, as it significantly simplified the reconstruction procedures4. However, due to the short anterior wall of the recipients' suprahepatic vena cava (SHVC), the cuff technique is very difficult to use for the reconstruction of the SHVC. Most researchers in this field still use the hand-suture technique for SHVC reconstruction, which makes it the bottleneck step in rat OLT5,6,7.
The magnetic anastomosis technique (i.e., magnamosis) is a method of connecting two vessels or other tubular structures using the attractive force between two magnets8,9,10,11. The magnetic force gradually compresses and remodels the tissue into a strong, sutureless anastomosis12,13. This compression anastomosis has been proven to be effective in humans14,15. We have designed a pair of magnetic rings specifically for the anastomosis of the SHVC in rats. Our recent study has shown that the magnetic anastomosis technique is superior to the hand-suture technique for SHVC reconstruction in rat OLT16. The purpose of this article is to provide a detailed, step-by-step protocol for SHVC reconstruction in rats using the novel magnetic anastomosis technique.
Protocol
The protocol was carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals and was approved by the Committee on the Ethics of Animal Experiments of Xi'an Jiaotong University, Xi'an, Shaanxi Province, China.
NOTE: None of the procedures in this protocol was performed under a surgical microscope.
1. Preoperative Preparation
- The Design and Shape of the Magnetic Rings (Figure 1)
- Assemble the magnetic anastomosis device by matching a pair of neodymium-iron-boron magnetic rings.
- Design the rings according to the parameters of rat SHVC with a major axis diameter, minor axis diameter, thickness and weight of 8 mm, 5.5 mm, 1 mm, 0.5 g, respectively.
- Curve the rings to the desired shape by a wire-electrode cutting technique, and then coat the obtained rings with a titanium oxide airbrush and sterilize with 500 mg/L ethylene oxide at 55-60 °C for 6 h.
- The cuffs (Figure 2)
- Prepare the cuff for anastomosis of the IHVC and PV by cutting the polyethylene tube. Ensure that the cuff body is 5 mm long with a 2 mm cuff extension.
NOTE: The inner and outer diameters of the PV cuff are 1.8 and 2.1 mm, while those of the IHVC cuff are 2.6 and 2.8 mm, respectively.
- Prepare the cuff for anastomosis of the IHVC and PV by cutting the polyethylene tube. Ensure that the cuff body is 5 mm long with a 2 mm cuff extension.
- The Stent (Figure 2)
- Prepare the stent for anastomosis of the BD by cutting 24-G intravenous cannula with a length of 5 mm to produce slant at both ends.
- Experimental Animals
- Use male Sprague Dawley rats weighing between 250 and 280 g as donors and recipients. Ensure that the recipient is the same weight or slightly heavier (< 10 g) than the donor.
- Keep the rats in climate-controlled rooms with free access to food and water before surgery.
2. Donor Operation
- Inject buprenorphine (0.05 mg/kg) subcutaneously as an analgesic 1 hour before operation.
- Anesthetize the donor rat by isoflurane inhalation. Use 5 vol% isoflurane at a flow rate of 1.5 L/min in a Plexiglas box for the induction of anesthesia and 2 vol% isoflurane at a flow rate 0.6-0.8 L/min on a cone mask for the maintenance of anesthesia. Confirm depth of anesthesia by performing a toe pinch and a skin pinch.
- Shave the entire abdominal skin of the rat using an electric shaver to allow for cleaner exposure. Disinfect the corresponding skin with a povidone-iodine solution.
- Open the abdominal cavity via a cruciform incision using surgical scissors from the root of the penis extending to 1 cm above the xiphisternum along linea alba, extending from the midpoint of the longitudinal incision to the midaxillary line. Exteriorize the gastrointestinal tract to the left and cover it with wet gauze.
- Clamp and pull the xiphisternum toward the head using a hemostatic forceps. Dissect the falciform ligament and the connective tissues around the liver. Isolate and ligate the left inferior phrenic vein with a 6-0 silk suture.
- Insert the stent into the bile duct.
- Clamp the BD just above the point where the gastroduodenal vein joins the portal vein and pull it to maintain a little tension using a microforceps. Then perform a "V" incision of 1 mm length on the anterior wall about 5 mm proximal to the biliary confluence by a micro scissors.
- Insert the stent into the lumen of the bile duct using a curved microforceps. Make sure at least a half of the stent lies outside the bile duct.
- Secure the stent with a 6-0 silk suture. Cut one end of the suture. Keep the other one to hold during later anastomosis. Transect the bile duct below the stent and make sure the bile can come out of the stent.
- Isolate the IHVC down to the left renal vein level. Free the IHVC from anterior and lateral surrounding tissues by cotton bud dissection until the IHVC is skeletonized. Separate and ligate the right suprarenal vein and the right renal vein with a 6-0 silk suture.
- Heparinize the rat by injecting 50 U of heparin diluted in 2 mL of normal saline solution through the dorsal vein of the penis.
- Perfuse the liver.
- Isolate the abdominal aorta below the left renal vein. Insert a 22-G catheter into the aorta.
- Perform a thoracotomy, clamp the thoracic aorta using a microforceps.
- Perfuse the donor liver through the catheter in the aorta with 20 mL of normal saline solution containing 25 U of heparin (4 °C) at the rate of 3 mL/min with an infusion pump. In the meantime, cut the vena cava below the right renal vein to allow the perfusion solution to flow out of the liver.
- Transect the SHVC along with part of the diaphragm and IHVC at the level of the left renal vein when the donor liver became pallid.
- Dissect the connective tissue surrounding the PV by two micro forceps. Ligate and divide the pyloric veins with an 8-0 prolene suture. Transect the PV at the level of the splenic vein. Excise the donor liver and rinse it with cold normal saline solution. Place it in a cold saline bath 4 °C.
3. Graft Preparation
NOTE: All procedures for the liver graft preparation are performed in a cold saline bath at 4 °C.
- Attach a cuff to the donor's IHVC (Figure 3).
- Insert the IHVC into its cuff with the cuff extension pointing towards the liver. Position the cuff extension on the posterior wall of IHVC. Fix the cuff extension and the IHVC with a curved microforceps itself attached using a file clip to the bath container.
- Evert the distal end of the IHVC over the cuff body using two micro forceps.
- Secure the edge with a 6-0 silk suture. Make sure the IHVC is not twisted during the process.
- Repeat step 3.1 on the PV to attach a cuff to the donor's PV.
- Attach a magnetic ring to the donor's SHVC (Figure 4).
- Pull the diaphragm through the four forceps and trim the SHVC.
- Pass the SHVC through the magnetic ring using the titanium-alloy micro forceps.
- Evert the distal end of the SHVC over the ring.
- Secure the ring with a 6-0 silk suture. Make sure the SHVC is not twisted during the process.
- Remove the excess diaphragm around the liver.
- Immerse the donor liver in a cold normal saline bath and store at 4 °C.
4. Recipient Operation
NOTE: The schema of the graft implantation in the recipient rat is shown in Figure 5.
- Inject buprenorphine (0.05 mg/kg) subcutaneously as an analgesic 1 hour before operation. Anesthetize the donor rat by isoflurane inhalation. Use 5 vol% isoflurane at a flow rate of 1.5 L/min in a Plexiglas box for the induction of anesthesia and 2 vol% isoflurane at a flow rate 0.6-0.8 L/min on a cone mask for the maintenance of anesthesia.
- Shave the entire abdominal skin of the rat. Disinfect the corresponding skin with a povidone-iodine solution.
- Open the abdominal cavity via a 4 cm midline incision. Place the abdominal retractors. Pull the ribs as far apart as possible from the midline.
- Clamp and pull the xiphisternum toward the head using a hemostatic forceps. Dissect the connective tissues and ligaments around the liver. Ligate and divide the hepato-esophageal ligament. Ligate and divide the hepatic artery with an 8-0 prolene suture.
- Isolate the common bile duct from the first hepatic portal. Place a 6-0 silk suture around the common bile duct just below its division and ligate the common bile duct.
- Dissect the IHVC down to the right renal vein. Ligate and divide the right adrenal vein. Place a strap in the SHVC of the liver.
- Clamp the IHVC just above the right renal vein with microvessel clips. Clamp the PV at the level of the pyloric vein with microvessel clips.
- To flush the blood out of the liver, slowly inject 2 mL of normal saline through the PV. Drag down the liver by pulling the previously placed strap. Clamp the SHVC and part of the diaphragm with a Satinsky clamp.
- Transect the IHVC close to the parenchyma and the PV at the porta hepatis. Excise the liver by transecting the SHVC just above the liver. Remove the recipient liver quickly.
- Attach a magnetic ring to the recipient's SHVC.
- Insert the recipient's remaining SHVC into a magnetic ring.
- Fix the magnetic ring on a Satinsky clamp by magnetic attraction.
- Evert the SHVC over the magnetic ring until the vessel covers the edge of the ring.
- Place the donor liver graft orthotopically and cover it with a cold wet gauze.
- Fill the lumen of the donor's and the recipient's SHVCs with normal saline solution. Remove air bubbles thoroughly to prevent air embolism. Finish the SHVC reconstruction by coupling the magnetic rings embedded in the donor's and recipient's SHVC together through magnetic force.
- Reconstruct the PV.
- Tract the right and left branch of the recipient's PV by pulling an 8-0 prolene suture to maintain the tension of the PV.
- Move the clamp of recipient's PV down from the pylorus vein to the splenic vein in order to expose the vein fully.
- Incise the anterior wall of the recipient. Fill the lumen of the PV with normal saline solution.
- Hold the cuff extension of the donor's PV with a curved forceps.
- Insert the cuff body of the donor's PV into the recipient's PV while keep flushing the PV lumen with normal saline.
- Secure it with a circumferential 6-0 silk ligature. Re-establish the blood flow to the graft liver by releasing the clamps on the PV and SHVC.
- Repeat step 4.11 on the IHVC to reconstruct the IHVC.
- Reconstruct the bile duct by a stent technique.
- Incise a "V" on the anterior wall of the recipient common bile duct.
- Insert the donor stent into the lumen of the recipient BD and fix it with a 6-0 silk circumferential suture. Pull the sutures on the two bile ducts together and tie the sutures to bring the BD close to each other.
- Wrap omentum tissue around the anastomotic site to prevent bile leak.
- Irrigate the abdominal cavity with warm normal saline solution. Close the abdomen incision in two layers with a continuous 3-0 non-absorbable surgical suture.
- Administer 16 mg/kg cefuroxime (an antibiotic) and 0.05 mg/kg buprenorphine (an analgesic) by subcutaneous injection after the operation immediately.
- Place the rat in a clean cage and allow food and water ad libitum.
- Inject cefuroxime (16 mg/kg) and buprenorphine (0.05 mg/kg) into the abdominal cavity every 12 hours for 3 days.
Representative Results
After approximately 10 attempts, the magnetic anastomosis technique for SHVC reconstruction was successfully mastered by a researcher who had no prior microsurgical training. The reconstruction of the SHVC took less than 2 min. The anhepatic phase for the recipient rats was approximately 10 min. No thrombosis, bleeding or angiostegnosis was observed at the SHVC anastomotic site at 1, 5 and 30 days after reperfusion. The inferior vena cavography was performed at 2 weeks after transplantation. As shown in Figure 6, the magnetic rings were intact, and the blood flow was patent through the SHVC anastomosis. The post-transplant survival rate was 95% at day 1, 90% at day 3, and 85% at days 7 to 30 (Figure 7).
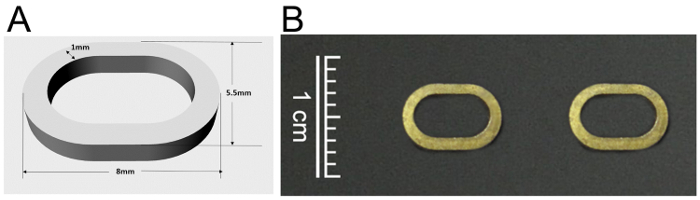
Figure 1: The design (A) and photo (B) of the magnetic ring for anastomosis of the SHVC.
SHVC: suprahepatic vena cava. Please click here to view a larger version of this figure.
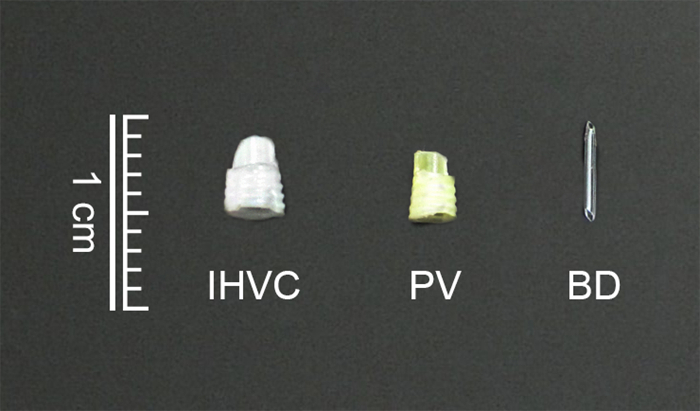
Figure 2: The cuffs and the stent for anastomosis of the IHVC (A), PV (B) and BD (C).
IHVC: infrahepatic vena cava; PV: portal vein; BD: bile duct. Please click here to view a larger version of this figure.
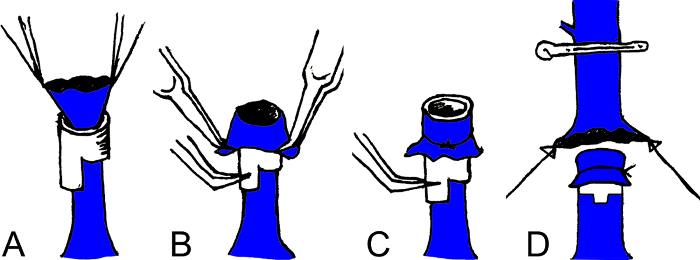
Figure 3: Schema of the cuff anastomosis of the IHVC and PV. Insert the IHVC or PV into a cuff (A); Evert the distal end of the IHVC or PV over the cuff body using two microforceps (B); Secure the edge with a 6-0 silk suture (C); Insert the cuff body of the donor's IHVC or PV into the recipient's IHVC or PV (D).
IHVC: infrahepatic vena cava; PV: portal vein. Please click here to view a larger version of this figure.
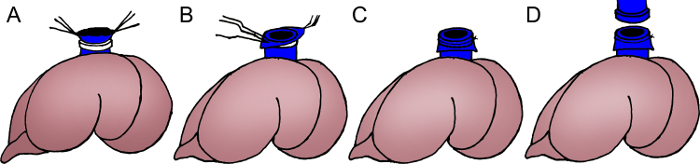
Figure 4: Schema of the magnetic anastomosis of the SHVC. Pass the SHVC through a magnetic ring using the titanium-alloy microforceps (A); Evert the distal end of the SHVC over the ring (B); Secure the ring with a 6-0 silk suture (C). Couple the magnetic rings embedded in the donor's and recipient's SHVC together through magnetic force (D).
SHVC: suprahepatic vena cava. Please click here to view a larger version of this figure.
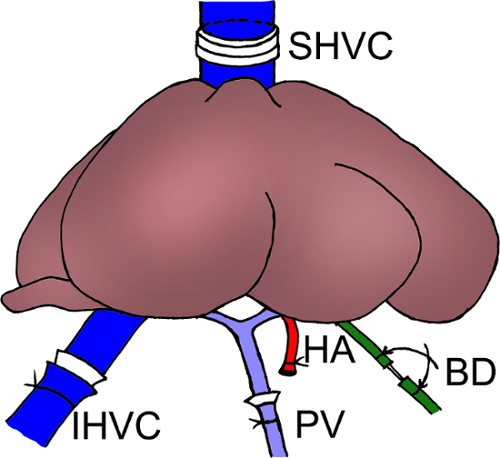
Figure 5: Schema of the graft implantation in the recipient rat. Reconstruction of the SHVC: magnetic anastomosis; Reconstruction of the IHVC and PV: cuff anastomosis; Reconstruction of the BD: stent anastomosis. Reconstruction of the HA: not performed.
SHVC: suprahepatic vena cava; IHVC: infrahepatic vena cava; PV: portal vein; BD: bile duct; HA: hepatic artery. Please click here to view a larger version of this figure.
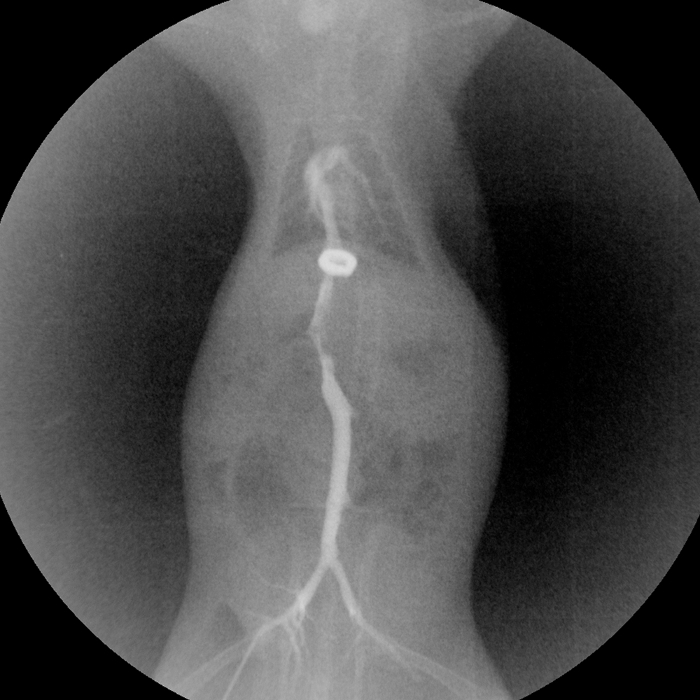
Figure 6: Inferior vena cavography of the rat at 2 weeks after transplantation. Please click here to view a larger version of this figure.
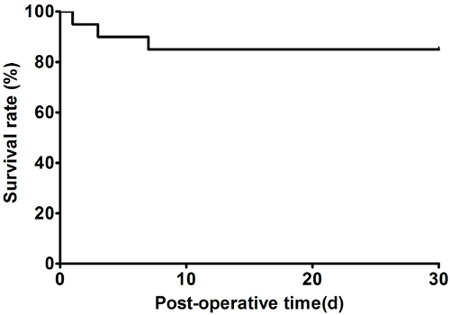
Figure 7: Representative survival cure after transplantation. Please click here to view a larger version of this figure.
Discussion
Many clinical progresses in liver transplantation can be attributed to animal studies. Rat OLT is a widely used and well accepted model in the research of organ preservation, transplant immunology, physiology and pathology. However, it is also a very complicated procedure and requires advanced microsurgical skill. Despite many improvements in the procedure of rat OLT, the SHVC anastomosis remained a challenging step. In this article, we describe a novel magnetic anastomosis technique for SHVC reconstruction in rat OLT. The application of this technique reduced the time for SHVC reconstruction to less than 2 min and the anhepatic phase of rat OLT to approximately 10 min. We believe this technique has great implications in transplant research.
The critical step in using this magnetic anastomosis technique for the reconstruction of the SHVC is to attach the magnetic rings to the donor’s and recipient’s SHVC. Every caution should be used to avoid twisting the SHVC. Moreover, when connecting the two magnetic rings, air bubbles in the SHVC should be removed thoroughly.
There are some limitations in using this technique. Some strains of rats such as Fischer-344 rats and Buffalo rats have an extremely short SHVC. It is impossible to attach the magnetic ring to their SHVC. Thus, they are not suitable for this technique. However, this technique can be easily applied for OLT in most strains of rats including Sprague Dawley rats, Lewis rats and brown Norway rats. Magnetic rings can interfere with the magnetic resonance imaging (MRI) scan. Therefore, this magnetic anastomosis technique cannot be used if an MRI exam is required for the experiment.
In summary, the magnetic anastomosis technique makes easy and fast SHVC reconstruction in rats possible. After a reasonable learning curve, even researchers without advanced microsurgical skills can produce reliable and reproducible results using this rat model of OLT.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the Ministry of Education Innovation Team Development Program of China (No. IRT16R57), National Natural Science Foundation of China (No. 81470896), and a research fund for Young Talent Recruiting Plans of Xi’an Jiaotong University (RW).
Materials
| Anesthesia Machine | Harvard | tabletop | Animal anaesthesia |
| PLX7000B HF Mobile Digital C-arm System | Perlong Medical | PLX7000B | It is mainly used for the angiography and photography of various operations |
| Syringe Pump | Mindray | BeneFusion SP5 | intravenous infusion |
| Isoflurane | RWD life Science Co. | anesthetic:for the induction and maintenanceof anesthesia | |
| iohexol | Shanghai General Pharmaceutical Co | intravascular contrast media | |
| heparin sodium injection | SPH No.1 Biochemical & Pharmaceutical Co., LTD | prevent the formation of thrombosis | |
| cefuroxime | Glaxo Operations UK Limited | an antibiotic | |
| buprenorphine | TIPR Pharmaceutical Responsible Co.,Ltd | an analgesic | |
| curved microforceps | Shanghai Medical Instruments (Group) Ltd., Corp. | W40350 | surgical tool |
| hemostatic forceps(straight) | Shanghai Medical Instruments (Group) Ltd., Corp. | J31010 | surgical tool |
| hemostatic forceps(curved) | Shanghai Medical Instruments (Group) Ltd., Corp. | J31020 | surgical tool |
| Satinsky clamp | Shanghai Medical Instruments (Group) Ltd., Corp. | XEC050 | surgical tool |
| needle holder | Shanghai Medical Instruments (Group) Ltd., Corp. | J32010 | surgical tool |
| microneedle holder | Shanghai Medical Instruments (Group) Ltd., Corp. | WBA040 | surgical tool |
| notched forceps | Shanghai Medical Instruments (Group) Ltd., Corp. | J42010 | surgical tool |
| tissue forceps(with hook) | Shanghai Medical Instruments (Group) Ltd., Corp. | J41010 | surgical tool |
| tissue scissor | Shanghai Medical Instruments (Group) Ltd., Corp. | Y00040 | surgical tool |
| surgical scissors | Shanghai Medical Instruments (Group) Ltd., Corp. | Y00030 | surgical tool |
| micro scissors | Shanghai Medical Instruments (Group) Ltd., Corp. | MR-S121T | surgical tool |
| microvessel clips | Shanghai Medical Instruments (Group) Ltd., Corp. | XEC240 | surgical tool |
| straight microforceps(titanium alloy) | Shanghai Medical Instruments (Group) Ltd., Corp. | WCC010 | surgical tool |
| curved microforceps (titanium alloy) | Shanghai Medical Instruments (Group) Ltd., Corp. | WCC020 | surgical tool |
References
- Aller, M. A., et al. A half century (1961-2011) of applying microsurgery to experimental liver research. World journal of hepatology. 4, 199-208 (2012).
- Aller, M. A., et al. The value of microsurgery in liver research. Liver international : official journal of the International Association for the Study of the Liver. 29, 1132-1140 (2009).
- Lee, S., Charters, A. C., Chandler, J. G., Orloff, M. J. A technique for orthotopic liver transplantation in the rat. Transplantation. 16, 664-669 (1973).
- Kamada, N., Calne, R. Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 28, 47-50 (1979).
- Oldani, G., Lacotte, S., Morel, P., Mentha, G., Toso, C. Orthotopic liver transplantation in rats. Journal of visualized experiments : JoVE. , (2012).
- Nagai, K., Yagi, S., Uemoto, S., Tolba, R. H. Surgical procedures for a rat model of partial orthotopic liver transplantation with hepatic arterial reconstruction. Journal of visualized experiments : JoVE. , e4376 (2013).
- Liu, X., He, C., Huang, T., Gu, J. Development of a New Technique for Reconstruction of Hepatic Artery during Liver Transplantation in Sprague-Dawley Rat. PloS one. 10, e0145662 (2015).
- Jamshidi, R., Stephenson, J. T., Clay, J. G., Pichakron, K. O., Harrison, M. R. Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. Journal of pediatric surgery. 44, 222-228 (2009).
- Pichakron, K. O., et al. Magnamosis II: Magnetic compression anastomosis for minimally invasive gastrojejunostomy and jejunojejunostomy. Journal of the American College of Surgeons. 212, 42-49 (2011).
- Gonzales, K. D., et al. Magnamosis III: delivery of a magnetic compression anastomosis device using minimally invasive endoscopic techniques. Journal of pediatric surgery. 47, 1291-1295 (2012).
- Wall, J., et al. MAGNAMOSIS IV: magnetic compression anastomosis for minimally invasive colorectal surgery. Endoscopy. 45, 643-648 (2013).
- Xue, F., et al. Choledochojejunostomy with an innovative magnetic compressive anastomosis: How to determine optimal pressure?. World journal of gastroenterology. 22, 2326-2335 (2016).
- Yan, X., et al. Portacaval shunt established in six dogs using magnetic compression technique. PloS one. 8, e76873 (2013).
- Dorman, R. M., Vali, K., Harmon, C. M., Zaritzky, M., Bass, K. D. Repair of esophageal atresia with proximal fistula using endoscopic magnetic compression anastomosis (magnamosis) after staged lengthening. Pediatric surgery international. 32, 525-528 (2016).
- Russell, K. W., Rollins, M. D., Feola, G. P., Scaife, E. R. Magnamosis: a novel technique for the management of rectal atresia. BMJ case reports. , (2014).
- Shi, Y., et al. Magnetic ring anastomosis of suprahepatic vena cava: novel technique for liver transplantation in rat. Transplant international : official journal of the European Society for Organ Transplantation. 28, 89-94 (2015).

