Multiphoton Intravital Imaging for Monitoring Leukocyte Recruitment during Arteriogenesis in a Murine Hindlimb Model
Summary
The recruitment of leukocytes and platelets constitutes an essential component necessary for the effective growth of collateral arteries during arteriogenesis. Multiphoton microscopy is an efficient tool for tracking cell dynamics with high spatio-temporal resolution in vivo and less photo-toxicity to study leukocyte recruitment and extravasation during arteriogenesis.
Abstract
Arteriogenesis strongly depends on leukocyte and platelet recruitment to the perivascular space of growing collateral vessels. The standard approach for analyzing collateral arteries and leukocytes in arteriogenesis is ex vivo (immuno-) histological methodology. However, this technique does not allow the measurement of dynamic processes such as blood flow, shear stress, cell-cell interactions, and particle velocity. This paper presents a protocol to monitor in vivo processes in growing collateral arteries during arteriogenesis utilizing intravital imaging. The method described here is a reliable tool for dynamics measurement and offers a high-contrast analysis with minimal photo-cytotoxicity, provided by multiphoton excitation microscopy. Prior to analyzing growing collateral arteries, arteriogenesis was induced in the adductor muscle of mice by unilateral ligation of the femoral artery.
After the ligation, the preexisting collateral arteries started to grow due to increased shear stress. Twenty-four hours after surgery, the skin and subcutaneous fat above the collateral arteries were removed, constructing a pocket for further analyses. To visualize blood flow and immune cells during in vivo imaging, CD41-fluorescein isothiocyanate (FITC) (platelets) and CD45-phycoerythrin (PE) (leukocytes) antibodies were injected intravenously (i.v.) via a catheter placed in the tail vein of a mouse. This article introduces intravital multiphoton imaging as an alternative or in vivo complementation to the commonly used static ex vivo (immuno-) histological analyses to study processes relevant for arteriogenesis. In summary, this paper describes a novel and dynamic in vivo method to investigate immune cell trafficking, blood flow, and shear stress in a hindlimb model of arteriogenesis, which enhances evaluation possibilities notably.
Introduction
Despite intensive research interest during recent years, cardiovascular diseases, e.g., ischemic heart disease and stroke, are still the leading global cause of death1. Current treatments for these diseases are highly invasive therapies such as percutaneous transluminal angioplasty, percutaneous transluminal coronary angioplasty, or bypass surgery2. Therefore, the development of alternative, non-invasive therapeutic options is urgently needed. The body can create natural bypasses around a stenosed or occluded vessel to redirect the interrupted blood flow to the distal part of the stenosis. This process is called arteriogenesis2. Many recent studies have shown that increased fluid shear impacts leukocyte recruitment, which plays an important role during arteriogenesis3. The main current options to analyze the recruitment of leukocytes during arteriogenesis are ex vivo (immuno-) histological analyses or fluorescence-activated cell sorting (FACS) methodology4. To enable the assessment of leukocyte dynamics during arteriogenesis, this paper presents an intravital imaging protocol with multiphoton microscopy.
Leukocytes are the major blood cells recruited during the process of arteriogenesis3. This protocol uses multiphoton imaging to show the crawling of adherent leukocytes labeled with injected anti-CD45-PE antibodies in collateral arteries, 24 h after the induction of arteriogenesis by femoral artery ligation (FAL) employing a murine hindlimb ischemia model5,6. Alternatively, immune cells can be labeled ex vivo and carefully injected into mice, as shown in studies on angiogenesis using intravital microscopy7. The blood flow inside vessels and arteries can be visualized by CD41-FITC (to label platelets), dextran-FITC (plasma), or by the second harmonic generation (SHG), which visualizes collagen type 1 present in the basement membrane of some part of the vascular tree. SHG is a unique free labeling effect of the multiphoton excitation. Multiphoton imaging allows long-term cell tracking without harming the tissue and activating the cells by laser power excitation. Multiphoton microscopy is the imaging method of choice for visualizing fluorescently labeled cells and structures in living animals due to its ability to excite the fluorophores deeper into the tissue/organs with minimal phototoxicity8.
The use of tuned infrared lasers with pulses delivered within femtosecond intervals excites the fluorochrome only at the focal plane, with no excitation above and below the focal plane8. Thus, multiphoton microscopy allows high spatio-temporal resolution, less photo-damage, and increased tissue penetration imaging for studying dynamic biological events inside the organs. It is an ideal microscopy tool for live-animal imaging. However, multiphoton and any other art of intravital microscopy is limited by tissue motion due to heartbeat, respiration, peristaltic movements, muscle tonality, and other physiological functions, which disturbs imaging acquisition and analysis. As these movements impair temporal and spatial resolution and sometimes even prohibit subsequent analysis, they must be addressed appropriately to enable accurate data analysis and interpretation. Several strategies have been developed to lower or prevent artifacts from tissue motion. This protocol applies an in situ drift correction software called VivoFollow9 to correct tissue drift during image acquisition. This approach provides the required image stabilization, enabling long-term imaging and cell-tracking analysis.
Protocol
This study was approved by the Bavarian Animal Care and Use Committee (approved by ethical code: ROB-55.2Vet-2532.Vet_02-17-99); these experiments were carried out in strict accordance with the German animal legislation guidelines.
1. Animals and femoral artery ligation (FAL)
NOTE: To induce sterile inflammation and arteriogenesis, 8-10 weeks old male C57BL/6J mice were used. None of the mice died or suffered from wound infection or wound healing disturbance during or post-FAL or sham surgery, respectively.
- Anesthesia
NOTE: Before injection of the MMF-mix anesthesia, it is recommended to first anesthetize with 5% isoflurane until the mouse is sleeping.- Weigh the mouse and prepare MMF mix with a dose of midazolam 5.0 mg/kg, medetomidine 0.5 mg/kg, and fentanyl 0.05 mg/kg.
- Fill a 1 mL syringe with MMF-mix and inject a final volume of 100 µL s.c. using a 30 G needle (after anesthesia with 5% isoflurane).
- Place the mouse on a warm pad and wait until the mouse is completely anesthetized. Set the timer to 35 min. After this time, inject s.c. half of the dose (50 µL) of the MMF-mix.
- Check the depth of anesthesia by testing the pedal and interdigital reflexes (by firm toe pinch) of the mouse.
NOTE: An absence of response indicates an adequate depth of analgesia.
- Femoral artery ligation (FAL)
NOTE: Use sterile surgical instruments and sutures. Disinfect the skin with disinfectant before opening and after closing.- Place the anesthetized mouse on a heating plate (35-37 °C) to maintain physiological body temperature. Apply eye cream (dexpanthenol) to each eye to prevent drying of the eyes under anesthesia.
- Transfer the anesthetized mouse to a Laser Doppler imaging (LDI) chamber before and after FAL to check blood flow and perfusion (Figure 1A-C).
- Remove hair, disinfect the skin, and make a cut to access the femoral artery. Under a surgical microscope, ligate the right femoral artery of the mouse hind limb using a braided silk suture (7-0) and perform a sham operation on the left femoral artery to serve as an internal control as previously described6 (Figure 1D-K).
NOTE: The incision in the skin was made with small scissors to avoid direct injury to the blood vessels close to the skin. - Close the skin using a braided silk suture (6-0). Administer buprenorphine (0.1 mg/kg) s.c. every 12 h, starting 10 min before antagonizing the anesthesia for pain relief. Observe the animal for signals of pain, i.e., reduced grooming activity, reluctance to move, or hunched posture for 24 h post-surgery.
- Inject (s.c.) a combination of naloxone (1.2 mg/kg), flumazenile (0.5 mg/kg), and atipamezole (2.5 mg/kg) to antagonize the anesthesia after finishing the FAL and closing the skin.
- After surgery, keep the mouse on the warm pad and continuously monitor the animal until it can maintain an upright posture and start walking normally around the cage.
- When the mouse is fully awake, place it back in the cage together with the other mice and return the cage to the animal housing room.
2. Tissue preparation for multiphoton intravital imaging
- Tail vein injection
NOTE: The animal needs to be under anesthesia before starting the preparation for intravital imaging. Before introducing the needle of the vein catheter, it is recommended to apply a skin disinfectant on the tail. This will also help to visualize the vessels.- Prepare a tail vein injection catheter using a 2 x 30 G needle, 10 cm of a fine bore polythene tubing (0.28 mm internal diameter and 0.61 mm outer diameter), a 1 mL syringe, and a histoacryl glue to fix the catheter on the mouse tail for further i.v. injections during imaging (Figure 2A,B and Figure 2F).
- Prepare the antibody solution for i.v. injection: CD45-PE and CD41-FITC (20 µg/mouse) at a final volume of 100 µL.
- Check the tail for the laterally located tail veins and dam the vein to identify the tail vein. Disinfect the tail, insert the vein catheter, and fix it using tissue histoacryl glue (Figure 2B).
- Inject the antibodies prior to imaging (at least 15 min before) and after finishing the tissue preparation.
- Tissue preparation
NOTE: Always use a warm pad and administer a drop of eye cream (dexpanthenol) in each eye before tissue preparation. Use sterile surgical instruments and sutures. Disinfect the skin with disinfectant before open and after closing.- Place the anesthetized mouse on a stable and portable pad. Fix the mouse in a supine position with a 4 point fixation on the pad using adhesive tape (Figure 2B).
- Mold two pieces of modeling clay and place the pieces under the upper hind limb of the mouse to ensure a plane position of the adductor muscle (Figure 2B, indicated by red arrows).
- To ensure a stable body temperature of 37 °C of the mouse, place the pad with the mouse on a heating plate under a surgical microscope with a 0.64-4.0-fold zoom (Figure 1L).
- Remove the hair from both legs, disinfect the skin, and cut the skin in a circle around the scar of the earlier femoral artery ligation of the right hind limb (Figure 1M).
- Remove the skin and fat layer from the top of the leg (Figure 1N,O), and pull aside the remaining skin using sutures to create a pocket around the adductor muscle (Figure 1P,Q).
- Remove subcutaneous fat to get a clear vision of the adductor muscle and the profound artery/vein as well as the collateral vessels (Figure 1Q,R).
- Carefully start removing the superficial muscle layer on top of the collateral vessels by carefully dissecting it out with fine forceps (Figure 1R).
NOTE: The collateral artery and the collateral vein are clearly visible and ready for imaging (Figure 1S). - Fill the prepared pocket with saline to avoid drying of the tissue and continue with the same procedure for the sham-operated left hind limb. Before imaging, add ultrasonic gel in both pockets, which prevents drying and serves as an immersion medium for optical coupling with the objective (Figure 2C and Figure 2F).
3. Intravital multiphoton microscopy
- Preparation
NOTE: Keep a syringe with ultrasonic gel inside the incubator chamber of the microscope to maintain a warm temperature for refilling the pockets during long-term imaging.- Remove the saline from the two prepared pockets and replace it with ultrasonic gel to cover the collateral vessels (Figure 2C).
- Inject the antibody solution through the tail vein catheter. 15 min after antibody injection, transfer the pad with the fixed mouse into the warmed multiphoton imaging chamber (Figure 2F).
- After completion of the image acquisition, euthanize the mouse by cervical dislocation under deep narcosis.
- Multiphoton microscopy image acquisition
NOTE: Turn on the microscope 30 min before imaging to allow for laser stabilization. For the best optical stability of the microscope, it is recommended to keep the microscope chamber permanently heated.- Turn on the key of the Ti:Sapphire laser box, the electronic interfaces boxes, the heating unit of the incubation chamber, the fluorescent lamp, and the computer (Figure 2D,E).
- Launch the acquisition software (Inspector software). Set the wavelength to 800 nm and open the microscope shutter (Figure 3A ii).
- In the Measurement Wizard dialog, choose the Instrument Mode (Single beam) and in Measurement Mode (3D-scan Time lapse) (Figure 3A i).
- Transfer the anesthetized mouse into the pre-warmed incubation chamber of the microscope. Position the area with the ultrasonic gel (step 2.2.8) directly in contact with the objective front lens (Figure 2F).
- Open the epifluorescence microscope shutter, choose an adequate filter/dichroic setting for FITC visualization (4) to find the area of interest by following the blood flow under epifluorescence illumination. After bringing the area of interest into the center field of view, close the epifluorescence microscope shutter.
- In the xy-Scanner dialog, set the following parameters: Image size (554×554), Pixel (512×512), Frequency (400), Line average (1) (Figure 3A iii). Adjust the laser power (5%) (Figure 3A iv), and set the photomultiplier (PMT) gain for the channels green (66), red (66), and blue (63) (Figure 3A v).
- Select anadequate objective for intravital imaging and drift correction settings (see details in 3.2.11).
NOTE: Here, the selected objective was the Nikon LWD 16x (Figure 3A vi). - Define the image stack range by starting the preview acquisition mode by pressing the red button on the top right corner of the Measurement Wizard dialog window (Figure 3A i).
- Define the area and structure of interest by focusing to obtain a good image. Then, while observing the screen, change focus by moving the objective up until the image disappears, and set it as zero (first= 0). Change focus in the opposite direction by moving the objective down until the image disappears from the screen again, and set it as the last (end= 40 µm) position in the axial direction (Figure 3A vii).
- Set the step size to 2 µm. Click the red button on the top right corner of the Measurement Wizard dialog to stop the preview scanning.
NOTE: The number of images will be calculated and set automatically (21 images), as indicated in Figure 3A vii. - If the microscope is equipped with live-drift correction9, start the drift correction software (e.g., VivoFollow) and follow the steps described below.
- In the Measurement Wizard dialog, choose the Python axis (Python Ax) (Figure 3A viii) as the first axis device; DO NOT activate the auto save mode. Check the auto save (AS) box only on the time axis (Time Time). Press the Python icon on the Available Devices window (Figure 3A ix) to open the Python dialog window.
- In the Python dialog Axis settings, enter in From (0 zero) and To (-40). Enter Number of steps (21) as indicated in Figure 3A x.
- Go to the xyz Stage Z dialog window and enlarge the Scan Range by 200 µm on both ends: in Start, enter (200 µm) and in End, enter (-240 µm), the range will automatically be set (-440 µm). Keep the step size (2 µm) as shown in Figure 3A xi.
- Open the VivoFollow Frontend dialog and click the Motion Profile box (Figure 3A xii).
- Start image acquisition by pressing the green arrowhead in the top left corner of the Measurement Wizard dialog of the Inspector Software (Figure 3A i).
- If live-drift correction is used, look for a dialog for the drift correction configuration to appear as shown in Figure 3A xiii. Set the acquisition channel that will be used as an immobile landmark reference (for this protocol, choose channel 2, where the vascular tracer signal is to be recorded). Enter the maximal correction offset in µm that was added to the first and last z positions (here, 200 µm) and click OK.
- Monitor the current drift offset in x, y, and z in real time during the image acquisition in the VivoFollow fronted dialog window Figure 3A xiv.
- Stop imaging acquisition after ~35 min when another cycle of anesthesia re-injection is required. Inject half a dose of the MMF-mix (50 µl s.c) and re-start imaging acquisition by pressing the green arrowhead again (step 3.2.11.5).
- Repeat this procedure until the experiment is finished.
Representative Results
Multiphoton microscopy offers a high spatio-temporal resolution for leukocyte tracking, wherein cell migration steps and speed can be tracked and monitored (Figure 4A,B). However, the physiological motion of the sample poses a challenge, especially for long-term intravital microscopy image acquisitions. Therefore, a good tissue preparation and holders to fix the tissue and tools, such as real-time imaging correction for tissue drift, are required for successful and stable imaging acquisition. Here, a live-drift tool, VivoFollow, was employed to correct for the tissue drift generated during long-term imaging acquisition.
The application of this tool allows long-term image acquisition and enables the collection of high-quality data, suitable for tracking of cells for speed measurements. As shown in Figure 3B, without drift correction, the region of interest progressively drifts away from the recording view, impacting the ability to track cells for speed analysis. However, as shown in Figure 3C, with the drift correction software, stable movies can be recorded, and more cells can be tracked over a long period. The drift correction software can also provide a visualization of the x, y, and z offsets over time that the system corrected for, as shown in Figure 3D.
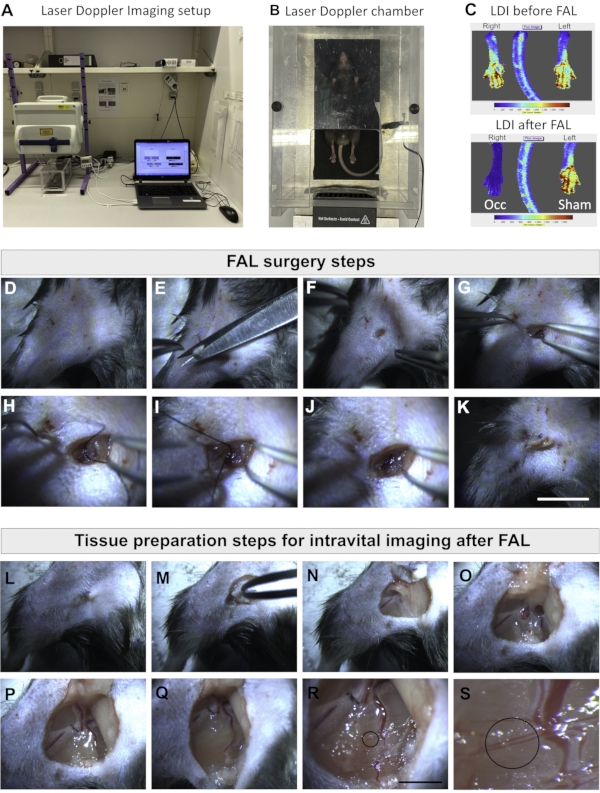
Figure 1: Laser Doppler Imaging setup and preparation for femoral artery ligation and tissue preparation for intravital imaging. (A) LDI imaging setup. (B) The mouse is placed inside the LDI warming chamber for image acquisition. (C) LDI images of the right and left legs before (upper panel) and after FAL (lower panel) to check the hindlimb perfusion after ligation. The right femoral artery was ligated/occluded, while the left femoral artery was sham-operated (Sham) and served as an internal control. In the color bar, blue indicates low blood perfusion, and red indicates higher perfusion. (D–K) Images represent the surgery steps of the femoral arterial ligation. Scale bar = 10 mm. (L–S) Tissue preparation for intravital imaging after FAL. The area to be imaged is indicated by the black circle, where the collateral vessels are located. Scale bar = 5 mm. Abbreviations: LDI = Laser Doppler imaging; FAL = femoral artery ligation; Occ = occlusion. Please click here to view a larger version of this figure.
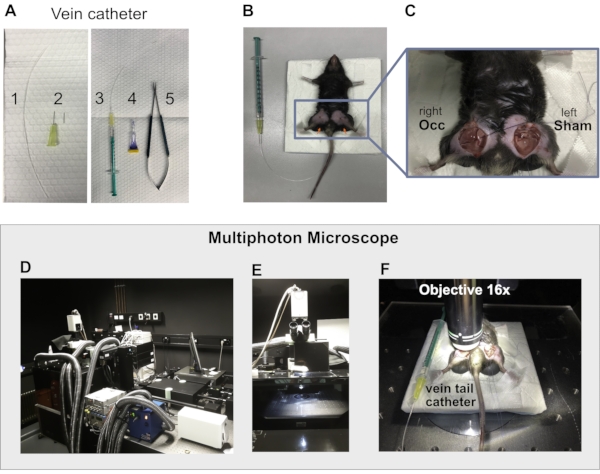
Figure 2: Setup for multiphoton intravital imaging. (A) Vein catheter preparation; 1-Fine polythene tubing of 10 cm, 2: 2x 30 G needle; 3: mounted catheter connected with 1 mL syringe; 4: tissue histoacryl glue; 5: needle holder. (B) The mouse positioned in supine position with both hindlimbs placed on pieces of black modeling clay (red arrows) prepared for imaging. (C) Occluded (Occ) right hindlimb and left, sham-operated hindlimb ready for imaging. (D) Multiphoton microscope setup showing laser boxes, fluorescence lamp box, tubes for heating the incubator chamber. (E) Incubator chamber of the multiphoton microscope. (F) Mouse placed inside the microscope chamber with the 16x objective touching the tissue covered with ultrasonic gel. Detail showing the vein catheter fixed on the tail vein for antibody injection. Please click here to view a larger version of this figure.
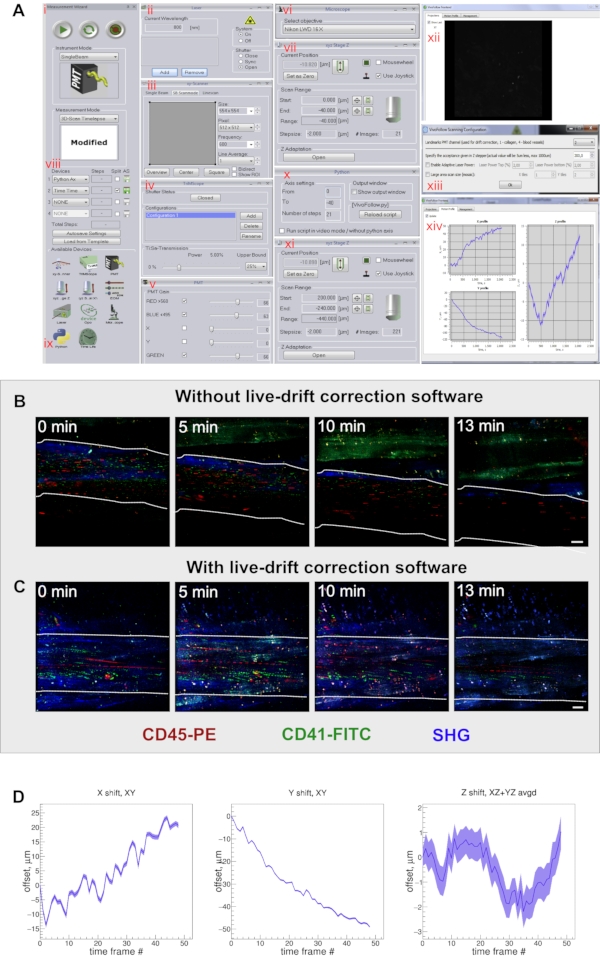
Figure 3: VivoFollow drift correction software setup. (A) (i-xiv) Software setup steps as described in protocol step 3.2.2-3.2.11.9. (B) Representative time series images showing the imaging drifting without drift correction software turned on. Collateral artery is delimited by the white discontinued lines (Video 1 and Video 2). (C) Representative time series images showing the stable time series when the live drift correction is applied during imaging acquisition. Leukocytes were labeled with CD45-PE antibodies (red), platelets were labeled by CD41-FITC antibodies (green) and collagen type 1 with the SGH (blue). The collateral artery is delimited by the white discontinued lines. (D) Line chart showing the live correction along x, y, and z directions. Abbreviations: PE = phycoerythrin; FITC = fluorescein isothiocyanate; SGH = second harmonic generation. Please click here to view a larger version of this figure.
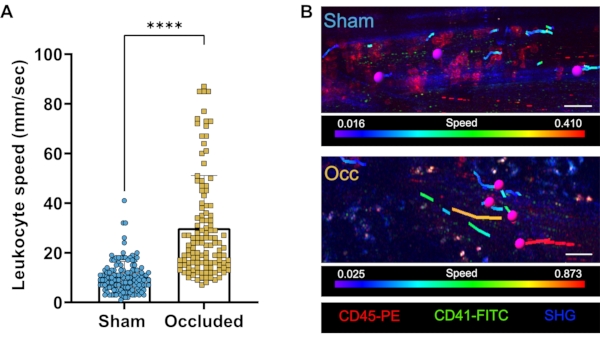
Figure 4: Representative results. (A) Leukocyte speeds measured in collateral arteries of sham-operated (Sham) and femoral artery-ligated hindlimbs. (B) Representative images show the cells tracked (magenta) with the tracks color-coded. The color code bar represents the cell speed with the slower cells shown by blue tracks and faster cells with red tracks. Leukocytes were labeled with injected CD45-PE antibodies (red), platelets were labeled by injected CD41-FITC antibodies (green), and the collagen type 1 with the SGH (blue). Results from three individual experiments. Scale bar = 20 µm. See Video 3 and Video 4. Abbreviations: Occ= occluded/femoral artery-ligated; PE = phycoerythrin; FITC = fluorescein isothiocyanate; SGH = second harmonic generation. Please click here to view a larger version of this figure.
Video 1: Multiphoton intravital imaging of a collateral artery without drift correction. Four-dimensional images were recorded with a pixel size of 554 x 554 µm, frequency 600 Hz, an interval of 1100 ms/frame, step size of 2 µm, and range of 40 µm. The video shows the image drifting without the application of the VivoFollow drift correction software. The collateral artery is shown in the lower part of the video, and the collateral vein appears after 3 min in the upper part of the movie. Scale bar = 50 µm. Please click here to download this Video.
Video 2: Multiphoton intravital imaging of a collateral artery with applied drift correction. Four-dimensional images were taken with a pixel size of 554 x 554 µm, frequency 600 Hz, an interval of 1100 ms/frame, step size of 2 µm, and range of 40 µm. The video was recorded with the live drift correction software, VivoFollow, and shows the imaging stability promoted by applying the drift correction software. Scale bar = 50 µm. Please click here to download this Video.
Video 3: Cell-tracking imaging in a collateral artery after sham operation. Leukocytes labeled with injected anti-CD45-PE antibodies were imaged in a resting collateral artery of the sham-operated leg and tracked using Imaris software with the plugin for tracking cells. Tracked cells were selected and represented by the magenta dots. The tracks were color-coded according to the cell speed. Slower cells are represented by blue tracks and faster cells by red tracks. Scale bar = 50 µm. Abbreviation = PE = phycoerythrin. Please click here to download this Video.
Video 4: Cell-tracking imaging in a collateral artery after FAL. Leukocytes labeled with anti-CD45-PE antibodies were imaged in a growing collateral artery 24 h after the induction of arteriogenesis by FAL and tracked by using Imaris software with the plugin for tracking cells. Tracked cells were selected and represented by the magenta dots. The tracks were color-coded according to the cell speed. Slower cells are represented by blue tracks and faster cells by red tracks. Scale bar = 50 µm. Abbreviations: FAL = femoral artery ligation; PE = phycoerythrin. Please click here to download this Video.
| Technical Problems | Proposed solutions | |
| In case the tail vessels are not visible for introducing the catheter | • Use a warm compress surrounding the mouse tail for 3-5 min to promote local vasodilation. It will help to make the vein visible. | |
| In case the tail catheter cannot be fixed for antibody injection | • Use a 1 mL syringe with 30 G needle to introduce into the vein and directly inject the antibodies and dyes before imaging. | |
| • In some cases (depending of the antibodies) the injection can be applied i.p. 1 h before imaging. | ||
| When the motion of tissue impedes imaging stability | • Try to re-position and re-fix the mouse upper hint leg to avoid the physiological abdominal movements. | |
| • Check whether the microscope table has enough air to cancel vibration. | ||
| • Make sure that the mouse is in deep anesthesia and does not wake up. | ||
| • Monitor the breathing of the mouse. When the mouse is breathing too fast, it can have an impact on the imaging motion. | ||
| When the image quality starts decreasing | • Ensure the ultrasonic gel does not dry out. | |
| • Re-fill the ultrasonic gel. | ||
| • Make sure that the objective is clean (clean up the dried ultrasonic gel on the objective whenever a new re-filling is done). | ||
| When the arteries are damaged during preparation for imaging | • In this case, it will not be possible to acquire images. It is not possible to run the experiment. | |
Table 1: Troubleshooting.
Discussion
The described method of multiphoton in vivo analysis of leukocyte recruitment represents an addition to commonly used tools for leukocyte recruitment studies such as (immuno-) histological or FACS analyses. With this imaging method, it is possible to visualize in greater detail the dynamic processes in leukocyte adherence and extravasation during arteriogenesis10. Despite the added value of this method, the offered protocol includes some critical steps and limitations. See Table 1 for more troubleshooting tips. During the removal of the superficial muscle layer on collateral arteries, these arteries could be damaged and will not be useful for intravital imaging. The catheter must be well-fixed for antibody injection; otherwise, slipping of the tail vein catheter can cause the extravasation of the antibodies into the perivascular space of the tail, which makes replacement and additional correction for injection of the antibodies a challenging task. The correct identification of the collateral artery and the collateral vein during intravital microscopy represents another critical step of this protocol.
Given that the mouse is not mechanically ventilated during intravital microscopy, the dynamic imaging time is limited to avoid physical injury to the mouse as a result of excessively long anesthesia. One of the shortcomings of intravital microscopy is the mouse physiologic motion, generated by respiration, heart beating, and peristaltic movements, that can impact image acquisition and therefore, the quality of data analyses. Improving tissue holders, mounting, and fixation are steps that further reduce the effects of motion. In addition to the physiological motion, long-term imaging may contribute to tissue drifting during imaging. As described in this protocol, a real-time tissue drift correction software, VivoFollow, was employed to obtain imaging data suitable for subsequent cell tracking and cell speed analysis. This VivoFollow software corrects for the sample displacement in x, y, and z directly during image acquisition. It relies on immobile anatomical structure, for example, when vessels are visualized with vascular tracers that serve as reference landmarks for the correction procedure.
Although there are other post-acquisition drift correction tools available, such as those within Bitplane Imaris or plugins for ImageJ, they are very limited in their capacity for correction as they do not allow for restoration of the region of interest. In principle, they cannot correct for severe offsets when the region of interest moves out of the field of view. However, this protocol can be applied even when the live-drift correction is unavailable, and post-acquisition correction becomes necessary. However, this requires frequent training of personnel in tissue mounting and fixation. In conclusion, multiphoton intravital microscopy is a useful dynamic method that can be applied in parallel to static established methods such as (immuno-) histological analyses to get a more detailed picture of the dynamic components of leukocyte recruitment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The study was funded by the Deutsche Forschungsgemeinschaft SFB 914 (HI-A/SM, project Z01). We thank Dr. Susanne Stutte for reading the manuscript.
Materials
| 1.0 mL Syringe | BD Biosciences, San Jose, CA, USA | 309628 | syringe for injection |
| 3M Durapore Surgical Tape 1538-0 | 3M, St. Paul, MN, USA | 1538-0 | fixation tape |
| Atipamezole | Zoetis, Berlin, Germany | antagonize anesthesia | |
| Buprenorphine | Reckitt Benckiser Healthcare, Slough, UK | antagonize anesthesia | |
| C57/B6J mouse | Charles River, Sulzfeld, Germany | used animals for surgery/imaging | |
| CD41-FITC ab | Biolegend | 133904 | Platelet labeling in vivo |
| CD45-PE ab | Biolegend | 368510 | Leukocytes labelling in vivo |
| Disinfectant Cutasept | Carl Roth GmbH, Karlsruhe, Deutschland | AK64.2 | Disinfection |
| Eye cream (Bepanthen) | Bayer Vital GmbH | 5g | |
| Fentanyl | CuraMED Pharma, Karlsruhe, Germany | anesthesia | |
| Flumazenile | Inresa Arzneimittel GmbH, Freiburg, Deutschland | antagonize anesthesia | |
| Fine bore polythene tubing | Smiths medical | Lot 278316 | 0.28 mm ID and 0.61 mm OD, tubing for the vein catheter |
| Histoacryl flexible | BRAUM | 1050052 | tissue glue |
| Imaris software | Oxford Instruments | version 9.2 | Used for cell tracking, cell speed analysis, 3D projection |
| Laser Doppler Imaging instrument | Moor LDI 5061 and Moor Software Version 3.01, Moor Instruments, Remagen, Germany | ||
| LEICA KL300 LED | Leica, Solms, Germany | light for microscope | |
| Leica M60 | Leica, Solms, Germany | microscope for surgery | |
| LEICA MC120 HD | Leica, Solms, Germany | camera for microscope | |
| Medetomidine | Pfizer Pharma, Berlin, Germany | anesthesia | |
| Midazolam | Ratiopharm GmbH, Ulm, Germany | anesthesia | |
| Multiphoton microscope | Lavision | TRIMScope II | WL 820 nm |
| NaCl 0.9% | Braun, Melsungen, Deutschland | 3570310 | saline for pocket |
| Naloxone | Inresa Arzneimittel GmbH, Freiburg, Deutschland | antagonize anesthesia | |
| Needle 30 G | BD Biosciences, San Jose, CA, USA | 305128 | needle for i.v. catheter |
| Silk braided suture (0/7) | Pearsalls Ltd., Taunton, UK | SUT-S 103 | suture for femoral artery ligation |
| Ultrason Gel | SONOSID-ASID BONZ 250 mL | 782012 | gel for imaging |
| Vicryl 6.0 suture | Vicryl, Johnson&Johnson, New Brunswick, NJ, USA | NW-2347 | suture to build pocket |
| VivoFollow drift correction software | Developed by Mykhailo Vladymyrov | Reference 9 |
References
- The top 10 causes of death. World Health Organization Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020)
- Deindl, E., Schaper, W. The art of arteriogenesis. Cell Biochemistry and Biophysics. 43 (1), 1-15 (2005).
- Lasch, M., et al. Extracellular RNA released due to shear stress controls natural bypass growth by mediating mechanotransduction in mice. Blood. 134 (17), 1469-1479 (2019).
- Kumaraswami, K., et al. A simple and effective flow Ccytometry-based method for identification and quantification of tissue infiltrated leukocyte subpopulations in a mouse model of peripheral arterial disease. International Journal of Molecular Sciences. 21 (10), 3593 (2020).
- Padgett, M. E., McCord, T. J., McClung, J. M., Kontos, C. D. Methods for acute and subacute murine hindlimb ischemia. Journal of Visualized Experiments: JoVE. (112), e54166 (2016).
- Limbourg, A., et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nature Protocols. 4 (12), 1737 (2009).
- Wagner, M., et al. Intravital microscopy of monocyte homing and tumor-related angiogenesis in a murine model of peripheral arterial disease. Journal of Visualized Experiments: JoVE. (126), e56290 (2017).
- Ishikawa-Ankerhold, H. C., Ankerhold, R., Drummen, G. P. Advanced fluorescence microscopy techniques–FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 17 (4), 4047-4132 (2012).
- Vladymyrov, M., Abe, J., Moalli, F., Stein, J. V., Ariga, A. Real-time tissue offset correction system for intravital multiphoton microscopy. Journal of Immunological Methods. 438, 35-41 (2016).
- Chandraratne, S., et al. Critical role of platelet glycoprotein ibalpha in arterial remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology. 35 (3), 589-597 (2015).

