Reconfigurable Microfluidic Channel with Pin-discretized Sidewalls
Summary
A microfluidic channel with deformable sidewalls offers flow control, particle handling, channel dimension customization and other reconfigurations while in use. We describe a method for fabricating a microfluidic channel with sidewalls made of an array of pins that allows their shape to change.
Abstract
Microfluidic components need to have various shapes to realize different key microfluidic functions such as mixing, separation, particle trapping, or reactions. A microfluidic channel that deforms even after fabrication while retaining the channel shape enables high spatiotemporal reconfigurability. This reconfigurability is required in such key microfluidic functions that are difficult to achieve in existing “reconfigurable” or “integrated” microfluidic systems. We describe a method for the fabrication of a microfluidic channel with a deformable sidewall consisting of a laterally aligned array of the ends of rectangular pins. Actuating the pins in their longitudinal directions changes the pins’ end positions, and thus, the shape of discretized channel sidewalls.Pin gaps can cause unwanted leakage or adhesion to adjacent pins caused by meniscus forces. To close the pin gaps, we have introduced hydrocarbon-fluoropolymer suspension-based gap filler accompanied by an elastomeric barrier. This reconfigurable microfluidic device can generate strong temporal in-channel displacement flow, or can stop the flow in any region of the channel. This feature will facilitate, on demand, the handling of cells, viscous liquids, gas bubbles, and non-fluids, even if their existence or behavior is unknown at the time of fabrication.
Introduction
Microfluidic devices – micro-sized devices that control small amounts of liquid and their flows – offer miniaturization of biomedical procedures into a "chip" format with increased portability and, often, affordability. As described in a recent review1, various microfluidic components consisting of spaces and positive features have been developed to realize basic and key fluidic functions such as mixing, separation, particle trapping, or reactions.
While the behavior of many microfluidic devices is determined at the design stage, some kinds of microfluidic devices allow post-fabrication changes of their structure or behavior. Here we refer to this feature as "reconfigurability". The reconfigurability of microfluidic systems generally reduces the time and cost required to design a device, and/or enables customization of the microfluidic layout or functions over time.
Previously described reconfigurable microfluidic devices fall into the following three categories. In the first, deformation of elastomeric channels allows flow rates and directions to be changed during use. To gain reconfigurability, elastomeric channels are deformed by various external and controllable forces such as pneumatic pressure sources2, Braille actuators3, or compression sealing4. In the second, reconfigurable devices rely on modular designs, such as multi-layer fluidic circuits, modular channels with magnetic interconnects, and tubing-based microfluidics5. In the third, the device itself is not reconfigurable, but microdroplet transportation on electrode arrays (often referred to as digital microfluidics)6,7 and hanging drop-based microfluidic devices8 enable on-demand switching of the flow or the route of fluid.
Nonetheless, many of these reconfigurations are limited at the topological and macroscopic levels. For example, many integrated microfluidic devices stop flow or change the flow direction by collapsing microchannels in predefined regions. However, the position and number of regions to be collapsed are not reconfigurable. Although the digital microfluidics has a variety of fluid handling abilities, possible flows should be largely limited by the volume of each droplet. In addition, when cells are cultured in such droplets of cell culture media, extra effort is needed to prevent evaporation and gas dissipation from droplets and avoid osmolality shock and sudden pH change.
To realize channel feature-level reconfigurability, we proposed a microfluidic device with movable sidewalls that consisted of arrays of machine elements to dynamically reconfigure them when in use9. To form a deformable sidewall, small rectangular pins were lined up so that each end of the pins defined a segment of the sidewall. Sliding the pins allowed the deformation of the sidewall which allowed transport or patterning of cells, bubbles, and particles inside the channel. To minimize dead volume and maximize reconfigurability, the distance between the adjacent pins had to be minimized. However, strong capillary action acting on the small gaps between pins connecting the inside and outside of the microchannel causes leakage of any liquid entering the pin gap, causing media evaporation, bacterial or cytotoxic contamination, and eventually cell death. Therefore, we have developed leak-free discretized sidewall-type reconfigurable microfluidic channels that withstand cyclic pin actions and long-term cell culture10.
In this article, we provide a protocol to build microfluidic cell culture device with a discretized sidewall that can be reconfigured following the gradual increase in the cell culture area. Airtightness of the discrete channel sidewalls is tested using fluorescence imaging. The cell-culture compatibility and the ability of cell patterning are evaluated using on-chip cell culture.
This microfluidic system is suitable whenever appropriate channel design cannot be predetermined and must be changed on demand. For example, this system could be used to adjust the channel width and flow rate based on the cell growth or migration, to flow or trap active nematodes or other small objects that behave unexpectedly in the channel, or to accept various raw samples or bioproducts that were not yet conceived at the time of design.
Protocol
1. Etching of Pins (Figure 2A)
- Degrease rectangular pins by immersion in acetone.
- Passivate the pins by immersing in 4 mL of 10% nitric acid, then heat the solution at 65 °C for 30 min in an oven.
- Sonicate the pins in deionized water for 5 min to remove residual acid and dry with a paper towel. Immerse the pins in 0.5 mL of mold release agent for 2 h.Sonicate the pins in deionized water for 5 min.
- Fabricate an etching dish (Figure 2C).
- Draw or inscribe two parallel lines with a 4 mm gap on a glass slide using a ruler.
- Apply a chemical-resistant and low viscosity adhesive to one surface of two rectangular cut coverglasses.
- Bond the two cut coverglasses on the glass slide at a 4 mm gap. Use the parallel line as a guide.
- Dispense two lines of silicone adhesive on the etching dish (see Figure 2C for the position and the size of the contour).
NOTE: A 3D-printed template (a STL model file is included as a supplemental material) will help draw lines easily and accurately. - Put the pins on the etching dish so that the 2 mm-long tips on their straight ends are immersed into the adhesive line pattern. Dispense the adhesive again to ensure that the pins are completely covered and to draw a contour. Transfer the etching dish to a humidified fermenter heated to 38 °C. Wait overnight to cure the adhesive.
- Add 0.2 mL of 0.5 M nitric acid to 0.2 mL of 5.0 M hydrochloric acid slowly in a glass vial.
CAUTION: The mixture, also known as Aqua regia, is very corrosive and potentially explosive. Wear acid-proof rubber gloves and safety goggles, and exercise extreme caution when handling acids. Never store the solution. Reduce nitric acid as possible to decrease its aggressiveness. - Put the etching dish on a hotplate heated to 65 °C. Pour 0.4 mL of the acid mixture over the uncovered region of the pins. Wait 10 min and transfer the acid to a beaker.
- Neutralize all the remaining acid including the etched region of the pins with 5 mL of 0.8 M sodium bicarbonate solution in deionized water.
- Remove the pins from the etching dish by pulling the pins longitudinally with tweezers. Sonicate the pins in deionized water for 5 min followed by sonication in acetone for 5 min.
- Passivate the etched region of the pins as in step 1.2.
- Check the width of the etched pins with a shop microscope with a reticle. Adjust the etching time described in 1.7 so that the width of the etched region is 0.2 ± 0.02 mm.
- Transfer the pins to a glass vial containing 5 ml of 70% ethanol. Bring the vial to a laminar hood. Pick up the pins out of the vial and allow them to dry.
2. Fabrication of Silicone slab with Reservoirs and a Space for Pins.
- Fabricate a mold for a space for pins and a fixed sidewall by typical lithographic processes.
- Coat a degreased glass slide with 1 mL of negative epoxy photoresist using a spin coater at 1,000 rpm. Dry the photoresist on a 95 °C hot plate for 15 min. Repeat this step once.
- Spin coat the third layer of the same photoresist at 2,000 rpm on the glass slide with coated photoresist. Dry the photoresist on a 95 °C hotplate for 30 min.
- Expose the photoresist layer to 450 mJ/cm2 of 365-nm ultraviolet light from a UV spot light source through a photoplotted film. Bake the exposed photoresist on a 95 °C hotplate for 15 min. Develop the photoresist by spraying a solvent (2-methoxy-1-methylethyl acetate) using a hand sprayer, and blow-dry with nitrogen gas.
- Place the glass slide with patterned photoresist at the bottom of a plastic dish.
- Pour prepolymer of polydimethylsiloxane (PDMS) onto the mold to a thickness of 3 mm.Debubble the PDMS prepolymer in a vacuum desiccator at -800 kPa for 10 min.
- Cure the PDMS prepolymer in an oven at 65 °C for 1 h. Demold the partially cured PDMS slab using a scalpel. Fully cure the PDMS in an oven at 120°C for 1 h.
- Along the guideline pattern, trim away irregular edges from the PDMS slab with the same scalpel. Make as precise and clean a cut as possible, particularly at the surface that defines the insertion slot (See Figure 1A) for the pins.
- Perforate 2 mm-diameter holes for the inlet/outlet into the ends of the main channel of the PDMS slab using biopsy punches. Similarly, perforate 1 mm-dimeter holes at the ends of the air vent channel. See Figure 1A for the channel layout and the position of these holes.
3. Assembly of the Device with In-Place Fabrication of Gap Filler and Barrier.
- Fabricate a microchannel assembly.
- Immerse a 10 × 20 mm No. 4 coverglass in a cleaning solution heated to 60 °C for 10 min.
- Rinse the coverglasses with deionized water twice and dry at 120 °C for 10 min.
- Plasma-bond the PDMS slab to a coverglass:
- Place the PDMS slab (channel feature side up) and the cleaned 10 × 20 mm No. 4 coverglass in the vacuum chamber of a sputter coater.
- Start pumping down the chamber to 60 Pa. Generate air vacuum plasma (20 mA) for 30 s.
- Immediately vent the chamber. Bond the channel feature side of the PDMS slab to the coverglass with their edges aligned.
- Place the bonded layers in a 65 °C oven for 10 min.
- Bring the bonded layers to a laminar hood using a sterile container. Sterilize them with UV light for 30 min.
- In the laminar hood, insert the pins into the slot so that their ends form the other sidewall of the microchannel. Adjacent pins should be different in length to avoid contact of both vertical ends (See Figure 1B). Large spacing between the vertical ends is preferable. A space of (N-1) × (pin width) is possible when N kinds of pins with different pin lengths (L in Figure 2A) are prepared.
- Fabricate a base ( Figure 2B).
- Make or read a part file of the base and make two numerical control (NC) files (containing toolpaths; included as supplemental material) using CAD/CAM software. The first supplemental NC file uses a 4 mm-diameter end mill and the second a 1 mm-diameter end mill.
- Clamp a 3 mm-thick clear polymethylmethacrylate (PMMA) board onto a CNC mill.
- Open the first NC file on the controller of a computer NC (CNC) mill. Install a 4 mm end mill to the CNC mill and locate part zero by touching the end mill to the PMMA board. Run the NC code to cut the board.
NOTE: Occasionally blow the end mill tip with compressed air for cooling and chip removal. - Repeat 3.2.3 using the second NC file and a 1 mm end mill.
- Degrease the machined parts with detergent and dry with a paper towel. Spray the parts with 70 % ethanol and bring them to a laminar hood.
- Fabricate a pin gap filler and elastomeric barrier:
NOTE: Steps 3.3.1 – 3.3.7 should be performed aseptically in a laminar hood.- Prepare gap filler by mixing white petrolatum and polytetrafluoroethylene powder at a 2:1 ratio by weight. Homogenize the mixture using an ultrasonic homogenizer.
- Pour gap filler into a dispenser syringe. Insert a plunger and push it to fill the tip of the syringe. Attach a needle and push the plunger again until the needle tip is filled. Likewise, prepare a dispenser syringe with a plunger and a needle, and fill with silicone adhesive.
- Connect each syringe to a pneumatic dispenser using an adapter tube. Adjust the dispense pressures for silicone adhesive and filler to 250 kPa and 280 kPa.
- Dispense silicone adhesive to the edge of a pocket of the base. Place a 10 × 20 mm No.4 coverglass on the pocket and press it firmly to bond.
- Dispense silicone adhesive to a depth of approximately 1 mm to draw two segments along two outer slots of the base. Dispense gap filler to a depth of approximately 1 mm, to draw segments along the other slot.
- Dispense silicone adhesive to the edge of another pocket. Place a microchannel assembly (3.1) on the pocket and press it firmly to bond.
- Repeat 3.3.5 to ensure that both gap filler and silicone adhesive fully embed the pins and that there is no opening at the slots.
- Put the device in a sterile container such as a stainless steel box with lid. Transfer the container to a humidified fermenter heated to 38 °C. In the laminar hood, cure the elastomeric barrier for one day.
- Move each pin up to 1 mm along adjacent pins to release the pins from the cured elastomeric barrier.
- Sterilize the device with UV light for 30 min.
4. Evaluation of the Microfluidic Device
- Detect leakage using fluorescence
- Open the microchannel using a fine tool or a desktop robot. Make the channel width as consistent throughout the channel as possible.
- Dilute a green fluorescent dye with deionized water at 10 µM to make fluorescence solution.
- Add fluorescence solution to one of the end ports of the microchannel with a micropipettor. This step will fill the channel with the solution.
- Put the microfluidic device and two pieces of absorbent paper wet with deionized water in a large plastic dish. Incubate the dish at 37 °C and 5% CO2 for at least 24 h.
- Record green fluorescence images of the microchannel with an inverted fluorescent microscope with a microscope camera.
- Open the fluorescent images with an appropriate image analysis software and confirm there is no leakage (green fluorescence) at the interface of the gap filler and the pins.
- Seed cells to the microchannel.
- Prepare a cell culture vessel containing 70 – 80% confluent cells (depending on cell types). Detach and suspend the cells in growth medium.
- Centrifuge the cells (the speed and time depend on cell types), and aspirate the medium.
- Resuspend the cells with a small amount of medium. Count the cells with a cell counter and adjust the cell density from 1.5 × 106 to 1.5 × 107 cells/mL.
- Open the microchannel using a fine tool or a desktop robot (Figure 1B) to make a straight 400-µm-wide channel. Adjust pin positions to make the sidewall as flat throughout the channel as possible. Add cell suspension to one of the end port of the microchannel and fill the channel.
- Locate one of the pins that defines the sidewall of the region to start culture. Under an inverted microscope, close the two adjacent pins to enclose cells in the cell culture region.
- Close all pins from in order from inner to outer to expel all cells from the channel. Gently aspirate suspension from the end ports, and add medium to them.
- Incubate the device as described in 4.1.4. When cells are about 70 – 80% confluent, slowly open a pin to widen the culture area.
Representative Results
The construction of the reconfigurable microchannel is shown in Figure 1. Multiple rectangular pins were placed on a glass substrate and were lined up so that the long side of the pins were in contact. A PDMS sheet with punched holes and a recess of the same depth as the pin height covered the ends of the pins to form the channel inlet/outlet reservoirs, channel ceiling, and another sidewall opposite to the channel wall that consisted of the pins. The region surrounded by pins, a wall (one of the faces of the PDMS sheet), and the glass substrate form one microfluidic channel.
As previously described, the reconfigurability of the proposed microfluidic system is achieved by many small pins placed in parallel with very small but non-zero gaps. The problem in previous reports was the strong flow generated through the gaps by the capillary effect. To overcome this problem, the gaps were first filled with a gap filler. In this protocol, a disperse mixture of viscous hydrocarbon and fluoropolymer powder was used as a gap filler. However, the gap filler itself is also subject to the capillary effect. Therefore, as shown in Figure 1, the resulting reconfigurable microchannel has both hydrocarbon/fluoropolymer gap filler and an elastomeric barrier formed around the outer perimeter of the gap filler. Thinning the middle of the pins is needed to accommodate a sufficient amount of gap filler to ensure the thickness and strength of the elastomeric barrier between two pins.
Figure 2A shows a drawing of a pin that forms a sidewall segment. Stainless steel grade 316L was selected as the material due to its corrosion-resistant and low leaching properties. However, an extra passivation process was required to make pins cell culture compatible. A pin must have a precisely rectangular tip without burrs to successfully form a sidewall segment. In addition, a pin must have a "handle" so that the pin can easily be moved by pushing the handle. Because each pin has a narrow middle, the thickness of elastomer between pins was enough to withstand shear caused by pin movement. Unlike other parts comprising the device, the fabrication of pins, except middle thinning, should be ordered from a company specializing in electrical discharge machining (EDM) because it is one of the most precise and cost-effective methods of machining small parts made of hard metals. Performing middle thinning by etching yourself reduces the cost of machining and the risk of bending or breaking during machining.
To confirm that the gap filler, the elastomeric barrier, and eventually the watertightness of the reconfigurable microchannel function properly, leak detection by fluorescence was used. Figure 3 shows a fluorescence image of the area near the edge of the elastomeric barrier 3 days after the microchannel was filled with water containing fluorescent tracer dye. The fluorescence image shows that the liquid filling the channel reached a depth of about 200 µm from the visible edge of the elastomeric barrier. However, the liquid did not reach the gap filler. Additionally, no leakage of gap filler through the elastomeric barrier was observed. This observation indicates that the tight fit between the narrow middle of the pins and elastomeric barrier prevented the migration of liquid through the gaps.
Finally, we performed long-term cell culture with the culture area adapted by gradually expanding the sidewall of the reconfigurable microfluidic device as shown in Figure 4A. At 0 d, a small number of cells were confined within a space equal to one pin-width and other cells were aspirated. At 2 d, the cells were attached to the bottom surface and started proliferating. Two pins were retracted so that all cells were clearly visible, although the confluency was still low. At 5 d, the cells continued to proliferate and the confluency increased. At 6 and 9 d, two other pins were retracted to keep the cells underconfluent. The effect of gradual expansion of the culture area is shown in Figure 4B. There were sudden changes in the cell density on the day the pin(s) were retracted. However, the growth rate of the cell count was kept constant, while that seen in typical cell culture is exponential.
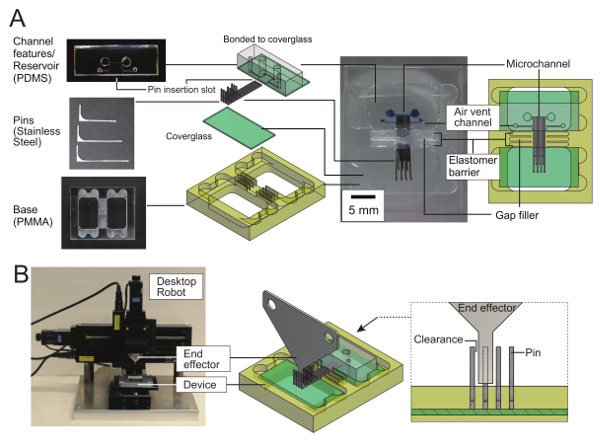
Figure 1: Reconfigurable microfluidic device with one pin-discretized sidewall. (A) Parts and construction of a reconfigurable microfluidic device. The device has one straight channel with one sidewall formed by the ends of 10 stainless steel pins inserted into PDMS/glass microchannel features. Gap filler and an elastomeric barrier prevents liquid from leaking through the pin gaps. Coverglasses, gap filler, and the elastomer barrier are fixed to a polymethylmethacrylate (PMMA) base. (B) Automated pin manipulator. An end effector made from a sheet of metal is fixed to a 3-axis desktop robot. To move one pin, the end effector pushes its vertical end. Pins with different lengths are placed at an interval of three times the pin width. The interval ensures that the end effector mates one pin at one time with enough clearance. Please click here to view a larger version of this figure.
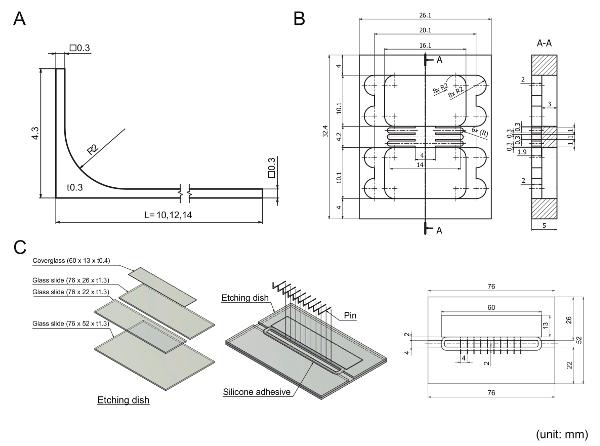
Figure 2: Mechanical drawing of machined parts used in the protocol. Units are in millimeters; R indicates a radius dimension; the square symbol (□) indicates square features; t indicates thickness. (A) A 316L stainless steel pin as a part of the sidewall. Pins can be ordered and machined as described. Thinning of the pin middle to make dog bone-like shapes is not reflected in this drawing because this was not ordered as part of the machining but was performed as part of the protocol. (B) A polymethylmethacrylate (PMMA) base that holds the coverglasses, gap filler and elastomeric barrier in place against pin movement. (C) An etching dish that is used to etch the middle of pins. To build an etching dish, four pieces of glass are bonded using silicone adhesive. A contour pattern of silicone adhesive is drawn on the dish followed by placement of the pins on the dish as shown in the drawing. Please click here to view a larger version of this figure.
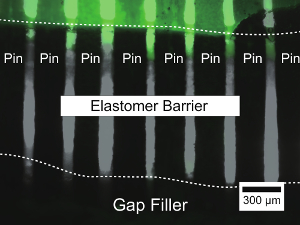
Figure 3: Fluorescence detection of leakage from a reconfigurable microchannel through pin gaps. Fluorescence image of green fluorescent dye filling the reconfigurable microchannel is overlaid on a phase contrast image of the seal structure, which consists of a gap filler (opaque) and elastomeric barrier (translucent). An edge of the elastomer barrier is visible as meniscus-like features and is denoted by an upper dotted line; the interface between elastomer barrier and gap filler is shown as meniscus-like features that contact the black area and is indicated by the lower dotted line. Please click here to view a larger version of this figure.
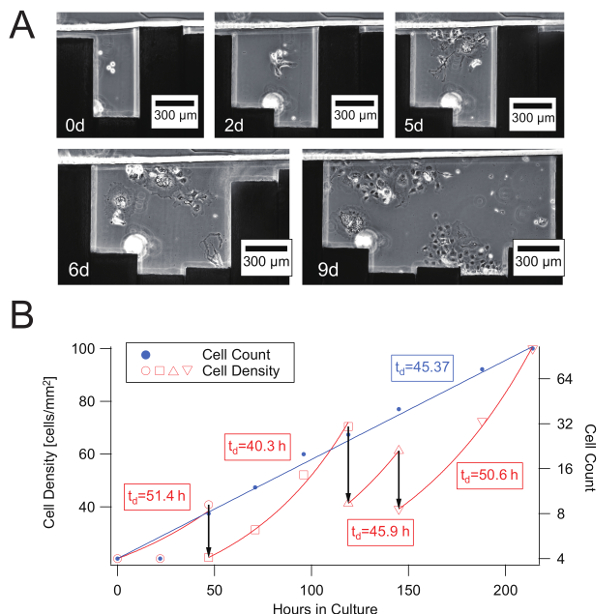
Figure 4: Progressive and continuous cell growth with variable cell culture area in a reconfigurable microchannel. (A) COS-7 cell growth in a cell culture area confined by moving sidewalls. (B) Growth curve and time evolution of the density of COS-7 cells confined in variable-size culture areas in the reconfigurable microchannel shown in A). Three vertical arrows denote expansion of the cell culture area at 2, 5, and 6 d, respectively. In addition to cell count, cell densities are shown for the same culture areas, fitted individually to each exponential growth curve, and used to estimate the local doubling time (td [h]) shown in the frames. Please click here to view a larger version of this figure.
Discussion
The pin-discretized microchannel is a full-featured microfluidic channel, and we believe that it has obviously high reconfigurability in channel shape compared with any existing microfluidic channels. The protocol we provided here will enable microfluidic devices capable of cell culture with gradually expanding cell culture surface area to keep the cultures under confluency for a long duration. The device will also provide in-channel patterning of cells without patterning proteins on the substrate beforehand or any other consideration at the time of design or fabrication. In addition, this reconfigurable microfluidic device easily generates strong in-channel displacement flow, which would help implement handling of such difficult-to-flow materials that very few existing microfluidic devices can handle. This means that the interaction between the cells and other microorganisms, gases, and other non-fluids can be evaluated using this device without large modifications in device design.
We have considered applying Laplace pressure or hydrostatic pressure to one inlet of the channel as external flow control methods. We do not recommend pushing liquid at a dead end because it will generate flow toward the air vent channel through the gaps between pins and the ceiling/floor of the channel. Many fluid operations do not require such pin operations. For example, mixing can be accomplished by mashing liquid by one pin (i.e., moving only one pin back and forth several times).
The most critical parts of the device are the pins. Precision in length, parallelism, perpendicularity and surface quality are required for the pins, as they must form a microchannel, must move smoothly, and must guide the movement of adjacent pins. Therefore, we recommend that the pins should be ordered from a company that specializes in precision machining by submitting a drawing similar to Figure 2A. There may be companies that require additional geometric dimensioning and explicit surface roughness directions. However, the pins are reusable if they are handled with care and occasionally passivated with nitric acid.
The elastomeric barrier is another critical feature, and its formation is the most critical step in the fabrication processes of the device. A precisely machined base will be needed to obtain repeatable and reliable results. Placing the pins on the uncured barrier is also a critical step. The pins should be kept well aligned, and embedded in the gap filler and the barrier without air bubbles. These steps prevent leakage through the pins, which is a common problem with this microfluidic device.
Other common issues in using this device are a) frictionally restrained pins, and b) cell death, and low growth rate. Possible causes for these in a) include uneven (tapered or wavy) etching of the pin middle, poor quality of the etched surface, and dimensional misfit between the pin tip height and the height of the photoresist layer on a mold for silicone slabs. Adjustment of etchant formulation, temperature, and agitation may help improve the pin movement. In addition, trial fitting without using wax or adhesive will provide hints to solve the problem. Possible factors in b) are insufficient passivation of the pins, errors in selection of adhesives for elastomeric barriers, and incomplete curing of the adhesives. Some cells may require coating inside the microchannel with fibronectin or other proteins or polymers that promote cell adhesion. In addition, optimization in cell culture practice such as trypsinization and centrifugation will decrease dead cells in the microchannel.
One of the limitations of the presented fabrication protocol is that only one of the sidewalls is discretized. The reconfigurability of the channel will further improve if the both sidewalls are built by pin arrays. Although it requires double the amount of pins and longer fabrication steps, this is a technically viable option.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by KAKENHI (20800048, 23700543).
Materials
| Oven | Yonezawa | MI-100 | |
| 10% Nitric Acid | Wako Chemicals | 149-06845 | |
| Stainless steel pins | Micro Giken | N/A | 0.3 mm crosssection, Grade 316L stainless steel, wire-cut EDM |
| Mold release agent | Fluoro Technology | FG-5093SH | |
| Polydimethylsiloxane (PDMS) | Shin-Etsu Chemicals | KE-106 | |
| Negative epoxy photoresist | Nippon Kayaku | SU-8 3050 | |
| Coverglasses (Rectangular) | Matsunami Glass | 26 x 60mm No.4 | |
| Acetone | Kanto Chemicals | 01060-79 | |
| Glass slides (Large) | Matsunami Glass | 76 x 52mm No.1 | |
| Silicone adhesive | Shin-Etsu Chemicals | KE-41 | |
| White petrolatum | Nikko Rica | Sun White P-1 | |
| Polytetrafluoroethylene (PTFE) powder | Power House Accele | Microfluon II | |
| Clear acrylic plate (3 mm-thick) | Various | N/A | |
| Pneumatic dispenser | Musashi Engineering | ML-5000XII | |
| Hydrochloric acid | Kanto Chemicals | 180768-00 | |
| Computer numerical control (CNC) mill | Pro Spec Tools | PSF240-CNC | |
| End mill (4 mm diameter) | Mitsubishi Materials | MS2MSD0400 | |
| End mill (1 mm diameter) | Mitsubishi Materials | MS2MSD0100 | |
| Adhesive (chemical-resistant and low viscosity ) | Cotronics | Duralco 4460 | |
| Borisilicate glass vials | Various | To prepare HNO3+HCl solution (Aqua regia). Always select borosilicate glass. | |
| Sodium bicarbonate | Kanto Chemicals | 37116-00 | |
| Ultrasonic cleaner | AS ONE | AS12GTU | |
| Ultrasonic drill | Shinoda Tools | SOM-121 | Used as a ultrasonic homogenizer. |
| Spin coater | Active | ACT-220DII | |
| Hotplate | AS ONE | ND-1 | |
| Photoplotted film (12,700 dpi) | Unno Giken | N/A | Negative image of the recess at the bottom of a PDMS slab are plotted. |
| 2-methoxy-1-methylethyl acetate | Wako Chemicals | 130-10505 | |
| UV spot light source | Hamamatsu | L8327 | Ultraviolet source |
| Nitrogen | Various | N/A | |
| Vacuum desiccator and pump | AS ONE | MVD-100, GM-20S | |
| Scalpels | Various | No.11 | |
| Biopsy punches (1.0mm and 2.0mm) | Kai Medical | BP-10F(1.0m), BP-20F(2.0mm) | |
| Glass engraving pen | Various | N/A | |
| Cleaning solution | Tama Chemicals | TMSC | Dilute 1:100 with deionized water |
| Sputter coater | San-yu Electron | SC-708 | For plasma bonding. |
| Dispenser syringe (5 ml) | Musashi Engineering | PSY-5E | |
| Plunger | Musashi Engineering | FLP-5E | |
| Blunt needle (21G) | Musashi Engineering | PN-21G-B | |
| Adapter tube | Musashi Engineering | AT-5E | |
| Fermenter | Japan Kneader | PF100 | |
| Green fluorescent dye (Alexa Fluor 488 carboxylic acid) | Thermo Fisher | A33077 | |
| Large plastic dish | Greiner bio-one | 688161 | |
| Absorbent paper | Asahi Kasei | BEMCOT M-1 | |
| Inverted microscope | Leica | DMi8 | |
| Microscope camera | Qimaging | Retiga 2000R | |
| Dulbecco modified Eagle medium (DMEM) | GE Health Care | SH30021.01 | |
| Antibiotic-antimycotic solution | Thermo Fisher | 15240-062 | |
| Trypsin/EDTA solution | Thermo Fisher | 25200-056 | |
| Phosphate buffered saline (PBS) | GE Health Care | SH30256.01 | |
| Fetal bovine serum (FBS) | Biowest | S1820 | |
| Cell counter | FPI | OC-C-S02 | |
| Cell culture vessel | VIOLAMO | VTC-D100 | |
| 15 ml conical tube | Corning | 352095 | |
| Shop microscope | PEAK | 2034-20 | |
| Hand sprayer | FURUPLA | No.3530 | |
| Coverglasses (Rectangular) | Matsunami Glass | 10 x 20mm No.4 | |
| CAD/CAM software | Autodesk | Inventor HSM | |
| Nitrogen gas pressure regulator | AS ONE | GF1-2506-RN-V | Set to 0.1 MPa |
References
- Nge, P. N., Rogers, C. I., Woolley, A. T. Advances in microfluidic materials, functions, integration, and applications. Chem Rev. 113 (4), 2550-2583 (2013).
- Araci, I. E., Brisk, P. Recent developments in microfluidic large scale integration. Curr Opin Biotechnol. 25, 60-68 (2014).
- Gu, W., Chen, H., Tung, Y. -. C., Meiners, J. -. C., Takayama, S. Multiplexed hydraulic valve actuation using ionic liquid filled soft channels and Braille displays. Appl Phys Lett. 90 (3), 033505 (2007).
- Konda, A., Taylor, J. M., Stoller, M. A., Morin, S. A. Reconfigurable microfluidic systems with reversible seals compatible with 2D and 3D surfaces of arbitrary chemical composition. Lab Chip. 15 (9), 2009-2017 (2015).
- Hahn, Y., Hong, D., Kang, J., Choi, S. A Reconfigurable microfluidics platform for microparticle separation and fluid mixing. Micromachines. 7 (8), 139 (2016).
- Kintses, B., van Vliet, L. D., Devenish, S. R. A., Hollfelder, F. Microfluidic droplets: new integrated workflows for biological experiments. Curr Opin Chem Biol. 14 (5), 548-555 (2010).
- Jebrail, M. J., Bartsch, M. S., Patel, K. D. Digital microfluidics: a versatile tool for applications in chemistry, biology and medicine. Lab Chip. 12 (14), 2452-2463 (2012).
- Frey, O., Misun, P. M., Fluri, D. A., Hengstler, J. G., Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat Commun. 5, 4250 (2014).
- Futai, N. Reconfigurable microchannels with discretized moving sidewalls. Chem Micro-Nano Syst. 10 (1), 24-25 (2011).
- Oono, M., et al. Reconfigurable microfluidic device with discretized sidewall. Biomicrofluidics. 11 (3), 034103 (2017).

