Unraveling the Role of Discrete Areas of the Rat Brain in the Regulation of Ovulation through Reversible Inactivation by Tetrodotoxin Microinjections
Summary
This protocol describes the construction of a low-cost microinjection system, its stereotaxic implantation into deep-brain structures and the procedure for timed microinjections of tetrodotoxin in awake and unrestrained rats. The goal is to reveal the participation of hypothalamic structures in the regulation of ovulation by inhibiting their neural activity.
Abstract
Many experimental approaches have been used for studying the role of the brain in the regulation of ovulation. Examples include the lesion and deafferentation of neuronal groups, which are both invasive methods that permanently impair the integrity of the target area. These methods are accompanied by collateral effects that can affect the analysis of acute and temporal regulatory mechanisms. The stereotaxic implantation of guide cannulas aimed at specific brain regions, followed by a recovery period, allows researchers to microinject different drugs after the disappearance of the undesired effects of the surgery. Tetrodotoxin has been used to determine the roles of several brain areas in diverse physiological processes because it transiently inhibits the sodium-dependent action potentials, thus blocking all neural activity in the target region. This protocol combines this method with strategies for the assessment of the estrous cycle and ovulation to reveal the role of discrete brain regions in the regulation of ovulation at particular times of any given stage of the estrous cycle. Awake and unrestrained rats (Rattus norvegicus) were used to avoid the blocking effects that anesthetics and stress hormones exert on ovulation. This protocol can be easily adapted to other species, brain targets and pharmacological agents to study different physiological processes. Future improvements to this method include the design of a microinjection system using glass capillaries of small diameter instead of guide cannulas. This will reduce the amount of tissue damaged during the implantation and decrease the spread of the infused drugs outside the target area.
Introduction
Ovulation is the process by which one or more mature oocytes are released from the ovaries once every estral/menstrual cycle. As all mammalian species depend on the production of gametes to breed, the understanding of the mechanisms that regulate ovulation has a huge impact in areas ranging from biomedicine, the livestock industry and the maintenance of endangered species. Ovulation is regulated by the hypothalamic-pituitary-ovarian axis, which involves several hypothalamic and extra-hypothalamic areas, the gonadotropes in the anterior pituitary and the theca and granulosa cells that, along with the oocytes, form the ovarian follicles inside the ovaries1.
Ovarian follicles grow, develop and eventually ovulate in response to the tonic and phasic secretion of the follicle-stimulating hormone and the luteinizing hormone, the two gonadotropins secreted by the gonadotropes. The pattern of gonadotropin secretion is pivotal for proper follicular development and ovulation and it is regulated by the gonadotropin-releasing hormone (GnRH)1,2. This neuropeptide is synthesized by neurons scattered throughout the basal diencephalon and then secreted to the portal vasculature that links the hypothalamus and the anterior pituitary. The secretory activity of the GnRH-neurons is in turn modulated by synaptic input arising from diverse brain structures. These structures convey information about the state of the external and internal environment of the organism including the availability of food, the length of the photoperiod and the concentration of hormones in the blood. In this sense, they shape the reproductive pattern of each species and the specific roles of such structures must be determined in order to properly understand the mechanisms governing ovulation. As an example, it has been shown that the fluctuation in estradiol levels during the estrous cycle regulates the secretion of GnRH; however, GnRH-neurons does not express the estradiol receptor isoform needed to detect such changes. Two populations of neurons expressing these receptors are located in the rostral periventricular region of the third ventricle and in the arcuate nucleus, respectively, and stablish synapses with GnRH-neurons. There is evidence to suggest that these neurons interpret the concentration of estradiol and then stimulate the activity of GnRH-neurons by releasing kisspeptin, a potent inductor of GnRH secretion3.
Experiments involving thermic or chemical lesions, as well as mechanical deafferentation, allowed researchers to determine the involvement of several brain structures in the regulation of ovulation4,5,6,7,8,9,10,11,12. These experiments, however, have the disadvantage of being invasive and traumatic, requiring several days of recovery before evaluating the effects of the treatment, impeding the analysis of the acute effects of the treatment. In addition, they permanently affect the targeted areas and disrupt other physiological processes in the long term. Due to these problems, the results of these experiments are usually obscured by the homeostatic compensatory mechanisms in the animal´s body and extracting accurate information about the temporal regulatory dynamics in which the area is involved is rather difficult.
The microinjection of drugs that transiently disrupt the activity of neurons through guide cannulas is a suitable alternative that surpasses the disadvantages mentioned above. The cannulas can be placed in any brain region by a stereotaxic surgery, allowing the researcher to start the drug treatment after the confounding effects of the surgery disappear. The timed microinjection of the drugs allows researchers to test hypotheses regarding the contribution of the region to a particular step of the process and can be performed in awake restrained or free-moving animals. A variety of drugs including local anesthetics, agonists, antagonists, inverse agonists and biological toxins such as tetrodotoxin (TTX) can be microinjected into the region of interest at specific times.
TTX is a biological toxin synthesized by bacteria living in the body of the pufferfish as well as other vertebrates and invertebrates. TTX silences neural activity through the selective and transient blockade of sodium channels, which results in the inhibition of sodium-dependent action potentials. In the presence of TTX, cells experience an alteration in the depolarization phase and thus are not excitable but remain alive. The blocking effect of TTX is explained by its molecular composition: a guanidinium group is able to pass through the extracellular aspect of the sodium channel, but the rest of the molecule cannot pass due to its size, so it is stuck and blocks the channel13,14,15,16,17. The mechanism of action of the TTX allowed its use as a tool to study the nervous system both in vitro and in vivo. Intracerebral injection of this toxin has been used to study the role of discrete brain areas in several processes like memory retention18, sleep and arousal19, place recognition20, spatial navigation21, drug abuse22, thermoregulation23, development of schizophrenia24, sexual behavior25 and regulation of ovulation26 among others. In this protocol we describe the effects on ovulation of the transient inactivation of hypothalamic nuclei by TTX microinjection in awake and unrestrained rats.
Protocol
Procedures involving animals were approved by the Ethics Committee of Facultad de Estudios Superiores Zaragoza, UNAM. This institution operates in strict accordance with the Mexican rules for animal handling, Official Norm: NOM-062-ZOO-1999, which agrees with international guidelines.
1. Construction of bilateral cannulas
- Extract the stainless-steel shaft from the hub of two 23 G hypodermic needles using pressure tweezers and then remove any remaining glue using a scalpel blade.
- Draw a line 15 mm apart from the blunt end of the shafts with a fine permanent marker. Use cutting tweezers to remove the beveled ends.
- Hold the 15 mm segments with fine hemostats and press them perpendicularly with a cut-off disc attached to a rotatory tool until 14 mm segments of tubing are obtained. This step is done in order to eliminate the occluded portion of the shafts, creating open and blunt ends. Finally, insert a 30 G needle through the segments to eliminate any internal obstruction.
- Determine the distance between the structures of interest in the left and right hemispheres of the brain with the aid of a brain atlas27. Use moldering clay to attach the two 14 mm segments to a microscope slide and ensure that both are at the same horizontal level. Observe through an ocular micrometer (10x) and adjust the segments with fine tweezers until the desired distance is obtained.
- Mix solder paste with 10% hydrochloric acid in a 2:1 proportion and add a drop of the mixture 2 mm below the blunt end of the segments. Solder both segments with a single point using a soldering iron and 0.5 mm-diameter solder wire. Ensure that the solder does not obstruct the lumen of the segments.
- Create a support to attach the cannula to the stereotaxic holder by cutting a 10 mm segment of resilient 0.3 mm stainless steel wire. Use moldering clay to place 2 mm of the wire in contact with the previous solder point and the rest laying above the blunt end of the cannulas. Solder the wire to the cannulas.
- Clean the surface of the cannulas with 70% ethanol to remove the excess of the solder paste. Flush the interior of the cannulas with sterile water to remove metallic particles. Repeat this process until no particles can be detected under the microscope at a 10x magnification.
2. Construction of obturators and caps
- To build the obturators cut two 16 mm segments of round stainless-steel soft wire (0.35 mm of diameter). Hold a bilateral cannula with hemostats, perpendicular to a lab bench, and insert one of these segments into each cannula until they reach the bench. Bend the remnant at a 90° angle.
- For the caps cut two 2 mm segments of silicone tubing (inner diameter=0.76 mm) and apply a drop of silicon glue at one of the ends of each segment. Do not let the glue to enter to the tubing. Let dry for at least 24 hours.
3. Construction of microinjectors
- Repeat step 1.1, using 30 G hypodermic needles instead to create the shafts.
- Draw a line 18.5 mm apart from the blunt end of the shafts and remove the remnant of the beveled ends with cutting tweezers.
- Repeat step 1.1 with a single 23 G needle to build adaptors by cutting two 6 mm-long segments starting from the blunt end.
- Eliminate the occluded portions of the 6 mm segments by pressing them perpendicularly against the cut-off disc until two 4 mm adaptors are obtained. Eliminate any internal obstruction.
- Insert the beveled end of the 30 G segments into the adaptors. Look through a stereomicroscope to ensure that the end of both, segments and adaptors, are at the same level. Apply cyanoacrylate glue to the distal joint using a cotton swab and let dry for 15 minutes.
- Soak two Teflon tubing connectors in 70% ethanol for 5 minutes and then attach them to the microinjectors through the adaptors. Wait until the diameter of the connectors shrinks for at least 24 hours and then sterilize the microinjector with a low temperature method to avoid damage of the connectors (ethylene oxide sterilization is recommended).
4. Animal maintenance and vaginal smears
- Use cyclic adult female hooded rats (Rattus norvegicus, CIIZ-V strain) weighing between 230 and 260 grams. House the rats in groups of four in standard polypropylene cages in a room with a 14:10 light–dark photoperiod. Set the temperature and humidity at 22 ± 2 °C and at 40%, respectively. Provide food and water ad libitum.
- Take vaginal smears every day between 10:00 and 12:00 h.
- Sterilize a modified bacteriological loop with an inner diameter of 1 mm using an alcohol lamp and cool it with sterile water. Hold the rat with a secure grip and introduce 5 mm of the bacteriological loop into the vagina, touching its internal walls. Remove the bacteriological loop. If successful, a cloudy drop will be seen at the tip. Place this drop on a microscope slide.
- Repeat this process for each rat sterilizing and cooling the loop between each animal.
- After the drops dry off, stain with hematoxylin-eosin and observe the samples under a microscope at 10x.
- Determine the proportion of leukocytes, epithelial nucleated cells and keratinized cells on each smear and classify it according to the criteria of estrous cycle stages reported in Figure 1, which agrees with previous literature28,29.
5. Stereotaxic implantation of the cannulas
NOTE: Perform the implantation of the cannulas following a regular aseptic stereotaxic surgery and abiding to institutional norms.
- Before surgery
- Sterilize surgical instruments, cannulas, surgical screws, obturators, gauze and wooden cotton swabs 24 h before surgery using a steam autoclave. Sterilize the tips of metallic instruments between consecutive surgeries by cleaning them with 10% hydrogen peroxide followed by water and then place them in a hot bead sterilizer.
- Prepare the working areas and the stereotaxic instrument before removing the animal from the homeroom. Prepare the animal for surgery in an area that is located as far as possible from the surgery table to avoid contamination during the procedure.
- Clean the preparation and surgery areas with 70% ethanol followed by an application of a 10% chloride solution for ten minutes. Clean the base, frame and manipulators of the stereotaxic instrument with 70% ethanol and sterilize the tips of the ear bars using a hot bead sterilizer and air-cool them before surgery.
- Perform the surgery wearing a dedicated lab coat, face mask, head bonnet, surgical sleeves, shoe covers and surgical gloves. Ask the assistant to prepare the animals for surgery and check their general state during the procedure.
- Attach the cannula to the stereotaxic holder. Ask the assistant to bring the first animal to be operated into the room. Select rats in diestrus for surgery, since observations from our lab indicate that these animals recover proper estrous cycles quicker than rats operated in other stages, probably because the response to stress changes along with the estrous cycle.
- Weigh the animal, and induce anesthesia with 4% isoflurane in 100% oxygen inside an induction chamber for rats connected to an isoflurane vaporizer. To avoid stressing the animals, do not prefill the chamber. Confirm a surgical plane of anesthesia by checking pupil dilation and the loss of pain as measured by the tail and ear pinch tests after you observe the loss of the righting reflex.
- Anesthetize the rat by an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) if an isoflurane vaporizer is not available.
- Remove the rat from the induction chamber and use a nose cone in the preparation table to maintain the effects of anesthesia with 2.5% isoflurane in 100% oxygen. Trim the hair of the scalp with clippers and remove loose hair with a lint roller. Inject a preoperative subcutaneous dose of 2 mg/kg of Meloxicam and 5 mg/kg of Enrofloxacin as a non-steroidal anti-inflammatory/analgesic and antibiotic, respectively.
- Apply hypromellose artificial tears to each eye to avoid desiccation during the surgery.
- Mount the animal in the stereotaxic instrument above a warming pad using non-rupture ear bars and an anesthesia mask. Adjust the nose clamp to ensure that the head of the animal is flat. Perform a surgical scrub in the shaved area alternating between povidone-iodine with soap and 70% ethanol one time and then using povidone-iodine and 70% ethanol two times. Insert a rectal thermometer to record the temperature of the animal during the procedure and then cover it with a sterile surgical field.
- During surgery
- Use a scalpel to make a 2 cm incision in the skin and muscle in the middle of the shaved area. Remove the periosteum from the skull using a cotton swab coated with 2% hydrogen peroxide to reveal bregma or lambda, the landmarks that will be used to calculate the target coordinates. Dry the skull with air for a better visualization of the landmark.
- Use the manipulators of the stereotaxic instrument to move the cannula to a position directly above the landmark of choice. Register the anterior-posterior and the medial-lateral coordinates from the stereotaxic instrument and use them to calculate the coordinates of the target area according to the brain atlas27.
- Set the calculated coordinates in the stereotaxic instrument and place the tip of the cannula on the surface of the skull. Register the dorsal-ventral coordinate and use it to calculate the depth to the target area.
- Remove the arm of the manipulator by unscrewing it from the frame. Use a dental burr attached to a rotatory tool at a speed of 15,000 rpm to make a mark in the place where the craniotomy will be performed. Then use the burr to make three holes, forming an equilateral triangle around the mark. These holes must not pierce the skull. Insert the surgical screws into the holes, ensuring that they are tightly placed.
- Make the craniotomy wide enough to accommodate the distance between the target areas in each hemisphere. It should be as small in diameter as possible but big enough to allow the lowering of the cannula without touching the borders of the skull, since this will modify the trajectory. Perform the craniotomy gradually, applying the lowest pressure possible. The rodent skull is just a few millimeters thick and it is pivotal to preserve the integrity of the tissues below.
- Once the dura mater is visible, use a sterile 21 G needle with the tip curved at a 90° angle to remove splinters of bone and make an anterior-posterior cut in the meninges. Use Wirtshafter and colleagues30 technique to avoid damage to the superior sagittal sinus if the target area is located close to the midline.
- Place the holder with the cannula in the frame and use the dorsal-ventral manipulator to reach the ventral coordinate. Check that the needle does not touch the borders while descending and increase the diameter of the craniotomy if needed.
NOTE: It is important that the tip of the guide cannulas targets a region ranging from 0.5 to 2.0 mm above the region of interest. This is due to the inflammatory reaction of the brain in response to the introduction of foreign bodies and to the destruction of the tissue. This is especially critical if the region of interest is small and the working distance must be calculated empirically in advance.
- Place the holder with the cannula in the frame and use the dorsal-ventral manipulator to reach the ventral coordinate. Check that the needle does not touch the borders while descending and increase the diameter of the craniotomy if needed.
- Apply dental cement to attach the cannula to the skull and let it dry. Ensure that the dental cement does not cover the blunt end of the cannula since this will irreversibly obstruct it. Wait until the cement solidifies completely.
- Insert the obturators to avoid further obstructions and to ensure patency during the study. Put on the silicone cap to avoid contamination.
- After surgery
- Replace lost fluids by an intraperitoneal injection of 1.0 mL of sterile saline solution at a physiological temperature. Place the animal in a recovery cage with thermal support until it recovers from anesthesia. Check the temperature and hearth/respiratory rate of the animal periodically. Isoflurane effects last for a few minutes after interruption of the gas flux while ketamine/xylazine effects lasts for 40-50 min.
- Provide post-operative injections of the antibiotic, analgesic and anti-inflammatory drugs (at the same doses and routes stated before) for 48-72 h and closely inspect the animals on a daily basis for the rest of the experiment. Report any sign of pain, stress or loss of weight to vet services or to a trained member of the lab. Do not perform any other manipulation to the rats during this period.
- Start taking vaginal smears after the post-operative treatment and continue doing it until the animal shows three consecutive estrous cycles of four days.
NOTE: Chronic jugular catheters can be surgically implanted, allowing the researcher to take serial blood samples to analyze the secretion of hormones such as estradiol, progesterone, testosterone, luteinizing hormone, follicle-stimulating hormone and corticosterone. We recommend placing such a catheter once the animal recovers from the brain surgery, since this will improve survival rate while also avoiding clogging of the catheter that may result from an extended recovery time.
6. Tetrodotoxin handling and preparation of solutions
CAUTION: TTX is one of the most toxic substances known. It acts on skeletal muscles and nervous tissue. Intoxication by contact is unlikely but any open wound is a potential pathway for inoculation into the body. The main concerns when working with TTX are the puncture of the skin with sharp instruments that have been in contact with the toxin and the generation of aerosols that may reach the mouth, eyes and mucous membranes. Depending on the dose, TTX may be lethal if inhaled, swallowed or inoculated by the skin. It will cause strong irritation of the eyes. The LD50 has been tested in mice by oral and intravenous administration and it is 334 µg/kg and 7.3 µg/kg, respectively. There is no antitoxin available at this time.
NOTE: Inactivating substance is a 1.0% NaOCl with or without 0.25 N NaOH, or a 10% bleach solution. Complete inactivation occurs after 30 min exposure. TTX cannot be completely inactivated by any chloride derivate at a concentration below 10%, by autoclaving nor by dry heat sterilization at a temperature lower than 500 °F. All the procedures involving TTX must be performed by two knowledgeable individuals wearing inner and outer pairs of nitrile gloves, dedicated lab coat, safety glasses, disposable face mask, closed shoes and full-length pants.
- Preparation of the stock solution
- Place a pad that is soaked with the inactivating substance in the fume hood to prevent contamination in case of a spill. Be sure that the fume hood is working properly before starting. Prepare a receptacle with the inactivating solution to dispose of pipette tips.
- Resuspend sterile TTX citrate lyophilized powder according to manufacturer instructions by an extremely careful and slow titration with sterile artificial cerebrospinal fluid, rinsing down the walls of the vial in the process. Avoid the formation of foam and aerosol. Follow an aseptic technique to avoid contamination of the TTX solution since it will be injected into the brain of live animals. Use a syringe with a needle instead of a micropipette if your TTX vials have an outer membrane to prevent aerosol formation.
- Aliquot the stock solution into sterile micro tubes. Make aliquots as small as possible since freezing/thawing cycles may alter the stability of the molecules. Do not fill more than half of the tubes because water increases in volume when frozen, which can lead to opening of the lid and contamination of the tube and other samples stored in the container.
- Decontaminate the exterior of the tubes and the surfaces where TTX was used with the inactivating substance. Store the tubes at -20 °C in a vial box inside a secondary container.
- Diluting the stock solution to a working concentration
- Prepare freshly diluted solutions the day they will be used since diluted solutions are less stable. Place a pad that is soaked with the inactivating substance in the fume hood and a micro tube-rack on top of it. Prepare a receptacle with the inactivating solution to dispose of pipette tips.
- Pipette the stock solution at the bottom of a sterile microtube and then add the calculated amount of artificial cerebrospinal fluid to achieve a concentration of 10 ng/µL. As described for the re-suspending of the TTX powder, perform an extremely careful and slow titration, rinsing down the walls of the vial and avoiding the formation of foam and aerosol.
- If samples that have been on the freezer will be used for microinjection, they must be allowed to equilibrate at room temperature for at least one hour prior to the experiment.
7. Microinjection of TTX or vehicle solutions into freely moving rats
- Configure the microinjection pump with the infusion rate and total time of injection according to the experiment. Calculate these parameters in advance considering the volume occupied by the target structure and the amount of solution to be injected. For this experiment inject 200 nL of solution at a rate of 50 nL per minute for a total infusion time of 4 minutes.
- Fill two 10 μL Hamilton syringes with sterile distilled water, insert a piece of sterile Teflon tubing into the tubing connector of each microinjector, ensuring that the length of the tubing does not constrain the movement of the rat (tubing can be sterilized using ethylene oxide).
- Place a pad that is soaked with the inactivating substance and a 2 cm x 2 cm square of paraffin film above it. Pipette a sufficient amount of TTX to fill the needle of the injector and 1 cm of the tubing above the film. Absorb the TTX drop by gently retracting the plunger. Mount the syringes in the microinjection pump and use its controls to manipulate the plunger until a drop of TTX can be seen at the tip of each microinjector. Discard the drops into the pad soaked with inactivating substance.
- Select the rats that will be microinjected. Use only rats that showed at least three consecutive cycles after the surgery. Consider their stage of the cycle and the time of the day. Both the time and the stage depends on the specific hypothesis that you will test, for this experiment 14:00 hours of proestrus selected since the nervous preovulatory signals governing phasic GnRH secretion occur at this moment. Transport them to the room where the injection will take place inside their own housing cages to avoid distress.
- Hold the rat with a firm grip, remove the cap and obturators from the cannulas, insert the microinjectors into the guide cannulas and return the animal to the cage.
- Turn on the pump and wait until the microinjection finishes. Observe the animal during this period in order to detect a possible detachment of the microinjectors and to avoid the twisting of the Teflon tubes.
- Leave the microinjectors in place for two additional minutes to avoid reflux of the solution. Remove them, clean the surface of the implant with iodopovidone antiseptic solution, avoiding its flow inside the guide cannulas. Insert sterile obturators, put on the cap and return the animal to the colony room. Observe the animal periodically to detect any possible side effects of the drug.
- Turn on the pump with the same parameters as during microinjection and check for a correct flow to ensure that patency of the system was retained during the procedure.
- Press the plunger to expel the TTX/vehicle out of the microinjector until the air bubble reaches the tip of the needle. Recollect the TTX in its microtube. Fill the syringe with distilled water and use it to clean the interior of the tubes. Discard the water into the inactivating substance and repeat this process at least three times. Clean the outside of syringes, tubes and microinjectors with a cloth soaked in the inactivating substance.
8. Euthanasia and tissue processing
- On the day of the predicted or vaginally confirmed estrus, inject the rat with an intraperitoneal overdose of sodium pentobarbital (75 mg/kg). Check for loss of consciousness and inject 200 nL of a 0.5% methylene blue solution through the guide cannulas as described in steps 7.1 to 7.9. Check for pain signs using the toe or tail pinch test and, if none are detected, decapitate the rat using a rodent guillotine.
- Use scissors to open the abdominal cavity and find the ovaries. Use fine iris scissors to dissect each ovary, cutting at the utero-tubal junction with the aid of a magnifying glass. Avoid damaging the oviduct because this will result in the loss of oocytes.
- Put the ovaries under a stereomicroscope and use a razor blade to remove all the fat tissue without damaging the organ. Remove the oviduct from each ovary by cutting with the razorblade as far as possible from the ampulla region. Mount each oviduct on a different slide and cover with a drop of water. Store the ovaries in a vial containing Bouin´s solution.
- Gently dry the oviducts with an absorbent paper towel and search for the ampulla under the stereomicroscope. With the non-dominant hand, hold the oviduct in place by pinching far from the ampulla with a 23 G needle, then use another 23 G needle in the dominant hand to make a 1 mm incision in the middle region of the ampulla. The cumulus-oocyte complexes will protrude from the incision as a drop of viscous fluid (Figure 2D). Use the needle in the dominant hand to gently and slowly pull the drop far from the oviduct. Press the remnant of the ampulla to ensure that no oocytes are left inside. Extract the oocytes from the oviducts as fast as possible as desiccation of the oviduct will irreversibly trap the oocytes inside.
- Remove the oviduct from the slide and wait until the drop of fluid containing the oocytes dries. Stain the sample with hematoxylin-eosin. Use mounting medium to coverslip. Observe under the microscope (10x) to determine the number of oocytes shed by each ovary.
NOTE: Sacrifice by cardiac perfusion is not performed in this protocol since the oocytes would be trapped in the oviducts and hence histological methods would be necessary to assess ovulation. If the user must perfuse to analyze other tissues, the ovaries can be removed first and the descending arteries clamped with thick hemostats below the liver to avoid leaking of fixative. - Remove the skin of the head and submerge the skull in a glass container with a 10% formalin solution. Allow fixation of the head for at least ten days before removing the cannula to avoid distortion of the tissue. To remove the cannula, use scissors to detach all the muscles in the region of the skull that surrounds it. Cut between the nasal and frontal bones with bone trimmers and then remove the occipital bone. Insert the tip of the trimmers into the foramen magnum and start cutting through the sagittal crests reaching the remnant of the nasal bone.
- The isolated region of the top of the skull must contain the frontal and parietal bones. Remove the implanted cannula attached to the top of the skull by slowly pulling it up perpendicularly to the head. Cut any attached portion of the meninges with fine iris scissors to avoid deformation of the brain.
- Make an anterior-posterior cut on the meninges and put them aside to remove the brain from the base of the skull. Insert blunt tweezers below the olfactory bulbs and gently pull up the brain until the optic nerves are visible. Cut the nerves with fine iris scissors and pull out the brain. Preserve the brain in fixative solution.
- Obtain 50 µm thick sections of the brain using a vibratome, mount them in poly-L-lysine coated slides (0.1% in distilled water) and stain with the Nissl technique. Observe the slides under the microscope (10x) to determine the final position of the cannulas (Figure 2A-2C) and the spread of the dye with the aid of a rat brain atlas27.
Representative Results
The protocol described above was tested by evaluating the effects of a single TTX or vehicle (artificial cerebrospinal fluid; ACSF) microinjection into one of two different nuclei known to be involved in the regulation of ovulation in the rat: the suprachiasmatic and the arcuate nucleus. The suprachiasmatic nucleus was chosen since it contains the central circadian pacemaker in mammals. It is involved in the regulation of cyclic events as the secretion of gonadotropins. The arcuate nucleus was chosen because it contains a population of neurons that express estradiol receptors, which stimulates GnRH secretion during most of the estrous cycle. The microinjection was performed at 14:00 hours of proestrus stage, which is a period known as the “critical window” due to the occurrence of many of the central mechanisms regulating the pre-ovulatory release of gonadotropins. After treatment, vaginal smears were taken every day and the rats were euthanized at 09:00 hours of the predicted day of estrus, which coincided with a vaginal smear characteristic of estrus. As an additional control group, five intact rats euthanized at the stage of estrus were used. The fraction of cycling animals after the surgery and the fraction of ovulating rats of each group (ovulating rats/n) was calculated and analyzed using Fisher´s exact probability test. The number of ova shed by both ovaries was analyzed by the Kruskal-Wallis test, followed by Dunn´s test. Appropriate statistical tests were performed to ensure that the sample size was adequate.
A total of 30 female rats were implanted with guide cannulas aiming at one of the two target areas. As shown in Figure 3, all the animals were cyclic before the surgery but only seven of them did not show alterations of the estrous cycle after the cannulation procedure. Twenty-three animals showed transient alterations of their cycles, characterized by an increase in the number of days with leukocytic smears. Such alterations are probably due to the stress induced by the surgery and gradually decreased. By the fifth cycle twenty-eight rats were considered cyclic and the remaining two were discarded from the experiment. This result shows that, after a recovery time of four estrous cycles (16 days), most of the cannulated animals are suitable for further experimentation.
Figure 4A and Figure 4B depicts the percentage of ovulating animals and the number of ova shed, respectively, by intact animals and by the groups treated with either ACSF or TTX into the suprachiasmatic nucleus. All the intact rats ovulated and the same was true for the ACSF group. However, none of the animals microinjected with TTX ovulated. The injection of the vehicle did not modify the number of ova shed. However, it would be interesting to assess the viability and quality of the oocytes released. Similar results can be observed in Figure 4C and Figure 4D for rats microinjected into the arcuate nucleus. In both cases, this method proved the participation of a discrete brain region in the regulation of ovulation at the critical window of the proestrus stage, which was first inferred from lesion studies but not confirmed. The effects of the TTX microinjection seems to be transitory rather than permanent since a previous experiment showed that rats injected in the suprachiasmatic nucleus outside the “critical window” ovulated at the expected day of estrus26. However, the inclusion of control groups in which the animals are sacrificed with a 24 hour or until a clear vaginal smear of estrus is attained is also recommended to address this issue.
The results in Figure 5A-B represent the ovulatory outcome of animals that were treated with ACSF or TTX. However, as determined after histological confirmation, their cannulas were located outside the intended region. Most of these cannulas were placed in the anterior commissure or the retrochiasmatic area, two areas that do not contribute to the regulation of ovulation. Both of these structures along with the suprachiasmatic and the arcuate nucleus are very close from the third ventricle, considering this, a blockade of ovulation would be expected in all animals if the drug leaked into the ventricle. The fact that TTX microinjection outside the targets failed to block ovulation suggests that the volume of TTX infused at the rate selected did not leak into the ventricle, hence revealing the region-specific effects of TTX. In support of this idea, histological analysis of the brain slices only showed stained neurons near the tip of the guide cannulas. However, we must clarify that no direct assessment of the spread of the drug was performed and hence this conclusion should be further addressed.
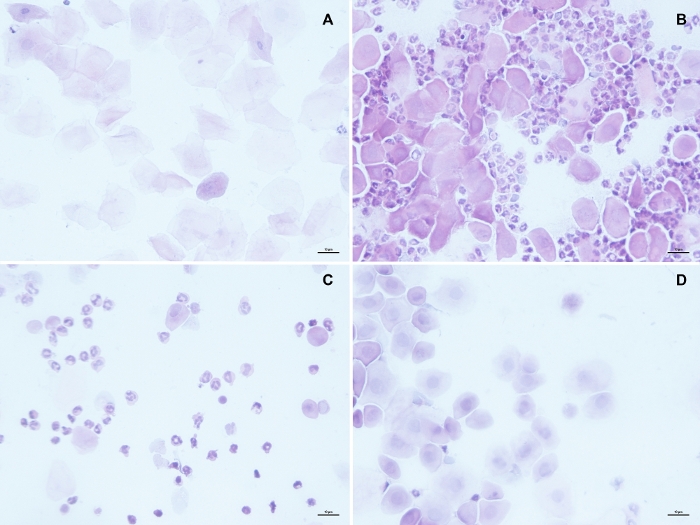
Figure 1: Vaginal smears representative of each stage of the rat estrous cycle. Estrus (A) is characterized for the presence of epithelial cornified cells without a nucleus that can be found either alone of forming cumulous as a result of epithelial desquamation. In metestrus (B) some of these cornified cells can still be present, but are outnumbered by leukocytes, which are also the predominant cell type in Diestrus (C). Proestrus samples (D) are characterized by they viscous consistence and the predominance of epithelial nucleated cells. Scale bar in each panel represents 10 µm. Please click here to view a larger version of this figure.
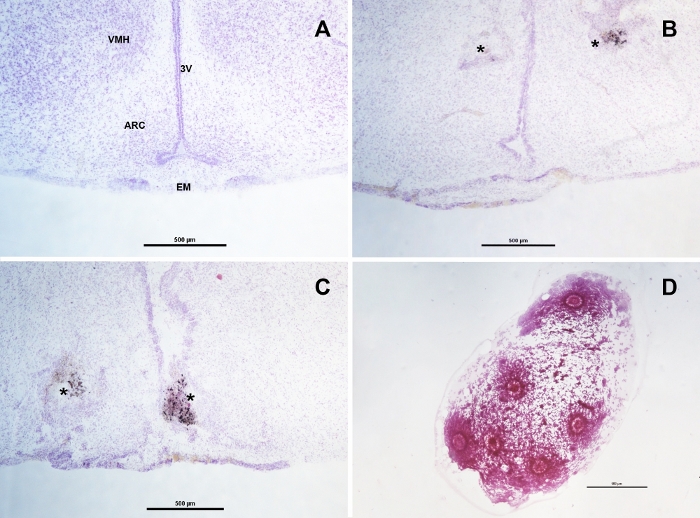
Figure 2: Histological examination of samples after euthanasia. Brain coronal sections at the arcuate nucleus (ARC) and median eminence (EM) region of an intact rat (A), a bilaterally-cannulated rat (B) and a rat with a misplaced cannula (C). The asterisks point at the area where the tip of the guide cannulas were located, in B the tips where at the basal margin of the ventromedial nucleus (VMH) allowing the protruded injectors to reach the upper margin of the ARC. In C one cannula was located inside the third ventricle (3V), resulting in a non-localized ventricular microinjection. This is a common mistake when the target is located near to the midline and data from these animals must be discarded. When performed properly, the extraction of oocytes should result in a single drop of viscous fluid containing all the oocytes from the examined oviduct as seen in panel (D). Scale bar in each panel represents 500 µm. Please click here to view a larger version of this figure.
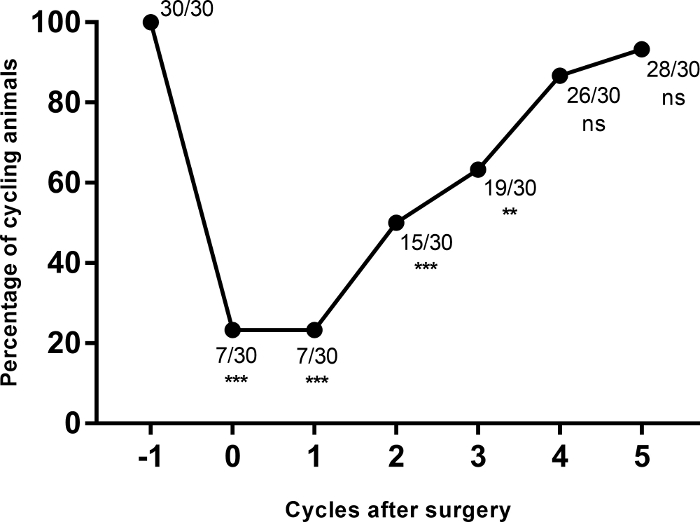
Figure 3: Cumulative percentage of cycling animals before and after the implantation of the cannula (***p≤0.0001; **p=0.0003 vs. the cycle before the surgery, Fisher´s exact probability test). Please click here to view a larger version of this figure.
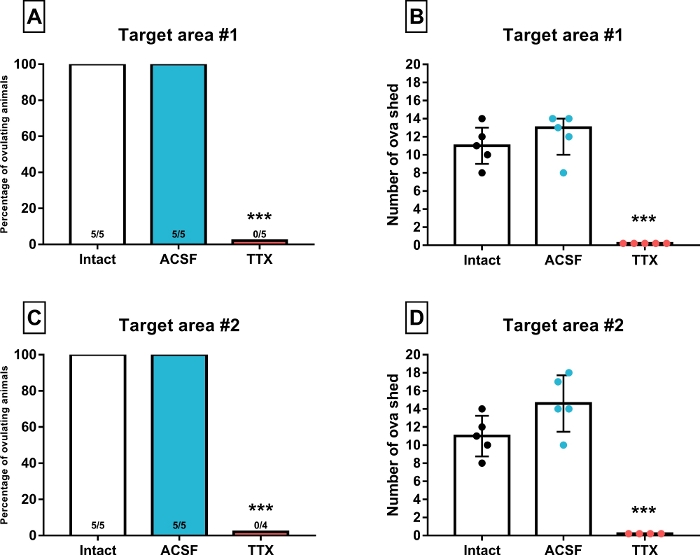
Figure 4: Percentage of ovulating animals and median with interquartile range (error bars) of the number of ova shed by animals microinjected into the target structure #1 (suprachiasmatic nucleus, A-B) or #2 (arcuate nucleus, C-D) with artificial cerebrospinal fluid (ACSF) or tetrodotoxin (TTX) at 14:00 of proestrus stage. The intact group was added to each graph for comparison purposes and the number inside the bars represent the fraction of ovulating females (***p≤0.01 vs. Intact and ACSF groups, Fisher´s exact probability test and Kruskal-Wallis test, respectively). Please click here to view a larger version of this figure.
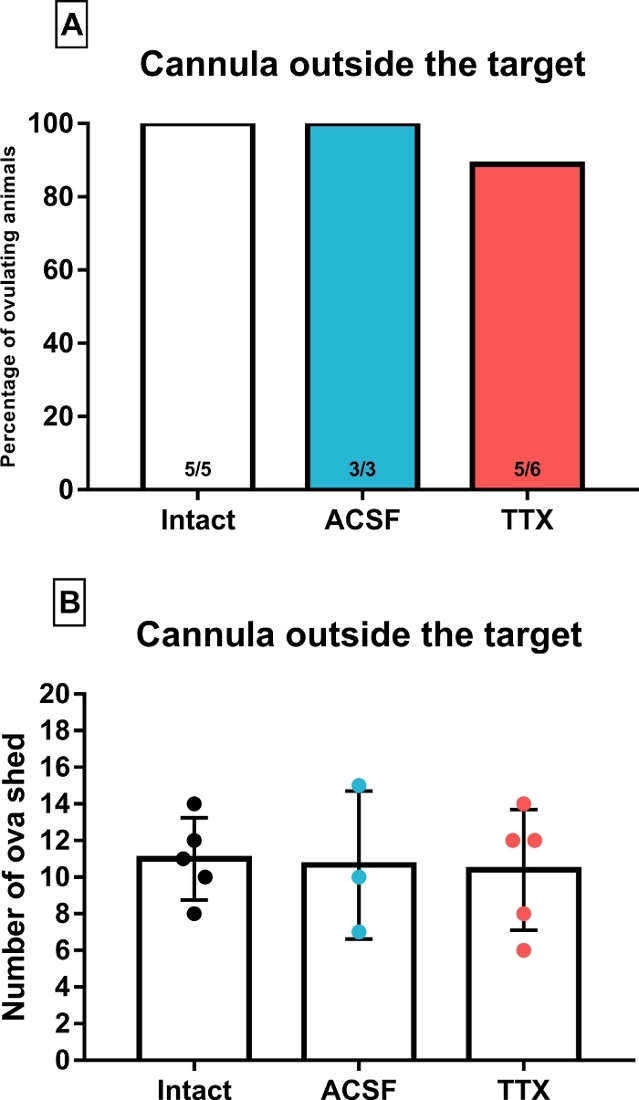
Figure 5: Percentage of ovulating animals (A) and median with interquartile range (error bars) of the number of ova shed (B) by animals whose cannulas were determined to be outside the target areas. These animals were microinjected with artificial cerebrospinal fluid (ACSF) or tetrodotoxin (TTX) at 14:00 of proestrus. The intact group was added to each graph for comparison purposes and the number inside the bars represents the fraction of ovulating females. Please click here to view a larger version of this figure.
Discussion
This article describes a method to transiently inactivate, at any given time, a discrete region in the brain of awake and unrestrained rats. A simple method to track their estrous cycle and assess ovulation is also provided. This protocol allows a straightforward analysis of the contribution of specific brain regions to the mechanisms that drive ovulation by comparing the ovulatory outcome of TTX-treated animals with that of vehicle-treated ones. With the exception of the stereotaxic instrument and the microinjection pump, which are common in neuroscience laboratories, this method does not require expensive materials. Commercial cannulas and microinjectors are the usual choice for this kind of experiment, but the easy-to-build system described here lower the costs and the results are indistinguishable. By using this protocol and the materials listed in the table that accompany this article, ten times more cannulas can be manufactured by spending the same amount of money that a commercial kit for ten animals would cost. The construction of ten bilateral cannulas, no matter the customization required for the study, can be performed in a few hours. In addition, all components are re-usable which increases cost effectiveness.
Along with the benefit-cost ratio, this protocol is suitable for any laboratory environment since it can be performed by any personnel, including students, with knowledge of rodent manipulation, stereotaxic surgery and handling of biological toxins and related wastes. In addition, it can be adapted to be used in any species of rodent and other small mammals including rabbits, ferrets, marmosets and even in non-mammalian species such as birds and reptiles. Besides the characteristics noted above, the main advantage of this protocol is that it can be easily adapted for the study of other processes under the regulation of the brain such as the activity of peripheral organs, the homeostatic response to environmental challenges and various behaviors.
There are several critical steps that must be performed before starting the experiment in order to attain the desired results. First, the coordinates of the targeted structure have to be calculated empirically since those reported in brain atlases will not necessarily match with the animals designated for the study as a result of their strain, sex or age. Several studies have shown that implanted electrodes and infusion probes alter the function of the injured zone not only by the destruction of neural bodies and fibers but also by the activation of glial cells. The inflammatory reaction that initiates immediately after the implantation typically causes encapsulation of the foreign body by glial cells. These cells form a tight sheath that impedes the flow of neurotransmitters and displaces neurons, initiating a process of neurodegeneration31. The deleterious effects of the implantation procedure cannot be avoided and care should be taken to ensure that such effects occur as far from the brain region of interest as possible. Coordinates should be calculated to place the guide cannulas into the target area only if it is big enough to ensure that no more than 10% of the area will be damaged by the cannula. This approach is useful, for example, if large areas of the cortex will be studied. Even in this case, a detailed analysis of the control group must be performed in order to account for the effects of the implant on the process of interest. For small structures such as discrete hypothalamic nuclei, we advise researchers to calculate the coordinates to place the guide cannulas at a distance ranging from 0.5 to 2 mm from the upper border of the target structure at the middle region in the anterior-posterior and medial-lateral planes according to the brain atlas (Figure 2B). For this approach the injectors must be designed to protrude enough to reach the border of the target. We have tested this for the two nuclei described in this protocol and found that these rats recovered their estrous cycle and the ability to ovulate quicker than the animals with cannulas implanted inside the targets.
The solubility of the pharmacological agents to be injected should be considered so that the vehicle solution is as similar to that of cerebrospinal fluid as possible. TTX can be purchased as a lyophilized powder, either alone or with citrate. The first one has low solubility in water and an acidic buffer with a pH around 5.0 is recommended to reconstitute it, but this can introduce the possibility of damaging the tissue as result of injections of the vehicle alone. On the other hand, TTX-citrate can be dissolved in water or 0.9% saline solution, which are both commonly used as vehicles. We recommend against the use of both of these vehicles, and suggest using artificial cerebrospinal fluid or Ringer´s lactate solution instead. A previous study showed that saline solution microinjected into the suprachiasmatic nucleus has deleterious effects on ovulation when performed at estrus or diestrus26. Several articles have reported that the perfusion of nervous tissue with solutions that do not match the physical and chemical characteristics of cerebrospinal fluid alters the anatomy and physiology of neurons and promotes inflammatory responses and cell death32,33,34,35. Considering these results, the use of artificial cerebrospinal fluid as the vehicle is strongly recommended in all experiments involving microinjection of drugs into the brain.
Before beginning experiments, researchers should empirically determine the optimal volume of solution to be injected, as both leaking into adjacent structures and insufficient coverage could confound the interpretation of results. This can be accomplished by microinjecting a water-soluble dye into clear agar or gelatin cubes. The dispersion of the dye can then be estimated and compared with the expected size of the target as reported in the brain atlas. Still, properties of the brain region of interest such as its cellular density and the presence of fiber tracts or ventricular borders will affect the diffusion dynamics, and the results may differ from those found in agar and gelatin. Accordingly, the researcher must then test the parameters obtained in vitro in the target area of a few animals. Several dyes have been successfully used for intracerebral microinjections in rats in order to delineate the diffusion of a given volume of solution. The most common examples are cresyl violet, thionine blue, methylene blue, Evan´s blue, fast green, and India ink. These dyes are dissolved in distilled water at a 0.5% or lower concentration. Evan´s blue has the advantage of being detectable in a fluorescence microscope (excitation at 620 nm and emission at 680 nm), allowing a more quantitative analysis of spread. Latex microbeads can also be suitable for this approach. It is important to mention that volumes greater than 2.0 µL should not be administered into the brain with this technique since mechanical damage and tissue displacement will occur36.
The flow rate of the solution is also an important factor to control, since the microinjection of high volumes at high speed causes tissue displacement and mechanical lesions40. In addition, a high rate of infusion also increases the spread of the drug, resulting in the inactivation of neighboring structures. As much as a threefold increase in the area covered by a solution can be caused by changing the infusion rate from 100 nL/5 minutes to 100 nL/1 minute36. Histological observations of rat brains injected with methylene blue indicate that an infusion rate of 50 nL/minute does not displace the tissue while confining the drug into the target area (personal observations). This was only tested with volumes equal to or lower than 200 nL and hence researchers must run pilot experiments if bigger volumes will be used.
Once the working volume has been determined, it is also recommended to assess the spread on each animal. This can be done by either co-injecting a dye with the drugs or by injecting it a few minutes before euthanasia, as described in this protocol. The former model allows the dye to spread along with the toxin, thus providing a more accurate assessment of the area affected. A downside of this method is that the dye may interfere with the activity of the drug, and may also be cytotoxic. Finally, clearance from the nervous tissue may occur if the animal needs to survive for several days after the injection procedure, which would affect the estimate of the spread. If this approach will be used, the effects of the dye on the process under study must be addressed by comparing the outcome of the vehicle+dye group with that of intact animals. If the injection of the dye will occur before euthanasia, doing so following the same parameters as during microinjection (infusion rate and volume) is recommended. This should be performed at least 10 minutes before euthanasia to allow spread of the solution. With this method, it was found that 200 nL of a TTX/methylene blue solution covers a sphere of 0.6 mm of diameter and the dispersion does not change significantly if microinjection occurs 1 hour before sacrifice (personal observation). This agrees with the study by Freund and colleagues37, as well as the one by Zhuravin and Bures38, which both found that 1 µL of TTX solution spreads in a spherical-shaped volume with a diameter of about 3 mm. In general, it has been shown that the dispersion of the drug depends on its molecular weight and the volume injected,39 and the results stated above match the diffusion of dyes with a molecular weight similar to that of the TTX. If a more accurate control of dispersion is needed, the injection of radiolabeled TTX or the implementation of immunohistochemistry against regular TTX with commercially available antibodies can be performed37. Besides a better delineation of the area covered by the drug, these two approaches are limited by the clearance of the TTX from the tissue, which must be considered if the animal should survive several days after the microinjection, on the other hand, working and disposing of radioactive material will introduce new steps and security issues that could not be compatible with all laboratories and protocols.
The inclusion of a control group that consist of animals injected into a brain area that does not have a role in the regulation of the process studied is recommended. This group will help the researcher to determine the specificity of the target area in the regulation of that process and will discard the possibility that the drugs, injected in any structure, could trigger a blocking signal based in a more widespread mechanism such as the activation of the stress or immune axis. Since even the most experimented stereotaxic surgeons are not able to obtain a 100% success rate when small structures are targeted, these experiments are usually accompanied by cannulas placed outside the desired structure and hence this control group is unintentionally attained. The results from the animals in which the cannula was misplaced are valuable and should not be discarded; instead, a comprehensive analysis must be carried on considering the area injected and the dispersion of the drug. Of special interest are the results that shows a different effect (or none) in animals injected in regions near to the targets.
This protocol has limitations inherent to the chronic placement of cannulas. It is not possible to avoid the permanent destruction of fibers of passage and neuronal bodies in the trajectory of the cannula. The use of glass capillaries with a tip diameter of a few micrometers is the method of choice to deliver drugs into the brain with minimal damage to the tissue41. This methodology, however, has been used mostly in experiments in which the animal is anesthetized and/or the head is attached to the stereotaxic apparatus or other holder devices. This is mainly due to the fragility of the capillary itself, which makes it difficult to attach it to an awake and unrestrained animal. A system using glass capillaries connected to osmotic pumps, instead of guide cannulas, has shown a considerable improvement in the amount of brain tissue damaged and in the glial response to the injury42. In this system, however, the infusion of the drugs is chronic rather than timed and hence not useful in experiments in which the injection time must be precisely controlled. A microinjection system based on a glass capillary inserted into the target area through a previously implanted guide cannula was described by Akinori and colleagues43. This system allows the injection of an awake animal at the desired time, but it is not very robust and the animal must be restricted during the procedure, which can lead to activation of the stress response. In this protocol, the effects of damage to nervous tissue in the trajectory of the guide cannula can be controlled for by comparing the intact and vehicle groups. Here it was shown a similar ovulatory outcome for both groups, and it was concluded that the tissue affected, as well as the injection of the vehicle did not interfere with ovulation. Researchers must run pilot studies to determine whether the structures located dorsal to their target area, which would be destroyed by the cannula, are involved in the regulation of the studied process. Still, a promising future direction for this work is to design a delivery system that capitalizes on the strengths of both guide cannulas and glass capillaries.
Another important consideration for researchers is the time of action of TTX. Zhuravin and Bures38 have shown that TTX takes a few minutes to reach a maximum blockade of neural transmission, which lasts for around one and a half hour, decreasing gradually over several hours. This makes TTX the drug of choice when long-lasting inactivation is required. If the experiment requires faster inactivation, local anesthetics such as lidocaine can be used since it takes only a few seconds to reach a maximum blockade. The effects of this drug last for less than 20 minutes, providing a finer temporal resolution than TTX. The characteristics stated above make TTX a good tool for exploratory experiments that study the presence of neural signals that are generated in less well-defined temporal windows. Lidocaine, on the other hand, is useful for delineating this window44. A disadvantage of both drugs is that they block the transmission of action potentials in fibers of passage, introducing artifacts in the interpretation of the data since the signal could potentially come from a different structure that sends axonal projections to the injected area. In this case, careful analysis of the injections determined to be outside of the target area are very important. For example, in this article it was shown that TTX infusion into the area just anterior to the suprachiasmatic nucleus and into the region between it and the arcuate nucleus did not block ovulation. Such results revealed that the fibers and cells in those regions are not involved in the regulation of ovulation, which was unexpected due to the presence of a reciprocal net of fibers linking the suprachiasmatic nucleus with both the arcuate nucleus and the anterior regions containing GnRH neurons.
An alternative that does not block the transmission along fibers of passage in the injected area is the use of divalent cations such as cobalt and magnesium, which both inhibit the release of neurotransmitters by presynaptic terminals. The problem with this approach, however, is the very short time of inactivating action and high toxicity44. Other options to avoid blockade of fibers are the optogenetic and chemogenetic approaches, both of which are strategies for the manipulation of the activity of specific neuronal populations. Selectivity is gained by transfecting neurons with viral vectors designed to insert sequences that codify for either opsins bound to ion channels or modified receptors expressed under specific promoters. The microinjection of the vector is performed in anesthetized animals using glass capillaries, causing little disruption of the surrounding tissue. The implantation of optic fibers or the systemic injection of synthetic drugs allows for a very precise manipulation of the target population45. These techniques proved useful in a study, either alone or in conjunction with techniques such as the TTX microinjection presented in this protocol46. Still, several aspects of these methods must be considered to design successful experiments, including the validation of the specificity of expression, controlling for the number of cells transfected, assessment of toxicity and evaluation of the side effects of light and chemical stimulation.
Critical components of this protocol included proper determination of the stage of the estrous cycle for each animal. To perform this procedure successfully, obtaining quality samples of the vaginal epithelium and correctly interpreting the results are pivotal (we advise following Figure 1). Additionally, researchers must carefully fill the injection system to prevent the incorporation of air bubbles that could result in the injection of inaccurate volumes of drugs. It is also important to monitor the animals during the microinjection to prevent them from biting the tubing or otherwise interfering with the system. Finally, researchers blind to experimental conditions should analyze the outcome of ovulation, the final position of the cannula and the dispersion of the solution into the target area to ensure that the interpretation of results is unbiased.
Here a reliable, cost-efficient and straightforward method to analyze the contribution of discrete areas of the brain in the regulation of ovulation was described. This procedure is performed in awake and unrestrained rats, surpassing the deleterious effects of the blockade of neurotransmission and also the inhibitory effects that stress hormones such as cortisol and corticosterone exerts on GnRH secretion. This method is fully customizable and can be used to deliver any drug into any structure of the brain. In addition, it can be easily adapted for common rodent and non-rodent species used in the laboratory. Finally, this protocol can be used in conjunction with methods for the quantification of substances such as hormones and metabolites in the blood, assessment of gene expression in cells and tissues, recording of neuronal activity and also the performance of awake animals in behavioral tests to determine the role of specific brain areas in diverse behavioral and physiological processes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are grateful to Raymond Sanchez at the University of Washington for his valuable help in manuscript editing and to M.Sc. Georgina Cortés and M.Sc. Cintia Javier for their technical support in the standardization of this technique. We are also grateful to the members of the vet services at Facultad de Estudios Superiores Zaragoza: MVZ. Adriana Altamirano, MVZ. Roman Hernández and MVZ. Dolores-Elizabeth Guzmán for the excellent maintenance and care of experimental animals. The experiments described in this protocol were supported by DGAPA-PAPIIT grant number: IN216015 and by CONACyT grant number: 236908 to Roberto Domínguez. Carlos-Camilo Silva is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM) and is supported by the Consejo Nacional de Ciencia y Tecnología (Grant number: 294555).
Materials
| 10 μL Hamilton syringes | Hamilton | 80314 | |
| 21 G x 1" stainless steel hypdermic needle | BD | 305165 | |
| 23 G x 1" stainless steel hypdermic needle | BD | 305145 | |
| 30 G x 1/2" stainless steel hypdermic needle | BD | 305106 | |
| Artificial cerebrospinal fluid | BASi | MD-2400 | |
| Bone trimer | Fine Science Tools | 16152-12 | |
| Burr for micro drill | Fine Science Tools | 19007-05 | |
| Clipper | Wahl | ||
| Cut-off disc | Dremel | SM5010 | |
| Cutting tweezers | Truper | 17367 | |
| Cyanocrylate glue | Kola loka | K-1 | |
| Dental cement | Nic Tone | ||
| Enrofloxasin | Senosiain | ||
| Eosin | Sigma | E4009 | |
| Estereoscope | Zeiss | ||
| Extra fine Bonn scissors | Fine Science Tools | 14084-08 | |
| Face mask | Lanceta HG | 60036 | |
| Graefe Forceps | Fine Science Tools | 11050-10 | |
| Hematoxilin | Sigma | H3136 | |
| Hemostats | Fine Science Tools | 13008-12 | |
| Hot bead sterilizer | Fine Science Tools | 18000-45 | |
| Hydrochloric acid | Sigma | 320331 | |
| Hypromelose artificial tears | Sophia Labs | 8950015 | |
| Isoflurane | Pisa Agropecuaria | ||
| Meloxicam | Aranda | 1183 | |
| Microinjection pump | KD Scientific | 788380 | |
| Monomer | Nic Tone | ||
| Mototool | Dremel | 3000 | |
| Nitrile gloves | Lanceta HG | 69028 | |
| Non-Rupture Ear Bars | David Kopf Instruments | 855 | |
| Poly-L lysine | Sigma | P4707 | |
| Povidone-iodine | Dermo Dine | ||
| Povidone-iodine with soap | Germisin espuma | ||
| Pressure tweezers | Truper | 17371 | |
| Rat anesthesia mask | David Kopf Instruments | Model 906 | |
| Saline solution | PISA | ||
| Scalpel | Fine Science Tools | 10004-13 | |
| Scalpel blade | Fine Science Tools | 10015-00 | |
| Sodium pentobarbital | Pisa Agropecuaria | ||
| Standard electrode holder | David Kopf Instruments | 1770 | |
| Stainless steel wire | American Orthodontic | 856-612 | |
| Stereotaxic apparatus | David Kopf Instruments | Model 900LS | |
| Surgical Sissors | Fine Science Tools | 14001-12 | |
| Teflon connectors | Basi | MD-1510 | |
| Teflon tubing | Basi | MF-5164 | |
| Tetrodotoxin | Alomone labs | T-500 | |
| Vaporizer | Kent scientific | VetFlo |
References
- Herbison, A. E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nature Reviews Endocrinology. 12 (8), 452-466 (2016).
- Fink, G., Conn, M., Freeman, E. Neuroendocrine Regulation of Pituitary Function. Neuroendocrinology in Physiology and Medicine. , 107-133 (2000).
- Herbison, A. E. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology. 159 (11), 3723-3736 (2018).
- Morello, H., Taleisnik, S. Changes of the release of luteinizing hormone (LH) on the day of proestrus after lesions or stimulation of the raphe nuclei in rats. Brain Research. 360 (1-2), 311-317 (1985).
- Slusher, M. A., Critchlow, V. Effect of Midbrain Lesions on Ovulation and Adrenal Response to Stress in Female Rats. Experimental Biology and Medicine. 101 (3), 497-499 (1959).
- Sawyer, C. H., Haun, C. K., Hilliard, J., Radford, H. M., Kanematsu, S. Further Evidence for the Identity of Hypothalamic Areas Controlling Ovulation and Lactation in the Rabbit. Endocrinology. 73 (3), 338-344 (1963).
- Schiavi, R., Jutisz, M., Sakiz, E., Guillemin, R. Stimulation of Ovulation by Purified LH-Releasing Factor (LRF) in Animals Rendered Anovulatory by Hypothalamic Lesion. Experimental Biology and Medicine. 114 (2), 426-429 (1963).
- Bagga, N., Chhina, G. S., Mohan Kumar, V., Singh, B. Cholinergic activation of medial preoptic area by amygdala for ovulation in rat. Physiology & Behavior. 32 (1), 45-48 (1984).
- Barraclough, C. A., Yrarrazaval, S., Hatton, R. A Possible Hypothalamic Site of Action of Progesterone in the Facilitation of Ovulation in the Rat. Endocrinology. 75 (6), 838-845 (1964).
- Critchlow, V. Blockade of ovulation in the rat by mesencephalic lesions 1, 2. Endocrinology. 63 (5), 596-610 (1958).
- Terasawa, E., Wiegand, S. J. Effects of Hypothalamic Deafferentation on Ovulation and Estrous Cyclicity in the Female Guinea Pig. Neuroendocrinology. 26 (4), 229-248 (1978).
- Halász, B., Köves, K., Molnár, J. Neural control of ovulation. Human Reproduction. 3 (1), 33-37 (1988).
- Narahashi, T. Pharmacology of tetrodotoxin. Journal of Toxicology: Toxin Reviews. 20 (1), 67-84 (2001).
- Narahashi, T., Moore, J. W., Scott, W. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. The Journal of General Physiology. 47 (5), 965-974 (1964).
- Narahashi, T., Deguchi, T., Urakawa, N., Ohkubo, Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. American Journal of Physiology-Legacy Content. 198 (5), 934-938 (1960).
- Narahashi, T. Chemicals as tools in the study of excitable membranes. Physiological Reviews. 54 (4), 813-889 (1974).
- Ritchie, J. M., Rogart, R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Reviews of Physiology, Biochemistry and Pharmacology. 79 (1), 1-50 (1977).
- Bermudez-Rattoni, F., Introini-Collison, I. B., McGaugh, J. L. Reversible inactivation of the insular cortex by tetrodotoxin produces retrograde and anterograde amnesia for inhibitory avoidance and spatial learning. Proceedings of the National Academy of Sciences. 88 (12), 5379-5382 (1991).
- Tang, X., Yang, L., Liu, X., Sanford, L. D. Influence of Tetrodotoxin Inactivation of the Central Nucleus of the Amygdala on Sleep and Arousal. Sleep. 28 (8), 923-930 (2005).
- Klement, D., Pašt’alková, E., Fenton, A. A. Tetrodotoxin infusions into the dorsal hippocampus block non-locomotor place recognition. Hippocampus. 15 (4), 460-471 (2005).
- Conejo, N. M., Cimadevilla, J. M., González-Pardo, H., Méndez-Couz, M., Arias, J. L. Hippocampal Inactivation with TTX Impairs Long-Term Spatial Memory Retrieval and Modifies Brain Metabolic Activity. PLoS ONE. 8 (5), 64749 (2013).
- Grimm, J., Ronald, E. Dissociation of Primary and Secondary Reward-Relevant Limbic Nuclei in an Animal Model of Relapse. Neuropsychopharmacology. 22 (5), 473-479 (2000).
- Hasegawa, H., et al. Inhibition of the preoptic area and anterior hypothalamus by tetrodotoxin alters thermoregulatory functions in exercising rats. Journal of Applied Physiology. 98 (4), 1458-1462 (2005).
- Meyer, F., Louilot, A. Early Prefrontal Functional Blockade in Rats Results in Schizophrenia-Related Anomalies in Behavior and Dopamine. Neuropsychopharmacology. 37 (10), 2233-2243 (2012).
- Rothfeld, J. M., Harlan, R. E., Shivers, B. D. Reversible disruption of lordosis via midbrain infusions of procaine and tetrodotoxin. Pharmacology Biochemistry and Behavior. 25 (4), 857-863 (1986).
- Silva, C., Cortés, G. D., Javier, C. Y., Flores, A., Domínguez, R. A neural circadian signal essential for ovulation is generated in the suprachiasmatic nucleus during each stage of the estrous cycle. Experimental Physiology. , (2019).
- Paxinos, G., Watson, C. . The Rat Brain in Stereotaxic Coordinates (7th Ed). , (2014).
- Cora, M. C., Kooistra, L., Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse. Toxicologic Pathology. 43 (6), 776-793 (2015).
- Byers, S. L., Wiles, M. V., Dunn, S. L., Taft, R. A. Mouse Estrous Cycle Identification Tool and Images. PLoS ONE. 7 (4), 35538 (2012).
- Wirtshafter, D., Asin, K., Kent, E. W. Simple technique for midline stereotaxic surgery in the rat. Physiology & Behavior. 23 (1), 409-410 (1979).
- Kozai, T. D., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C., Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chemical Neuroscience. 6 (1), 48-67 (2015).
- Kazim, S. F., Enam, S. A., Shamim, M. S. Possible detrimental effects of neurosurgical irrigation fluids on neural tissue: An evidence based analysis of various irrigants used in contemporary neurosurgical practice. International Journal of Surgery. 8 (8), 586-590 (2010).
- Miyajima, M., et al. Role of cerebrospinal fluid as perfusate in neuroendoscopic surgery: A basic investigation. Acta Neurochirurgica. 113, 103-107 (2012).
- Mori, K., et al. Potential risk of artificial cerebrospinal fluid solution without magnesium ion for cerebral irrigation and perfusion in neurosurgical practice. Neurologia Medico-Chirurgica. 53 (9), 596-600 (2013).
- Oka, K., Yamamoto, M., Nonaka, T., Tomonaga, M. The significance of artificial cerebrospinal fluid as perfusate and endoneurosurgery. Neurosurgery. 38 (4), (1996).
- James, T. A., Starr, M. S. Effects of the rate and volume of injection on the pharmacological response elicited by intraingral microapplication of drugs in the rat. Journal of Pharmacological Methods. 1 (3), 197-202 (1978).
- Freund, N., Manns, M., Rose, J. A method for the evaluation of intracranial tetrodotoxin injections. Journal of Neuroscience Methods. 186 (1), 25-28 (2010).
- Zhuravin, I. A., Bures, J. Extent of the tetrodotoxin induced blockade examined by pupillary paralysis elicited by intracerebral injection of the drug. Experimental Brain Research. 83 (3), 687-690 (1991).
- Myers, R. Injection of solutions into cerebral tissue: relation between volume and diffusion. Physiology and Behavior. 1 (2), 171-174 (1966).
- Gonzalez-Perez, O., Guerrero-Cazares, H., Quiñones-Hinojosa, A. Targeting of deep brain structures with microinjections for delivery of drugs, viral vectors, or cell transplants. Journal of Visualized Experiments. (46), e2082 (2010).
- McCluskey, L., Campbell, S., Anthony, D., Allan, S. M. Inflammatory responses in the rat brain in response to different methods of intra-cerebral administration. J Neuroimmunol. 194 (1-2), 27-33 (2008).
- Cunningham, M. G., O’Connor, R. P., Wong, S. E. Construction and implantation of a microinfusion system for sustained delivery of neuroactive agents. Journal of VisualizedExperiments. (13), e716 (2008).
- Akinori, A., Masamichi, S., Hiroshi, T. A new device for microinjection of drugs into the lower brain stem of conscious rats: Studies on site of action of morphine. Journal of Pharmacological Methods. 2 (4), 371-378 (1979).
- Malpeli, J. G. Reversible inactivation of subcortical sites by drug injection. Journal of Neuroscience Methods. 86 (2), 119-128 (1999).
- Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M., Deisseroth, K. Optogenetics in neural systems. Neuron. 71 (1), 9-34 (2011).
- de Sousa, A. F., et al. Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proceedings of the Natural Academy of Sciences. 116 (17), 8576-8581 (2019).
- Beppu, K., et al. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron. 81 (2), 314-320 (2014).

