A Double Humanized BLT-mice Model Featuring a Stable Human-Like Gut Microbiome and Human Immune System
Summary
We describe a novel method for generating double humanized BLT-mice that feature a functional human immune system and a stable engrafted human-like gut microbiome. This protocol can be followed without the need for germ-free mice or gnotobiotic facilities.
Abstract
Humanized mice (hu-mice) that feature a functional human immune system have fundamentally changed the study of human pathogens and disease. They can be used to model diseases that are otherwise difficult or impossible to study in humans or other animal models. The gut microbiome can have a profound impact on human health and disease. However, the murine gut microbiome is very different than the one found in humans. There is a need for improved pre-clinical hu-mice models that have an engrafted human gut microbiome. Therefore, we created double hu-mice that feature both a human immune system and stable human-like gut microbiome. NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice are one of the best animals for humanization due to their high level of immunodeficiency. However, germ-free NSG mice, and various other important germ-free mice models are not currently commercially available. Further, many research settings do not have access to gnotobiotic facilities, and working under gnotobiotic conditions can often be expensive and time consuming. Importantly, germ-free mice have several immune deficiencies that exist even after the engraftment of microbes. Therefore, we developed a protocol that does not require germ-free animals or gnotobiotic facilities. To generate double hu-mice, NSG mice were treated with radiation prior to surgery to create bone-marrow, liver, thymus-humanized (hu-BLT) mice. The mice were then treated with broad spectrum antibiotics to deplete the pre-existing murine gut microbiome. After antibiotic treatment, the mice were given fecal transplants with healthy human donor samples via oral gavage. Double hu-BLT mice had unique 16S rRNA gene profiles based on the individual human donor sample that was transplanted. Importantly, the transplanted human-like microbiome was stable in the double hu-BLT mice for the duration of the study up to 14.5 weeks post-transplant.
Introduction
Humanized mice (hu-mice) have transformed the study of many aspects of human health and disease including hematopoiesis, immunity, cancer, autoimmune disease, and infectious disease1,2,3,4,5,6,7,8,9. These hu-mice have the distinct advantage over other mouse models in that they have a functional human immune system and can be infected with human specific pathogens. Nevertheless, the importance of the gut microbiome has been demonstrated by its role in many human diseases such as obesity, metabolic syndrome, inflammatory diseases, and cancer10,11,12,13. The mucosal immune system and gut microbiome are reciprocally regulated to maintain gut and systemic homeostasis. The immune system is shaped by antigens presented by the gut microbiome and reciprocally the immune system plays an important regulatory role in promoting commensal gut bacteria and eliminating pathogens14,15,16. However, the gut microbiome of hu-mice has not been well characterized and the murine gut microbiome differs substantially in composition and function from humans17. This is due to evolutionary, physiological, and anatomical differences between the murine and human gut as well as other important factors such as diet, which may influence the experimental results of hu-mice disease models18. Therefore, beyond classification of murine gut microbiome of hu-mice, an animal model featuring both a human immune system and human gut microbiome is needed to study the complex interactions of human disease in vivo.
The study of human diseases directly in human subjects is often impractical or unethical. Many animal models cannot be used to study human pathogens like human immunodeficiency virus type 1 (HIV-1). Non-human primate models are genetically outbred, very expensive, and are not susceptible to many human pathogens. Mice that have been derived as germ free (GF) and reconstituted with human-like gut microbiomes have been widely used to study human health and disease19,20. However, these animals do not have a human immune system and working with GF animals requires specialized facilities, procedures, and expertise. Therefore, there is a need for improved pre-clinical models to study the complex relationship of the gut microbiome and the human immune system. Many strains of mice, such as NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG), are not commercially available as GF. GF animals also may suffer from long-lasting immune deficiencies that are not completely reversed by the engraftment of microbes21. Therefore, we created a double hu-mice featuring both a functional human immune system and stable human-like gut microbiome under specific pathogen free (SPF) conditions. To generate double hu-mice, surgery was performed on NSG mice to create bone-marrow, liver, thymus humanized mice (hu-BLT). The hu-BLT mice were then treated with broad spectrum antibiotics and then given fecal transplants with a healthy human donor sample. We characterized the bacterial gut microbiome of 173 fecal samples from 45 double hu-BLT mice and 4 human fecal donor samples. Double hu-BLT mice have unique 16S rRNA gene profiles based on the individual human donor sample that is transplanted. Importantly, the transplanted human-like microbiome was stable in the mice for the duration of the study up to 14.5 weeks post-transplant. In addition, the predicted metagenomes showed that double hu-BLT mice have different predicted functional capacity than hu-mice that is more similar to the human donor samples.
Protocol
All methods described here were conducted in accordance with Institutional Animal Care and Research Committee (IACUC)-approved protocols at the University of Nebraska-Lincoln (UNL). The IACUC at UNL has approved two protocols related to generating and using hu-BLT mice, including double hu-mice. Additionally, the Scientific Research Oversight Committee (SROC) at UNL has also approved the use of human embryonic stem cells and fetal tissues, which are procured from the Advanced Bioscience Resources for humanized mice studies (SROC# 2016-1-002).
1. Mice housing and maintenance
- Purchase 6-8-week old NSG mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ).
- House the mice under SPF conditions with air exchange, prefilters, and HEPA filters (0.22 μm) in a room with controlled temperature, humidity, and pressure.
- House and maintain the mice in autoclaved individual microisolator cages in a rack system capable of managing air exchange with prefilters and HEPA filters (0.22 μm).
- Perform all procedures and manipulations of the mice in a Class II Type A2 biological safety fume hood that has been pre-treated with 70% ethanol. Before working in the animal room, shower and change into clean scrubs, tyvek suit, boot covers, hair cap, face mask, and gloves. Wear a surgery gown over the tyvek suit during surgical procedures.
NOTE: Ultraviolet (UV) light decontamination is also preferred prior to procedures.- Autoclave all instruments and reagents if possible and then disinfect prior to transferring to the fume hood.
- During procedures, remove the animals' individually-ventilated cages from the rack and disinfect prior to transferring to the fume hood.
- Feed the mice an irradiated diet and provide autoclaved water. Provide irradiated food ab libitum and supplement with additional irradiated food as needed. Change autoclaved water weekly or as needed.
NOTE: Autoclaved acidified water can also be used. The irradiated diet provided had a shelf-life of 6 months after manufacture.
2. Generation of humanized BLT mice
NOTE: Generation of hu-BLT mice has been described previously22,23,24.
- On the day of surgery, give the mice whole-body irradiation at a dose of 12 cGy/g of body weight.
- Prepare the mice for surgery.
- Give each irradiated mouse a mixture of Ketamine at the working concentration of 100 mg/kg and Xylazine at the concentration of 12 mg/kg, which ranged from 130-170 μL per mouse based on body weight by intraperitoneal (IP) injection for anesthesia. To prepare 3 ml of the Ketamine and Xylazine mixture, add 0.27 mL of Ketamine at the stock concentration of 100 mg/mL and 0.03 mL of Xylazine at the concentration of 100 mg/mL to 2.7 mL of sterile saline and mix well. Disinfect the skin with 70% isopropanol prior to injection.
- Give each irradiated mouse Buprenorphine 1 mg/kg of body weight (half-live 72 h, SR-LAB) by subcutaneous injection for long lasting pain management.
- Give each irradiated mouse 100 μL (858 μg) of Cefazolin by IP injection for preoperative antibiotic prophylaxis.
- Shave the hair of the mice using electric hair clippers around the left lateral and medial side of the mouse at the later surgical site used to expose the left kidney.
- Verify proper level of anesthesia by pedal reflex (firm toe pinch).
- Give isoflurane gas at 3-5% if additional anesthesia is needed at any point during surgery.
- Apply ophthalmic ointment to both eyes to prevent corneal desiccation. Apply ear tag if needed.
- Perform surgery to implant the liver and thymus tissues into the left kidney capsule.
- Disinfect the skin at the surgical site by applying iodine scrub starting from the center of the surgical site and moving towards the outside in a circular manner. Repeat this process with 70% isopropanol and a third time with iodine.
- Using forceps, first load one human fetal liver tissue fragment into a trocar. Then using forceps, load one human fetal thymus tissue fragment into the trocar. Then using forceps, load another human fetal liver tissue fragment into the trocar. Tissues should be cut to 1-1.6 mm3 to fit the inner dimensions of the trocar.
- Use forceps to lift the skin and use scissors to make a small cut longitudinally. Extend the cut to 1.5-2 cm in the left side of the mouse.
- Use forceps to lift the muscle layer and use scissors to make a small cut longitudinally. Extend the cut as needed to expose the kidney.
- Expose the kidney by gently grasping the fatty tissue surrounding the kidney. Do not directly touch the kidney parenchyma.
- Make a 1-2 mm incision at the posterior end of the kidney capsule using a scalpel.
- Slowly insert the preloaded trocar through the incision parallel to the long axis of the kidney and release the tissues between the kidney capsule and kidney.
- Carefully return the kidney and bowel to their normal position. Use absorbable 5/0 P-3 (P-13) sutures with 13mm 3/8 circle needle to close the muscle layer and surgical staples to close the skin.
- After surgery, put the mouse into a clean autoclaved microisolator cage for recovery.
- To minimize heat loss during post-surgical recovery, put the cage containing the mice on a heating pad that is connected to a water pump that warms and circulates the water.
- Monitor the mice until they have regained sufficient consciousness to maintain sternal recumbency.
- Within 6 h of surgery completion, via the tail-vein, inject CD34+ hematopoietic stem cells isolated from human fetal liver tissue.
- Warm up the mice with a heat-lamp. Disinfect the tail with 70% isopropanol and then inject 1.5 to 5 × 105 stem cells/200 μL into the tail vein.
- Stop any bleeding from the injection and return mice to the microisolator cage and the microisolator cage to the cage rack.
NOTE: Post-surgery mice are typically housed together, five per microisolator cage, during and after recovery. However, mice are only housed together if they all received surgery on the same day.
- Check the mice daily. Carefully monitor the surgical staples and replace them as needed. Closely monitor the mice for any sign of infection or discomfort. Supply autoclaved food on the floor of the microisolator cage for a few days post surgery.
- Remove the surgical staples 7-10 days after surgery. Give isoflurane gas at 3-5% to anesthetize the mice. Carefully remove the staples and then apply antibiotic and pain-relieving ointment onto the site.
- Allow 9-12 weeks for reconstitution of human immune cells, then collect peripheral blood from the medial saphenous vein from each of the humanized mice.
- Restrain conscious mice using an appropriately sized plastic cone restraint with an opening near the head of the mouse for breathing and an opening near the back of the mouse to isolate one leg. Put the mice into the restraint cone head first and then gently pull one leg through the leg opening.
- Spray the medial side of the isolated leg with 70% isopropanol, then spread antibiotic and pain-relieving ointment onto the same site.
NOTE: The ointment helps to reveal the location of the vein without the need for hair removal and also assists in blood droplet formation. - Using a 25-gauge needle at a 90° angle, puncture the vein and collect 50-100 μL of blood using an EDTA coated blood collection tube. Stop the bleeding by applying pressure to the site with sterile gauze. Once the bleeding has stopped, return the mice to their cage.
NOTE: The maximum blood volumes collected are typically 50 μL per week or 100 μL every two weeks. - Use the collected peripheral blood to test the level of human immune cell reconstitution using flow cytometry with antibodies for hCD45, mCD45, hCD3, hCD4, hCD8, hCD19.
3. Antibiotic treatment
- Prior to antibiotic treatment, collect pre-treatment fecal samples. Move the mice to a new autoclaved microisolator cage.
- Prepare a fresh cocktail of broad-spectrum antibiotics daily.
- Prepare 250 mL of water containing freshly prepared Metronidazole (1 g/L), Neomycin (1 g/L), Vancomycin (0.5 g/L), and Ampicillin (1 g/L) for each microisolator cage of mice.
NOTE: Use autoclaved or sterile water for the antibiotic supplemented drinking water. - Add 9.2 g of grape sugar sweetened drink mix to 250 mL of antibiotic supplemented water.
NOTE: The use of grape sugar sweetened drink mix masks the bitter taste of the antibiotics and helps prevent dehydration in the mice. - Change the antibiotic and grape sugar sweetened drink mix supplemented water and place the mice in a new autoclaved cage daily.
NOTE: Mice are coprophagic and changing the cages daily prevents the mice from re-inoculating themselves with gut bacteria. - For maximal engraftment of the human-like gut microbiome, provide antibiotics for 14 days. During antibiotic treatment, monitor the mice for weight loss and dehydration. Weight loss is expected during the first 3-4 days and plateaus after that for the duration of the treatment. If dehydration occurs, give the mice saline or Ringers Solution via intraperitoneal injection.
NOTE: Other gut microbiome humanization protocols call for oral gavage of antibiotics. While effective at depleting the murine gut bacteria, we found that the less invasive method of adding antibiotics to the drinking water put less stress on our humanized mice and led to better outcomes.
- Prepare 250 mL of water containing freshly prepared Metronidazole (1 g/L), Neomycin (1 g/L), Vancomycin (0.5 g/L), and Ampicillin (1 g/L) for each microisolator cage of mice.
4. Donor samples and fecal transplant
- Prepare human donor fecal samples.
- Use properly prepared sources of fecal microbiota transplant (FMT) material for fecal transplant into the humanized mice.
- Thaw FMT preparations and aliquot them under anaerobic conditions in an anaerobic chamber.
- If desired, at this step mix equal parts of the fecal samples together to create an unbiased "human" sample.
- Keep freezing and thawing of the FMT material to a minimum, if aliquoting or mixing of samples is not needed, only thaw immediately before the procedure.
- Human fecal transplant
- After completion of 14 days antibiotic treatment, change the drinking water to autoclaved water and move the mice into a new autoclaved cage. Stop daily cage changes and implement a once every 1-2 weeks cage changing schedule.
- Give two fecal transplants at 24 and 48 h post cessation of antibiotics.
- 24 h after antibiotics treatment, thaw the needed amount of FMT material, give each mouse 200 μL of FMT material via oral gavage. Repeat procedure again at 48 h post antibiotics.
- Spread any remaining or leftover thawed FMT material on the fur of the humanized mice or onto the cage bedding.
5. Fresh fecal sample collection
- Autoclave individual paper bags prior to transferring to the fume hood.
- In the fume hood, put one mouse into each individual paper bag and allow the mouse to defecate.
- Using sterile forceps, collect the fecal sample into 1.5 mL plastic tubes and freeze at -80 °C. Return the mice to microisolator cages.
NOTE: This method of collecting fecal samples allows for fresh fecal sample collection from individual mice without any stress inducing manipulations.
Representative Results
Figure 1 shows an outline of the methods used to create double hu-BLT mice and briefly describes the process of adding a functional human immune system and stable human-like gut microbiome to the NSG mice. Figure 2 shows an example of flow cytometry analysis of peripheral blood from a humanized BLT-mouse 10 weeks post-surgery. Figure 3 shows the relative abundance of the human fecal donor samples used to transfer a gut microbiome to create double hu-mice. Figure 4 shows the phenotypic changes induced by antibiotic treatment to the spleen and cecum, similar to what is observed in germ-free animals. Figure 5 shows a principal component analysis (PCA) plot of the 16S rRNA sequencing data revealing double hu-mice have human-like gut microbiomes that are unique to the human donor sample.
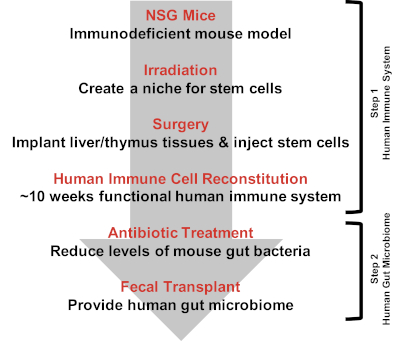
Figure 1: Creating double humanized BLT-mice. Creating double hu-BLT mice is a two-step process. The first step is to engraft the human immune system to the NSG mice. On the day of surgery, NSG mice are given irradiation to create a niche for stem cells. The mice are then implanted with human fetal liver and thymus tissues and injected with human hematopoietic stem cells. Human immune cell reconstitution is checked around 10 weeks post-surgery. The second step is to engraft the human gut microbiome. Mice are treated with antibiotics to reduce the pre-existing murine gut bacteria. Mice are then given fecal transplants to provide the human gut microbiome. Please click here to view a larger version of this figure.
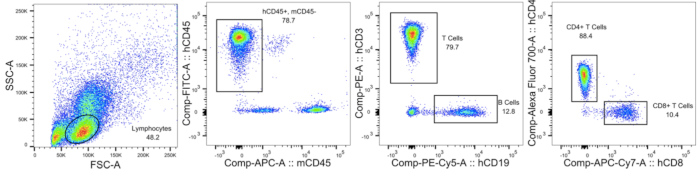
Figure 2: Testing human immune cell reconstitution in double humanized BLT-mice. An example of flow cytometry analysis of a humanized BLT-mouse peripheral blood 10 weeks post-surgery. The figure shows the gating strategy used to identify the lymphocyte population, mCD45- hCD45+ cells, CD19+ B cells, CD3+ T cells, CD4+ T cells, and CD8+ T cells. Please click here to view a larger version of this figure.
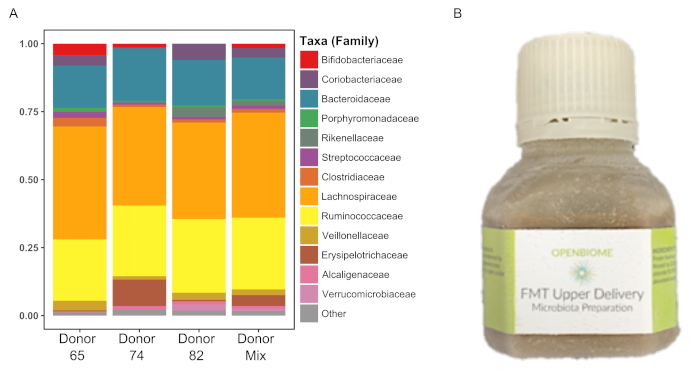
Figure 3: Human donor fecal sample profiles. Relative abundance of the 3 human donor and mixed (all donors) samples shown at the family level. Please click here to view a larger version of this figure.
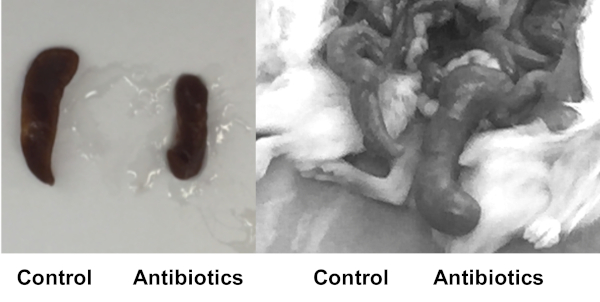
Figure 4: Antibiotic treated mice resemble germ-free phenotypes. Hu-mice were sacrificed after 9 days of antibiotic treatment (Antibiotics) or no antibiotic treatment (Control). After antibiotic treatment, the phenotype of the humanized mice begins to resemble those seen in germ-free animals. As a result of antibiotic treatment there is a reduction in the size of the spleen (left) and the cecum is enlarged (right). Please click here to view a larger version of this figure.
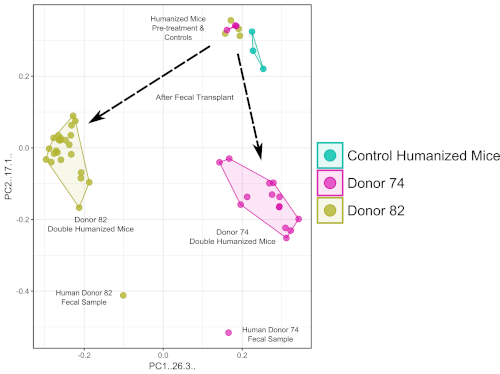
Figure 5: Double humanized BLT-mice feature fecal donor specific gut microbiomes. PCA plot of 16S rRNA sequencing data show after human fecal transplant the double hu-BLT mice feature gut microbiomes that are unique to the individual human fecal donor. Please click here to view a larger version of this figure.
Discussion
The protocol described here is for the creation of double hu-BLT mice that feature both a functional human immune system and a stable human-like gut microbiome. This protocol can be adapted to other humanized or non-humanized mice models without the need for GF animals and gnotobiotic facilities. While the methods described here are relatively simple, there are several critical details that are important for the successful creation of double hu-BLT mice. NSG mice are extremely immunodeficient and preventing infections is key to long-term survival of the mice. We took following measures to prevent infection. First, animals were housed in individual microisolator cages with HEPA filters (0.22 μm) in a rack system with air exchange rate management in a dedicated suite. The air handler for the rack contained pre-filters along with HEPA filtered (0.22 μm) supply and exhaust air, as well as real-time on-line monitoring of cage exhaust air temperature and relative humidity. Second, everyone who entered the animal room had to shower and wear clean scrubs and shoes as well as put on gloves, disposable tyvek suit, booties, hair bonnet, and face mask. Third, all procedures, including cage changes and addition of food and water, were performed within a Class II Type A2 biological safety fume hood that was pre-sterilized with 70% ethanol and UV light. Fourth, aseptic surgical technique was used during survival surgery, which included the surgeon and assistants wearing an additional layer of protection including a surgery gown and gloves. Surgery was conducted in the disinfected fume hood, using only sterile instruments, gauzes, and wound closure materials, while maintaining the sterility of gloves and instruments throughout the surgery. Finally, to prevent infection and ensure the stability of the engrafted human-like gut microbiome, all food and water given to the mice was sterile. All food should be irradiated and all water should be autoclaved. To minimize pain and distress, we administered long-acting buprenorphine subcutaneously to mice before surgery. The combination of Ketamine and Xylazine for mouse surgical anesthesia is very reliable and can last about for 30 min. If that is not long-enough, we give isoflurane gas to further anesthetize the mice. It is also very important to maintain mouse body temperature post-surgery. We put the cage on the heated warming pad until the hematopoietic stem cell injection via the tail vein. At that time, the mice are recovered from anesthesia and returned to rack.
To deplete the murine gut microbiome and prepare for human fecal transplant, it is important to always use freshly prepared antibiotics and to change antibiotic supplemented water and cages daily. This will use many microisolator cages throughout the 14-day antibiotic treatment, but it ensures the mice are not being re-inoculated through coprophagia. During antibiotic treatment, it is also critical to monitor the body weight and health of the mice. After fecal transplant, the mice quickly regain any lost weight. It is important to minimize any freeze-thaw cycles for the fecal transplant material and to make sure to use an anaerobic chamber if aliquoting samples is needed. While creating double hu-BLT mice it is important to minimize handling and stress induced on the mice. This helps to prevent infection and improves long-term survival.
We initially tried to pre-treat mice with anti-fungal amphotericin B but found the mice did not tolerate the treatment very well and it is no longer used. We also experimented with different durations of antibiotic treatment. We found that while a majority of murine gut bacteria appear to be depleted after 7 days of antibiotics, the level of donor engraftment is much higher after 14 days of treatment. We also tried administering antibiotics through twice-daily oral gavage. However, we found that this method was too invasive for our hu-mice. We switched to a single daily gavage schedule but the mice still appeared to be stressed and unhealthy. We found that providing antibiotics in the drinking water was the best method. It reduced the amount handling and stress to the mice while still adequately reducing the murine gut bacteria. We provided grape sugar sweetened drink mix in the drinking water to ensure the mice received an adequate dosage of antibiotic and to prevent dehydration. The mice do experience a reduction in body weight during the first 3-4 days of antibiotic treatment, but providing extra fluids via intraperitoneal injection does not increase body weight. After fecal transplant, the mice quickly regain the lost weight.
While this method is able to reproducibly generate double hu-BLT mice, there are some limitations to the model. The first thing to consider is hu-mice have less organized lymphoid structure, including the germinal center, leading to reduced antibody class switching and limited affinity maturation. However, NSG hu-BLT mice have systemic immune reconstitution and translatable T cell responses and can be used to model many human diseases. Another issue is the potential development of graft-versus-host disease (GVHD) in some hu-mice after several months of excessive human immune reconstitution. We and others have observed GVHD manifestations such as blepharitis, alopecia, weight loss, and malocclusion that must be carefully monitored25.
There are several documented regimens for depleting gut bacteria in mice with antibiotics26,27,28,29,30,31. We chose our cocktail of antibiotics due to their known capacity to target a broad range of bacteria in the gut and because we found several examples of successful bacterial depletion in the literature. Many published cases use a far less rigorous course of antibiotics but in our study, we found that 14 days is needed for optimal engraftment of a human-like gut microbiome. While we initially tried a protocol based on Hintze et al., we found that oral gavage was too invasive and that anti-fungal treatment was detrimental to mice26. We believe that NSG hu-BLT mice are unique and less invasive procedures are preferred compared to other more robust mice. We did not use GF animals in our study. The use of GF mice to study the effects of the gut microbiome have been well-documented, however, these animals do not have a human immune system19,32. Further, we admit that working with a GF NSG hu-BLT mice model would create interesting opportunities for research. For one, studying human immune cell reconstitution and the pathogenesis of human specific pathogens like HIV-1 without the presence of the gut microbiome could provide interesting results. Further, GF models may allow for a more complete reconstitution of a human-like gut microbiome following fecal transplant. However, GF mice have long-lasting immune deficiencies, even after gut microbiome reconstitution21. Our model has the advantage of using SPF housing conditions, which are widely available and less expensive compared to GF facilities. Our model also has the advantage of not perturbing the normal procedures of surgically generating hu-BLT mice because there is no need for a completely GF environment.
We believe that this double hu-BLT mouse model is unique in that it not only can be used to study human immune function and human diseases, but also determine the impact of the gut microbiome on disease pathogenesis and treatment in vivo. With this protocol, we can reproducibly create double hu-BLT mice with human donor specific gut microbiome profiles. Therefore, we believe that using double hu-BLT mice will be beneficial to future personalized medicine applications designed to test the impact of the gut microbiome on treatments for various human diseases like HIV-1 and cancer. In summary, our double hu-BLT mice model is an important and novel pre-clinical model that features both a functional human immune system and a stable human-like gut microbiome to study human health and disease.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Yanmin Wan, Guobin Kang, and Pallabi Kundu for their assistance in generating BLT-humanized mice. We would like to acknowledge the UNMC Genomics Core Facility who receives partial support from the Nebraska Research Network In Functional Genomics NE-INBRE P20GM103427-14, The Molecular Biology of Neurosensory Systems CoBRE P30GM110768, The Fred & Pamela Buffett Cancer Center – P30CA036727, The Center for Root and Rhizobiome Innovation (CRRI) 36-5150-2085-20, and the Nebraska Research Initiative. We would like to thank University of Nebraska – Lincoln Life Sciences Annex and their staff for their assistance. This study is supported in part by the National Institutes of Health (NIH) Grants R01AI124804, R21AI122377-01, P30 MH062261-16A1 Chronic HIV Infection and Aging in NeuroAIDS (CHAIN) Center, 1R01AI111862 to Q Li. The funders had no role in study design, data collection and analysis, preparation of the manuscript or decision for publication.
Materials
| Animal Feeding Needles 18G | Cadence Science | 9928B | |
| Clidox-s Activator | Pharmacal Research Laboratories | 95120F | |
| Clidox-s Base | Pharmacal Research Laboratories | 96125F | |
| DGM 108 cage rack | Techniplast | ||
| Flat Brown Grocery Bag 3-5/8"D x 6"W x 11-1/16"L | Grainger | 12R063 | |
| FMT Upper Delivery Microbiota Preparations | OpenBiome | FMP30 | |
| Grape Kool-Aid | Kraft Foods Inc. | ||
| hCD19-PE/Cy5 | Biolegend | 302209 | |
| hCD3-PE | Biolegend | 300408 | |
| hCD4-Alexa 700 | Biolegend | 300526 | |
| hCD45-FITC | Biolegend | 304006 | |
| hCD8-APC/Cy7 | Biolegend | 301016 | |
| Lactate Buffered Ringer's Solution | Boston BioProducts Inc | PY-906-500 | |
| mCD45-APC | Biolegend | 103111 | |
| Microvette 100 K3E | Microvette | 20.1278.100 | |
| Neosporin First Aid Antibiotic/Pain Relieving Ointment | Neosporin | ||
| NSG mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) | The Jackson Laboratory | 005557 | |
| PrecisionGlide 25 G Needle | BD | 305127 | |
| RS200 X-ray irradiator | RAD Source Technologies | ||
| Sealsafe Plus GM500 microisolator cages | Techniplast | ||
| Sterile Non-woven Gauze | Fisherbrand | 22-028-558 | |
| Teklad global 16% protein irradiated mouse chow | Teklad | 2916 |
References
- Simpson-Abelson, M. R., et al. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2R gamma(null) mice. Journal of Immunology. 180 (10), 7009-7018 (2008).
- Bankert, R. B., et al. Humanized Mouse Model of Ovarian Cancer Recapitulates Patient Solid Tumor Progression, Ascites Formation, and Metastasis. PLoS One. 6 (9), (2011).
- Vudattu, N. K., et al. Humanized Mice as a Model for Aberrant Responses in Human T Cell Immunotherapy. Journal of Immunology. 193 (2), 587-596 (2014).
- Whitfield-Larry, F., et al. HLA-A2 Matched Peripheral Blood Mononuclear Cells From Type 1 Diabetic Patients, but Not Nondiabetic Donors, Transfer Insulitis to NOD-scid/gamma c(null)/HLA-A2 Transgenic Mice Concurrent With the Expansion of Islet-Specific CD8(+) T cells. Diabetes. 60 (6), 1726-1733 (2011).
- Yi, G. H., et al. A DNA Vaccine Protects Human Immune Cells against Zika Virus Infection in Humanized Mice. EBioMedicine. 25, 87-94 (2017).
- Stary, G., et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 348 (6241), (2015).
- Sun, Z. F., et al. Intrarectal transmission, systemic infection, and CD4(+) T cell depletion in humanized mice infected with HIV-1. Journal of Experimental Medicine. 204 (4), 705-714 (2007).
- Wang, L. X., et al. Humanized-BLT mouse model of Kaposi’s sarcoma-associated herpesvirus infection. Proceedings of the National Academy of Sciences of the United States of America. 111 (8), 3146-3151 (2014).
- Ernst, W. Humanized mice in infectious diseases. Comparative Immunology Microbiology and Infectious Diseases. 49, 29-38 (2016).
- Turnbaugh, P. J., et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 444 (7122), 1027-1031 (2006).
- Gopalakrishnan, V., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 359 (6371), 97-103 (2018).
- Routy, B., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 359 (6371), (2018).
- Clemente, J. C., Manasson, J., Scher, J. U. The role of the gut microbiome in systemic inflammatory disease. Bmj-British Medical Journal. 360, (2018).
- Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L., Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature. 474 (7351), 327-336 (2011).
- Hooper, L. V., Littman, D. R., Macpherson, A. J. Interactions Between the Microbiota and the Immune System. Science. 336 (6086), 1268-1273 (2012).
- Maynard, C. L., Elson, C. O., Hatton, R. D., Weaver, C. T. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 489 (7415), 231-241 (2012).
- Xiao, L., et al. A catalog of the mouse gut metagenome. Nature Biotechnology. 33 (10), 1103 (2015).
- Nguyen, T. L. A., Vieira-Silva, S., Liston, A., Raes, J. How informative is the mouse for human gut microbiota research. Disease Models & Mechanisms. 8 (1), 1-16 (2015).
- Turnbaugh, P. J., et al. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Science Translational Medicine. 1 (6), (2009).
- Hazenberg, M. P., Bakker, M., Verschoor-Burggraaf, A. Effects of the human intestinal flora on germ-free mice. Journal of Applied Bacteriology. 50 (1), 95-106 (1981).
- Hansen, C. H. F., et al. Patterns of Early Gut Colonization Shape Future Immune Responses of the Host. PLoS One. 7 (3), (2012).
- Lan, P., Tonomura, N., Shimizu, A., Wang, S. M., Yang, Y. G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34(+) cell transplantation. Blood. 108 (2), 487-492 (2006).
- Li, Q. S., et al. Early Initiation of Antiretroviral Therapy Can Functionally Control Productive HIV-1 Infection in Humanized-BLT Mice. Jaids-Journal of Acquired Immune Deficiency Syndromes. 69 (5), 519-527 (2015).
- Brainard, D. M., et al. Induction of Robust Cellular and Humoral Virus-Specific Adaptive Immune Responses in Human Immunodeficiency Virus-Infected Humanized BLT Mice. Journal of Virology. 83 (14), 7305-7321 (2009).
- Greenblatt, M. B., et al. Graft versus Host Disease in the Bone Marrow, Liver and Thymus Humanized Mouse Model. PLoS One. 7 (9), (2012).
- Hintze, K. J., et al. Broad scope method for creating humanized animal models for animal health and disease research through antibiotic treatment and human fecal transfer. Gut Microbes. 5 (2), 183-191 (2014).
- Ericsson, A. C., Personett, A. R., Turner, G., Dorfmeyer, R. A., Franklin, C. L. Variable Colonization after Reciprocal Fecal Microbiota Transfer between Mice with Low and High Richness Microbiota. Frontiers in Microbiology. 8, 1-13 (2017).
- Ellekilde, M., et al. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Scientific Reports. 4, (2014).
- Staley, C., et al. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome. 5, (2017).
- Zhou, W., Chow, K. H., Fleming, E., Oh, J. Selective colonization ability of human fecal microbes in different mouse gut environments. ISME J. , (2018).
- Lundberg, R., Toft, M. F., August, B., Hansen, A. K., Hansen, C. H. F. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 7 (1), 68-74 (2016).
- Wos-Oxley, M., et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes. 3 (3), 234-249 (2012).

