A Method to Preserve Wetland Roots and Rhizospheres for Elemental Imaging
Summary
We describe a protocol to sample, preserve, and section intact roots and the surrounding rhizosphere soil from wetland environments using rice (Oryza sativa L.) as a model species. Once preserved, the sample can be analyzed using elemental imaging techniques, such as synchrotron X-ray fluorescence (XRF) chemical speciation imaging.
Abstract
Roots extensively interact with their soil environment but visualizing such interactions between roots and the surrounding rhizosphere is challenging. The rhizosphere chemistry of wetland plants is particularly challenging to capture because of steep oxygen gradients from the roots to the bulk soil. Here a protocol is described that effectively preserves root structure and rhizosphere chemistry of wetland plants through slam-freezing and freeze drying. Slam-freezing, where the sample is frozen between copper blocks pre-cooled with liquid nitrogen, minimizes root damage and sample distortion that can occur with flash-freezing while still minimizing chemical speciation changes. While sample distortion is still possible, the ability to obtain multiple samples quickly and with minimal cost increases the potential to obtain satisfactory samples and optimizes imaging time. The data show that this method is successful in preserving reduced arsenic species in rice roots and rhizospheres associated with iron plaques. This method can be adopted for studies of plant-soil relationships in a wide variety of wetland environments that span concentration ranges from trace-element cycling to phytoremediation applications.
Introduction
Roots and their rhizospheres are dynamic, heterogeneous, and critically important for understanding how plants obtain mineral nutrients and contaminants1,2,3. Roots are the primary pathway by which nutrients (e.g., phosphorus) and contaminants (e.g., arsenic) move from soil to plants and thus understanding this process has implications for food quantity and quality, ecosystem functioning, and phytoremediation. However, roots are dynamic in space and time growing in response to nutrient acquisition needs and they often vary in function, diameter, and structure (e.g., lateral roots, adventitious roots, root hairs)2. Heterogeneity of root systems can be studied on spatial scales from cellular to ecosystem-level and on temporal scales from hourly to decadal. Thus, the dynamic and heterogeneous nature of roots and their surrounding soil, or rhizosphere, poses challenges for capturing rhizosphere chemistry over time. Despite this challenge, it is imperative to study roots in their soil environment to characterize this critical plant-soil relationship.
The rhizosphere chemistry of wetland plants is particularly challenging to investigate because of steep oxygen gradients that exist from bulk soil to the roots, which change in space and time. Because roots need oxygen to respire, wetland plants have adapted to the low oxygen conditions of wetland soils by creating aerenchyma4, 5. Aerenchyma are hollowed cortical tissues that extend from shoots to roots, allowing the diffusion of air through the plant into the roots. However, some of this air leaks into the rhizosphere in less suberized parts of the roots particularly near lateral root junctions, less mature root tips and elongation zones6,7,8,9. This radial oxygen loss creates an oxidized zone in the rhizosphere of wetland plants that affects rhizosphere (bio-geo)chemistry and is distinct from the reduced bulk soil10,11,12. To understand the fate and transport of nutrients and contaminants in wetland rhizospheres and roots, it is critical to preserve the chemically reduced bulk soil, the oxidized rhizosphere, and roots of wetland plants for analysis. However, because the bulk soil contains reduced soil constituents that are oxygen-sensitive, root and soil preservation methods must preserve root structures and minimize oxygen-sensitive reactions.
Methods exist to fix plant tissues and preserve the ultrastructure for imaging, but those methods cannot be applied to chemically preserve roots growing in wetland soil. For investigations where only the elemental distribution within plant cells is desired, plants are typically grown hydroponically and roots can be easily removed from solution, fixed under high-pressure freezing and freeze substitution and sectioned for a variety of imaging applications including high-resolution secondary ion mass spectrometry (nanoSIMS), electron microscopy, and synchrotron X-ray fluorescence (S-XRF) analysis13,14,15. To investigate Fe plaque on the outside of wetland roots, these hydroponic studies must artificially induce Fe plaque formation in solution16, which does not accurately represent the heterogeneity of the distribution and mineral composition of Fe plaque formation and associated elements in situ17,18,19,20. Methods exist to preserve wetland soil and associated microorganisms with freeze-coring21, but it is difficult to obtain roots with this technique. Current methods to visualize roots growing in soil and their rhizospheric chemistry consist of two primary measurement types: elemental fluxes and total elemental concentration (and speciation). The former is typically measured using diffusive gradients in thin films (DGT)22,23,24, in which soil is placed into rhizoboxes to support plant growth in a laboratory setting and labile elements in the soil diffuse through a gel into a binding layer. This binding layer can then be imaged to quantify the labile elements of interest. This technique can successfully illustrate relationships between roots and the rhizosphere24,25,26,27, but artefacts from root-bounding may exist by growing plants in rhizoboxes, and information on the root interior is not captured with DGT. The latter involves sampling of the roots and rhizosphere, preserving the sample, and directly analyzing elemental distribution on a sample section. For this environmental sampling of wetland plant roots and their surrounding rhizosphere, careful sample handling is required to avoid artefacts from sample preparation.
Here a protocol is described that effectively preserves root structures and rhizosphere chemistry of wetland plants by slam-freezing and freeze drying. Flash-freezing can drastically slow down transformations of oxygen sensitive solutes but may damage roots and may cause mobilization when samples dry out. However, slam-freezing where the sample is frozen between copper blocks pre-cooled with liquid nitrogen minimizes root damage and sample distortion28. The preserved samples are then embedded in an epoxy resin that preserves As speciation20, 29 and can be cut and polished for imaging of roots within their rhizosphere soil. The samples in this report were analyzed by S-XRF chemical speciation imaging after thin sectioning. However, other imaging techniques could also be used, including laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS), particle induced x-ray emission (PIXE), secondary ion mass spectrometry (SIMS), and laser induced breakdown spectroscopy (LIBS) imaging.
Protocol
1. Preparation of slam-freezing equipment
- Place two copper blocks (~5 cm x 5 cm x 15 cm) horizontally inside of a clean cooler capable of holding liquid nitrogen and pour enough liquid nitrogen to submerge the blocks. Once the bubbling subsides, place two spacers on top of one copper block at each end.
NOTE: The spacer height determines the height of the sample to be frozen; this example uses a 2 cm spacer to create cubes approximately 3 cm x 3 cm x 2 cm. The volume of the liquid nitrogen will depend on the cooler size. This example uses approximately 1 L for approximately 5 cubes in series.
CAUTION: Use proper personal protective equipment and ventilation as liquid nitrogen is a cryogen and an asphyxiant. - Using tongs and cryogenic gloves, stand up the other copper block on its end, to make retrieval easier when the sample is in place.
2. Sample collection and slam-freezing
- Extract the desired plant and rhizosphere from the wet soil using a shovel and ensure that the dug hole is much larger than the desired root volume. Place the soil and plant into a container and place it on a benchtop.
NOTE: The entire potted soil and plant from a pot study can also be used. - Determine the desired soil location where roots are to be taken (i.e., depth and proximity to the shoot). Cut away excess soil using a steel blade, taking care not to disturb soil in the desired area. When the desired area is reached, cut a root "cube" approximately 3 cm x 3 cm x 2 cm and immediately place the cube between the two spacers on the horizontal copper block. Using cryogenic gloves, pick up the vertical copper block and place it on top of the spacers to slam-freeze the rhizosphere cube.
- After bubbling subsides (~5 min), retrieve the slam-frozen rhizosphere cube from the copper blocks and wrap inside of a pre-labeled aluminum foil square. Mark the orientation of the block on the foil if desired. Place in a second container of liquid nitrogen until storage in a -80 °C freezer.
- Repeat as needed to obtain the desired number of root cubes from the field site or the experiment. Ensure both copper blocks are given time to cool between samples.
3. Freeze-drying and embedding rhizosphere cubes
- Prepare the freeze dryer according to the manufacturer's instructions. Take care to ensure it has obtained the proper vacuum pressure and temperature prior to removing samples from the -80 °C freezer.
- When freeze dryer is ready to receive samples, place one frozen rhizosphere cube inside of a clean and acid-washed 50 mL tube and cover loosely with a clean disposable wipe. Secure the wipe with a rubber band. Repeat as needed to ensure one cube per tube.
NOTE: If the sample is too large for a tube, it can be placed directly into the freeze dryer vessel using the aluminum foil as a sample holder. - Place tubes containing samples in freeze dryer vessels and freeze dry for several days. The exact drying time will depend on soil properties.
NOTE: Store dried samples in the freeze dryer or a desiccator to avoid rehydration. - Use a steel blade to cut dried soil cubes to size so that they fit into the desired form (e.g., 25 mm diameter form is ideal for most applications). Label each form, place the soil cubes in the forms and place the forms inside a vacuum desiccator.
- Prepare epoxy according to manufacturer's instructions. Ensure that the chosen epoxy is not contaminated with and does not cause speciation changes of desired elements 20, 29, 30.
- Use a dropper to add epoxy to the form on one side of the soil, till it entirely covers the sample. The soil will darken in color as the epoxy wets the soil.
NOTE: Add the epoxy slowly to allow the air in the soil to escape. - Once forms are filled with epoxy, close the vacuum desiccator and turn on the vacuum. Depending on the amount of air trapped in the soil, more epoxy may need to be added to the forms periodically. Check the level of epoxy every 30-90 min for the first 1-4 h and add epoxy as needed.
- Remove the sample from the form once the epoxy has hardened (~5 days).
4. Cutting and sectioning the rhizosphere cubes
- Cut the sample using a diamond blade precision wet saw. Cut the samples in different locations if no roots are obtained in the previous cut.
- Manually sand the cut samples with progressively finer sandpaper (e.g., 220, 500, 1000, and 1500 grit) on the cut side for ~30 s.
- Perform surface imaging of the samples using techniques such as LA-ICP-MS.
NOTE: To prepare thin sections for S-XRF, either send the samples out to a company capable of preparing the thin sections (single or double side polishing) or follow the steps 4.4 – 4.6 as described below. - Glue the desired sample side to a quartz slide using super glue and allow to cure overnight.
- Using a thin sectioning machine, cut soil on slides to 2 mm thick and then grind to the desired thickness (typically 30 µm). The sample surface can be polished if desired.
- Perform S-XRF imaging of the sections. Follow the appropriate steps at the desired synchrotron facility and beamline to apply for and utilize imaging time.
Representative Results
This method allows for preservation of roots and chemical species in the roots and rhizosphere of wetland plants and into the bulk soil. In this work, the method was used to evaluate As speciation and co-localization with Fe and Mn oxides and plant nutrients in the rhizosphere of rice (Oryza sativa L.). Rice was grown at the RICE Facility at the University of Delaware where 30 rice paddy mesocosms (2 m x 2 m, 49 plants each) are used to grow rice under various soil and water management conditions with the goal of lowering As and Cd uptake into rice grain. This experiment provided 1470 individual plants from which rhizospheres could be sampled throughout the growing season.
Given a sufficient number of samples, thin sections were able to capture a variety of root morphologies. Figure 1A shows several root diameters present within the soil matrix as transverse sections. However, some soil sections may contain few, if any, roots. In this work, 63 soil blocks were processed and cut once on the wet saw to determine which subset of samples were suitable for thin sectioning. Of the 63 samples, 14 contained no roots, 31 contained 1-3 roots and 18 contained more than 3 roots. Note that the roots may be present in varying levels of quality. Figure 1B shows a well-preserved root, a root distorted by the freeze-drying process, and a root that was pulled out during the thin sectioning process.
Root thin sections were analyzed using S-XRF to map the location of elements of interest. Figure 2B shows a root transverse section with a lateral root in longitudinal section. Figure 2C shows this root section analyzed by XRF with a tricolor plot of Fe, Mn, and As. The Fe is present in the soil and surrounding the root in the Fe plaque, and Fe plaque is also visible on the light micrograph images. Manganese is uniquely present in the cortex of the lateral root, but also co-locates with Fe in some areas in the Fe plaque, appearing as a green-blue hue. Arsenic was mostly present in the vasculature of the lateral root, merging into the vasculature of the primary root.
Chemical speciation imaging separated the various As species of interest by taking repeated XRF maps at multiple incident beam energies and using linear combination fitting to standard As XANES spectra. The As speciation maps are shown in Figure 2D and show variability in the localization of As species. Figure 2E shows the same data as a tricolor plot. The tricolor plot shows arsenite and arsenite glutathione closely associated in the vasculature, while arsenate is primarily located on the exterior of the root associated with Fe plaque.
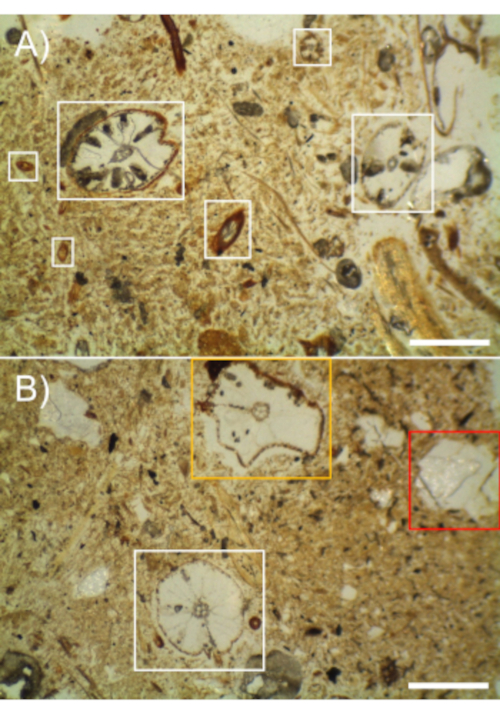
Figure 1 – Rice root thin sections showing a variety of methodological outcomes in a silty loam soil (scale bars are 0.5 mm). A) Numerous different root diameter cross-sections are evident (in white boxes). B) Roots may be damaged during the process. The root in the white box has remained intact and circular, while the root in the orange box has been compressed during the freeze-drying process. The red box shows where a root was pulled out during the thin sectioning process. Please click here to view a larger version of this figure.
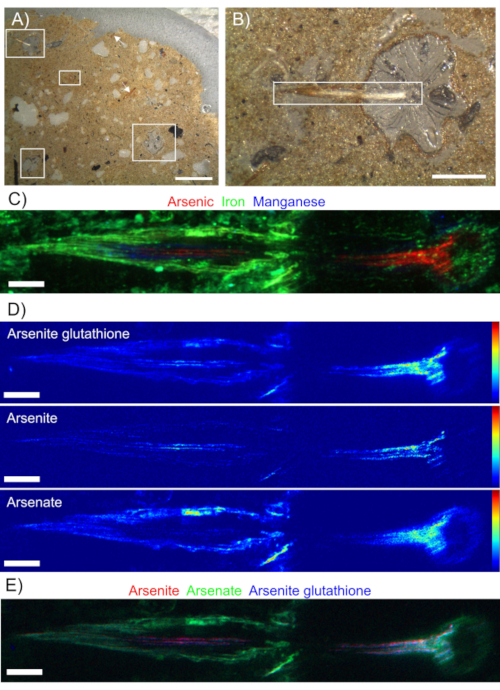
Figure 2 - Transverse section of a rice root with a longitudinal section of a lateral root in a silty loam soil. A) Soil section showing several root transverse sections in white boxes. White arrows denote longitudinal sections. Scale bar is 2 mm. B) Soil section showing root from upper left corner of panel A. White rectangle denotes area imaged by synchrotron XRF. Scale bar is 0.5 mm. C) Tricolor XRF image of arsenic (red), iron (green), and manganese (blue). The maximum scale of As, Fe, and Mn are in a ratio of 1:50:2.5. Scale bar is 100 µm. D) Arsenic XRF speciation maps for arsenite glutathione, arsenite, and arsenate, where warmer colors indicate higher concentrations of As. Scale bar is 100 µm. E) Tricolor plot of As species, where the maximum intensity is scaled to arsenite (red) = arsenate (green) = 0.5 arsenite glutathione (blue). Scale bar is 100 µm. Please click here to view a larger version of this figure.
Discussion
This paper describes a protocol to obtain preserved bulk soil + rhizospheres of wetland plant roots using a slam-freezing technique that can be used for elemental imaging and/or chemical speciation mapping.
There are several benefits of this method over existing methods. First, this method allows the simultaneous investigation of roots and the surrounding rhizospheres. Methods currently exist to preserve and chemically image roots out of their soil environment by washing away the soil and preserving roots31, 32 or by growing plants in artificial environments (e.g., rhizoboxes) and using DGT methods to examine root-soil interactions24, 33, 34 but without the ability to observe the root itself. The method described here allows for the direct investigation of the root and surrounding rhizosphere soil in situ for observation of root-soil relationships. A similar technique has been used to examine in situ rice roots and surrounding rhizosphere but with plunging the sample into liquid nitrogen11, 35, 36 rather than slam-freezing described here. The faster tissues are frozen, the less likely they are to form ice crystals37. Slam-freezing between pre-cooled copper blocks rapidly cools the sample and therefore minimizes the formation of ice crystals and subsequent plant tissue damage that can occur with flash-freezing using liquid nitrogen at ambient temperatures11, 38. Using the method described here, several samples can be taken from the same plant, multiple plants from a field, and/or the environment in a relatively short period of time. Once obtained, many samples can be freeze-dried, embedded in epoxy, and cut with a diamond blade wet saw with minimal cost. These samples can then be investigated with a light microscope to identify promising samples that can be directly imaged (e.g., LA-ICP-MS) or further processed for thin sectioning and sXRF imaging. One thin section can capture multiple roots of various sizes on the same slide, which helps to capture the heterogeneous rhizosphere and maximize imaging time on the instrument. This method also can be used to directly observe plant-soil relationships such as As sequestration in Fe plaques without disturbing the rhizosphere. Existing methods to induce Fe plaque formation on wetland roots like rice using hydroponic experiments39,40,41 fail to capture the heterogeneity of Fe plaque in terms of root coverage and mineral composition that occurs in soil-grown plants11, 18, 20, 42,43,44.
For the method to be successful, it is critical to follow a few key steps. First, ensure that the sample location and orientation selected addresses the desired question. Second, use an epoxy that is free of trace element contamination and has been shown to preserve chemical speciation of As elements of interest20, 29, 30. Third, add epoxy slowly and place the sample in epoxy under vacuum to facilitate epoxy wetting of the sample and removal of entrapped gas. Following these steps will provide a high-quality sample of bulk soil, rhizosphere, and roots that can be used for image analysis.
Several limitations of the method should be considered. First, drying the frozen sample on a freeze-dryer can cause deformation of the soil, which may affect roots. This is likely to be particularly challenging in soils with high clay content and thus propensity for collapse as clays dry. As an example, a rice rhizosphere sample obtained from a silty clay was prepared and cracking of the soil is apparent whereas the Fe plaque-coated rice root is unaffected (Figure 3).
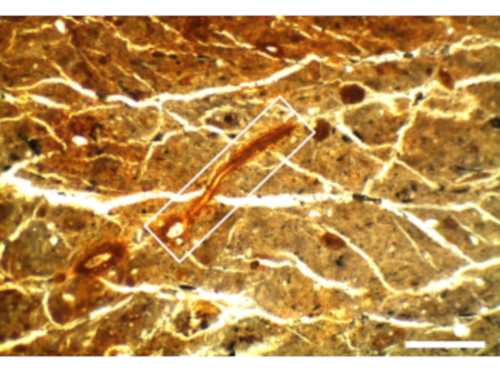
Figure 3 - Rice root thin section from a silty clay paddy soil. Numerous cracks in the soil have occurred during freeze-drying, but these cracks did not distort the lateral root longitudinal section, which is depicted by the white rectangle. Scale bar is 0.5 mm. Please click here to view a larger version of this figure.
The data show that this technique can successfully obtain micron-scale information in a silt loam (Figures 2, 3), and it is likely that the technique can be successful in coarser textured soils; however, soils with higher clay content might pose challenges and should be investigated further. Second, roots can be pulled out of the soil upon sectioning. This challenge is not unique to the protocol described in this paper but should be considered. Third, roots may not be present in every soil cube, so many samples need to be obtained and cut to capture the rhizosphere of the desired plant. Fourth, the preservation method requires liquid nitrogen, which might pose challenges for remote field studies. Here, the protocol has been successfully used in the field, which was less than 2 miles from a liquid nitrogen Dewar. However, if liquid nitrogen is not available within a short drive from a remote field site, several options exist to obtain the sample. This includes using another source to cool the copper blocks or excavating the entire plant and surrounding soil with a large PVC ring, placing this into gas-impermeable material, and transporting to the nearest liquid nitrogen source for preservation. For this, it is important to ensure that the plant shoot is not cut from its roots prior to obtaining the rhizosphere sample. If needed, the sample can also be placed under refrigeration and shipped overnight to the laboratory for preservation. Once received in the laboratory, sections can then be preserved using liquid nitrogen-cooled copper blocks. Finally, speciation changes are possible with any preservation method of wetland soils and rhizospheres. To avoid this, samples must be obtained and slam-frozen quickly or other measures taken as above to avoid exposure to oxygen. The edges of freeze-dried samples can then be shaved to avoid edges that may have had higher exposure to oxygen. The preservation of reduced arsenic species in the root and rhizosphere samples here (Figure 1D, E) and in previous work28 suggests that this slam-freezing technique is able to preserve oxygen-sensitive chemical species if carefully performed.
This method can be used to address several key questions in rhizosphere science. These include applications related to studying nutrient and contaminant interactions in the rhizosphere that may include interactions of contaminants and nutrients with Fe or Mn plaques. The method allows for the study of temporal and spatial heterogeneity of plant-soil relationships and the examination of how root morphologies interact with elements in the rhizosphere in situ. It can be used in applications related to food security such as in understanding arsenic uptake by rice, nutrient dynamics in the rhizosphere, or applications related to phytoremedation such as metal(loid) uptake into wetland plants.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge a joint seed grant to Seyfferth and Tappero to support collaboration between the University of Delaware and Brookhaven National Laboratory. Parts of this research used the XFM (4-BM) Beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
Materials
| Copper blocks | McMaster Carr | 89275K42 | |
| Diamond blade | Buehler | 15 LC, 102 mm x 0.3 mm | operation speed: 225 rpm |
| Epoxy forms | Struers | 40300085 | FixiForm |
| Epoxy | Epotek | 301-2FL | |
| Superglue | Loctite | 404 | |
| Thin sectioning machine | Buehler | PetroThin | |
| Wet saw | Buehler | IsoMet 1000 |
References
- Ahkami, A. H., White, R. A., Handakumbura, P. P., Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere. 3 (2), 233-243 (2017).
- McNear, D. H. The rhizosphere – roots, soil and everything in between. Nature Education Knowledge. 4 (3), 1 (2013).
- Berendsen, R. L., Pieterse, C. M. J., Bakker, P. A. H. M. The rhizosphere microbiome and plant health. Trends in Plant Science. 17 (8), 478-486 (2012).
- Armstrong, W., Justin, S., Beckett, P. M., Lythe, S. Root adaptation to soil waterlogging. Aquatic Botany. 39 (1-2), 57-73 (1991).
- Armstrong, W. Oxidising activity of roots in waterlogged soils. Physiologia Plantarum. 20 (4), 920-926 (1967).
- Armstrong, W. Oxygen diffusion from roots of some Brittish bog plants. Nature. 204 (496), 801-802 (1964).
- Li, H., Ye, Z. H., Wei, Z. J., Wong, M. H. Root porosity and radial oxygen loss related to arsenic tolerance and uptake in wetland plants. Environmental Pollution. 159 (1), 30-37 (2011).
- Kotula, L., Ranathunge, K., Steudle, E. Apoplastic barriers effectively block oxygen permeability across outer cell layers of rice roots under deoxygenated conditions: roles of apoplastic pores and of respiration. New Phytologist. 184 (4), 909-917 (2009).
- Mei, X. Q., Ye, Z. H., Wong, M. H. The relationship of root porosity and radial oxygen loss on arsenic tolerance and uptake in rice grains and straw. Environmental Pollution. 157 (8-9), 2550-2557 (2009).
- Khan, N., et al. Root Iron Plaque on Wetland Plants as a Dynamic Pool of Nutrients and Contaminants. Advances in Agronomy. 138, 1-96 (2016).
- Yamaguchi, N., Ohkura, T., Takahashi, Y., Maejima, Y., Arao, T. Arsenic Distribution and Speciation near Rice Roots Influenced by Iron Plaques and Redox Conditions of the Soil Matrix. Environmental Science and Technology. 48 (3), 1549-1556 (2014).
- Frommer, J., Voegelin, A., Dittmar, J., Marcus, M. A., Kretzschmar, R. Biogeochemical processes and arsenic enrichment around rice roots in paddy soil: results from micro-focused X-ray spectroscopy. European Journal of Soil Science. 62 (2), 305-317 (2011).
- Moore, K. L., et al. Combined NanoSIMS and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytologist. 201 (1), 104-115 (2014).
- vander Ent, A., et al. X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants. New Phytologist. 218 (2), 432-452 (2018).
- Sarret, G., Smits, E. A. H. P., Michel, H. C., Isaure, M. P., Zhao, F. J., Tappero, R. Use of Synchrotron-Based Techniques to Elucidate Metal Uptake and Metabolism in Plants. Advances in Agronomy. 119, 1-82 (2013).
- Moore, K. L., et al. High-Resolution Secondary Ion Mass Spectrometry Reveals the Contrasting Subcellular Distribution of Arsenic and Silicon in Rice Roots. Plant Physiology. 156 (2), 913-924 (2011).
- Seyfferth, A. L. Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant and Soil. 397 (1-2), (2015).
- Seyfferth, A. L., Webb, S. M., Andrews, J. C., Fendorf, S. Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L) roots having variable Fe coatings. Environmental Science and Technology. 44 (21), (2010).
- Amaral, D. C., Lopes, G., Guilherme, L. R. G., Seyfferth, A. L. A new approach to sampling Iintact Fe plaque reveals Si-induced changes in Fe mineral composition and shoot As in rice. Environmental Science and Technology. 51 (1), 38-45 (2017).
- Seyfferth, A. L., Webb, S. M., Andrews, J. C., Fendorf, S. Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochimica et Cosmochimica Acta. 75 (21), (2011).
- Franchini, A. G., Zeyer, J. Freeze-Coring Method for Characterization of Microbial Community Structure and Function in Wetland Soils at High Spatial Resolution. Applied and Environmental Microbiology. 78 (12), 4501-4504 (2012).
- Smolders, E., Wagner, S., Prohaska, T., Irrgeher, J., Santner, J. Sub-millimeter distribution of labile trace element fluxes in the rhizosphere explains differential effects of soil liming on cadmium and zinc uptake in maize. Science of the Total Environment. 738, 140311 (2020).
- Santner, J., et al. High-resolution chemical imaging of labile phosphorus in the rhizosphere of Brassica napus L. cultivars. Environmental and Experimental Botany. 77, 219-226 (2012).
- Williams, P. N., et al. Localized Flux Maxima of Arsenic, Lead, and Iron around Root Apices in Flooded Lowland Rice. Environmental Science and Technology. 48 (15), 8498-8506 (2014).
- Yin, D. X., et al. Localized Intensification of Arsenic Release within the Emergent Rice Rhizosphere. Environmental Science and Technology. 54 (6), 3138-3147 (2020).
- Santner, J., et al. High-resolution chemical imaging of labile phosphorus in the rhizosphere of Brassica napus L. cultivars. Environmental and Experimental Botany. 77, 219-226 (2012).
- Smolders, E., Wagner, S., Prohaska, T., Irrgeher, J., Santner, J. Sub-millimeter distribution of labile trace element fluxes in the rhizosphere explains differential effects of soil liming on cadmium and zinc uptake in maize. Science of the Total Environment. 738, 140311 (2020).
- Seyfferth, A. L., Ross, J., Webb, S. M. Evidence for the root-uptake of arsenite at lateral root junctions and root apices in rice (Oryza sativa L.). Soil Processes. 1, 3 (2017).
- Masue-Slowey, Y., Kocar, B. D., Jofre, S. A. B., Mayer, K. U., Fendorf, S. Transport Implications Resulting from Internal Redistribution of Arsenic and Iron within Constructed Soil Aggregates. Environmental Science and Technology. 45 (2), 582-588 (2011).
- Root, R. A., Fathordoobadi, S., Alday, F., Ela, W., Chorover, J. Microscale Speciation of Arsenic and Iron in Ferric-Based Sorbents Subjected to Simulated Landfill Conditions. Environmental Science and Technology. 47 (22), 12992-13000 (2013).
- Blute, N. K., Brabander, D. J., Hemond, H. F., Sutton, S. R., Newville, M. G., Rivers, M. L. Arsenic sequestration by ferric iron plaque on cattail roots. Environmental Science and Technology. 38 (22), 6074-6077 (2004).
- Hansel, C. M., La Force, M. J., Fendorf, S., Sutton, S. Spatial and temporal association of As and Fe species on aquatic plant roots. Environmental Science and Technology. 36 (9), 1988-1994 (2002).
- Yin, D. X., et al. Localized Intensification of Arsenic Release within the Emergent Rice Rhizosphere. Environmental Science and Technology. 54 (6), 3138-3147 (2020).
- Maisch, M., Lueder, U., Kappler, A., Schmidt, C. Iron Lung: How Rice Roots Induce Iron Redox Changes in the Rhizosphere and Create Niches for Microaerophilic Fe(II)-Oxidizing Bacteria. Environmental Science and Technology Letters. 6 (10), 600-605 (2019).
- Voegelin, A., Weber, F. -. A. A., Kretzschmar, R. Distribution and speciation of arsenic around roots in a contaminated riparian floodplain soil: Micro-XRF element mapping and EXAFS spectroscopy. Geochimica Et Cosmochimica Acta. 71 (23), 5804-5820 (2007).
- Smith, E., Kempson, I., Juhasz, A. L., Weber, J., Skinner, W. M., Grafe, M. Localization and speciation of arsenic and trace elements in rice tissues. Chemosphere. 76 (4), 529-535 (2009).
- Thompson, R. F., Walker, M., Siebert, C. A., Muench, S. P., Ranson, N. A. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods. 100, 3-15 (2016).
- Echlin, P., Lai, C., Hayes, T., Saubermann, A. Cryofixation of Lemna-minor roots for morphological and analytical studies. Cryoletters. 1 (9), 289-300 (1980).
- Ma, R., Shen, J. L., Wu, J. S., Tang, Z., Shen, Q. R., Zhao, F. J. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environmental Pollution. 194, 217-223 (2014).
- Chen, Z., Zhu, Y. G., Liu, W. J., Meharg, A. A. Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytologist. 165 (1), 91-97 (2005).
- Lee, C. H., Hsieh, Y. C., Lin, T. H., Lee, D. Y. Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice. Plant and Soil. 363 (1-2), 231-241 (2013).
- Seyfferth, A. L., Amaral, D. C., Limmer, M. A., Guilherme, L. R. G. Combined impacts of Si-rich rice residues and flooding extent on grain As and Cd in rice. Environment International. 128, 301-309 (2019).
- Seyfferth, A., Limmer, M., Wu, W. Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice. Soil Systems. 3 (3), (2019).
- Limmer, M. A., Mann, J., Amaral, D. C., Vargas, R., Seyfferth, A. L. Silicon-rich amendments in rice paddies: Effects on arsenic uptake and biogeochemistry. Science of the Total Environment. 624, 1360-1368 (2018).

