Gathering Self-Initiated Rat Behavioral Data to Characterize Post-Stroke Deficits
Summary
A system for acquiring data from self-initiated individual behavior sessions within a social colony cage setting is presented. The efficacy of this system is demonstrated using an automated skilled reach assessment, enabling the characterization of post-stroke motor impairments, potential behavioral alterations related to motivation, circadian variations, and other innovative dependent variables.
Abstract
Behavioral testing in rat models is frequently utilized for diverse purposes, including psychological, biomedical, and behavioral research. Many traditional approaches involve individual, one-on-one testing sessions between a single researcher and each animal in an experiment. This setup can be very time consuming for the researcher, and their presence may impact the behavioral data in unwanted ways. Additionally, traditional caging for rat research imposes a lack of enrichment, exercise, and socialization that would normally be typical for the species, and this context may also skew the results of behavioral data. Overcoming these limitations may be worthwhile for several research applications, including the study of acquired brain injury. Here, an example method is presented for automatically training and testing individual rat behavior in a colony cage without the presence of humans. Radio frequency identification can be utilized to tailor sessions to the individual rat. The validation of this system occurred in the example context of measuring skilled forelimb motor performance before and after stroke. Traditional characteristics of post-stroke behavioral impairments and novel measures enabled by the system are measured, including success rate, various aspects of pull force, bout analysis, initiation rate and patterns, session duration, and circadian patterns. These variables can be collected automatically with few limitations; though the apparatus removes experimental control of exposure, timing and practice, the validation produced reasonable consistency in these variables from animal to animal.
Introduction
Behavioral training and testing with rat models are important in countless research areas, from the exploration of cognitive processes to disease states and more1. Typically, this training and testing is conducted with single animals in one-on-one sessions, with a researcher manually removing the animal from their home caging and temporarily placing them in some kind of apparatus. Unfortunately, there are several difficulties and limitations with this approach. First, behavioral testing can take a great deal of time for researchers, and when training is necessary, that time requirement becomes even greater. Second, this approach automatically affects-or even potentially confounds-the acquired data, as has been established elsewhere2. These confounds are especially salient when considering enrichment-related variables. Specifically, laboratory rats are traditionally housed in small cages that are just big enough for one or two rats3, and if running wheels are not provided, they may go a lifetime without meaningful opportunities to exercise. Additionally, isolated housing can be a major source of stress in a social species such as the rat4. Some of these welfare-related drawbacks likely impact rat physiology5,6, which may preempt the development of species-typical behavioral expression4 and impact the quality of rodent models as applied to human contexts.
Researchers have pursued several types of solutions to these problems in recent years. The simplest type of solution has been to automate behavioral testing and training7,8,9,10, thus removing the requirement for a single researcher to attend to a single animal. An additional solution has been to automate animal transfer to experimental chambers11,12, further removing the need for human involvement. Last, several setups have been explored which allow animals to be housed in colony caging with other animals and with more room for exploration and enrichment13. Despite these advantages, such colony setups can limit or complicate the efforts to gather individually differentiated behavioral data (though see efforts to use computer vision)14,15. If individual behavioral data is required, it can be more difficult or complex to identify and retrieve animals from colony caging for behavioral sessions as well. At present, few systems exist for collecting individual behavioral data from (enriched) colony housing16,17,18.
These drawbacks may specifically impact research on the behavioral effects of acquired brain injury. First, it is clear that the presence and/or sex of humans as well as handling practices affect rodent behavior2,19, and these variables may differentially impact the behavior of rats before vs. after stroke. Second, human behavioral outcomes after stroke can be worsened by voluntarily decreased engagement with the recommended dosage of rehabilitation exercises20. Currently, rodent experiments tend to not model this sort of context, because rats are not free to choose to engage or abstain from behavioral sessions.
This article introduces a protocol designed to facilitate individual behavioral testing within the framework of enriched colony caging. This approach not only addresses the constraints of current practices but also opens avenues for the exploration of innovative measures. A one-rat turnstile (ORT) has been developed and can be affixed to a colony cage, enabling animals to enter behavioral chambers independently and initiate their own training and testing sessions. The system is affordable; each ORT can be assembled at low cost (given access to a 3D printer). In the past, validation of this system was carried out using a basic operant chamber, showing that animals could be consistently trained to perform a simple operant lever press without the presence of an experimenter16. Nevertheless, the question of whether this configuration is applicable to other scenarios remains unresolved. The aim is to validate the effectiveness of the ORT-colony caging setup, which was previously established, for training and quantifying skilled reach behavior relevant to motor impairment following a stroke. The configuration was utilized to generate novel variables that are typically not explored in stroke research. These variables include performance metrics for the skilled reach task and measurements of self-initiation, which could be pertinent to motivation and decision-making. Furthermore, stroke-induced changes in the circadian patterns of daily self-initiation across the entire 24 h period were effectively detected.
Protocol
All procedures and animal care were approved by the University of North Texas institutional animal care and use committee (IACUC) and adhered to National Institutes of Health guide for the care and use of Laboratory animals. Adult male and female Long-Evans rats (400-800 g, 1.5 years old), used in the present study, were housed in colony caging.
1. Equipment preparation
- Obtain or assemble the one-rat turnstile (ORT) according to the design files and instructions for construction (see Supplementary File 1 and Supplementary Coding File 1). Refer to Butcher et al.16 for further details.
NOTE: ORTs are specific to rat size, so a colony cage should include animals that are approximately the same size. If one doesn't wish to self-assemble ORTs, they may be purchased pre-assembled (see Table of Materials). - Obtain and attach a radio-frequency identification (RFID, see Table of Materials) reader and obtain and inject animals with RFID tags.
NOTE: When injecting RFID full duplex (FDX) tags, the orientation must be perpendicular to the RFID antenna as the rat walks through the ORT. In this validation, tags were implanted subcutaneously between the shoulder blade on a plane parallel with the spine. - Affix the RFID antenna to the tube of the ORT.
- Construct and/or obtain the behavioral apparatus(es) and colony caging appropriate for the experimental question. In this example, custom-built colony caging21,22 was used in conjunction with commercially available operant chambers (see Table of Materials), though any equipment could theoretically be used.
NOTE: Competition of colony-housed animals for access to the behavioral apparatus(es) via the ORT should be considered. Anticipate needing one ORT + behavioral apparatus for every 4 to 6 animals. - Attach the ORT(s) between the behavioral apparatus and colony caging.
- Cut a portal hole in the behavioral apparatus and colony caging using a Dremel rotary tool (see Table of Materials) or similar instrument. The internal diameter should be equal to the outer diameter of the constructed ORT tunnel.
NOTE: The ORT must be elevated a few inches to operate, so a small platform or stand will be needed to align the colony caging and apparatus heights. - Install RFID system to read animals as they pass through the ORT and, if desired, integrate it with the behavioral apparatus.
2. Presurgical behavioral training
- Obtain same-sized cohort of rats and introduce them into the colony caging.
NOTE: Animals that have been reared or housed extensively in isolation or with few conspecifics may have more trouble exploring the chamber, especially when it involves traversing social areas of the colony caging. Animals should be exposed to group caging early in life to avoid this pitfall. - Remove access to any manipulanda within the behavioral apparatus and set the chamber to auto-deliver rewards every 60 s, on average, when occupied.
NOTE: This study used sucrose water (30% to 40%) as the reward, but sweetened condensed milk is also effective. - Train all rats to regularly enter the behavioral apparatus(es) via the ORT.
- At least once per day, check the data to ensure all animals are entering the ORT. If animals are not entering, insert a pen-sized object into the locking mechanism to prevent it from locking temporarily allow animals to explore more freely. If animals are still not entering, remove the turnstile and attach a temporary sidewall to allow free tunnel access to the chamber.
- Once all animals are regularly entering the chamber, return the lock (and turnstile) and reassess.
NOTE: Animals may also occupy the ORT and chamber as a temporary reprieve from other rats. One way to preempt this sort of monopolization of the chamber is to attach an additional ORT that bridges to a simple isolation chamber. - Introduce the manipulandum-the pull handle, in this example case-and set to the highest sensitivity. Insert the handle just inside the box (up to 2 cm) or just outside the box.
NOTE: Painter's tape can evoke reach attempts if affixed to the back of the handle, just out of reach. - Reduce the frequency that the reward (i.e., 30% sucrose water) is auto-delivered (e.g., every 90-120 s). Remember that any reward can be utilized that fits the needs of the experimenter and the animals' preferences.
- Check the data daily to ensure that all animals have learned to activate the lever. Bait the lever and/or change insertion level until all animals are pulling.
- Discontinue auto-delivery of rewards so that they are only available via activation of the pull handle.
- If previously inserted, retract the lever each day (provided that all rats continue to pull at that retraction level) by 0.25 mm to 0.5 mm until the lever is in its final position, 1 cm to 1.25 cm outside the chamber.
NOTE: The exact position of the lever depends on the size of the rats. Ensure to choose a position which results in the desired reaching topography. - Initiate a percentile or other training program to progressively increase required pull forces to activate the handle.
NOTE: This study used a percentile schedule that sets the criterion for reinforcement at the upper quartile of the previous 15 responses. Alternatively, stepwise increases of the pull criterion can be used7. - Once animals reliably reach the final criterion range of 120 g pulls, remove the percentile training program and fix the criterion for handle activation at a constant of 120 g.
- Collect baseline data at this force requirement until success rates have been steady (non-trending) for about a week.
3. Inducing stroke
- Surgically induce stroke in all colony caged animals at the same time.
NOTE: To induce the stroke an endothelian-1 model of stroke was used, which has been described elsewhere23. - Allow animals to recover in traditional caging, isolated individually, for 3-7 days.
4. Postsurgical behavioral testing
- After recovery, return animals to the colony caging with the ORT-attached skilled reach apparatus.
- Perform the behavioral testing, keeping the pull requirements at the final of 120 g (follow step 2) until sufficient data is collected to evaluate post-stroke deficits (from one to several days).
- Implement any post-stroke or recovery-related independent variables during subsequent days while animals are accessing the chamber.
Representative Results
The animals were trained and tested with four female rats in one colony cage and four male rats in a separate colony cage. All rats learned to pass through the ORTs in four days or less. The four female rats reached >85% successful bouts at the 120 g force requirement in approximately 6 weeks of training and the male rats reached the same criterion in 10 weeks (compared to roughly 3 weeks with standard training with deprived rats)7. This training duration was greatly lengthened due to several hardware and software glitches that required continuous troubleshooting, between weeks 2 to 6. Once these glitches were addressed, training proceeded smoothly, and it is expected that subsequent training timelines will be comparable to the current literature7. The male rats were also trained longer to provide more opportunities for one male rat to begin pulling; however, he never did and was excluded from further analysis and surgery after week 7. Once rat performance steadied in baseline, 5 days of pre-stroke baseline data was obtained for each cage. The data was limited to days during which the ORT remained connected to the cage for the entire day (some days required husbandry-related temporary disconnections). For the female cage, baseline days were 7, 8, 9, 10, and 12 days before stroke. For the males, baseline days were 8, 9, 10, 11, and 13 days before stroke.
During their stroke induction surgeries, the animals in this validation were simultaneously implanted with electrodes connected to wireless receiving chips in either their Basal Forebrain (coordinates -5.8 mm anterior/posterior, 0.7 mm left medial/lateral, 8.3 mm dorsal/ventral) or their Ventral Tegmental Area (coordinates -2.3 mm anterior/posterior, 3.3 mm left medial/lateral, 7.0 mm dorsal/ventral). These implants were for use in a subsequent recovery experiment and are not relevant to the colony cage-ORT validation reported here. The implants were designed so that the skin could be closed over them, with the receiving chips located subcutaneously under the left arm.
One female animal died during stroke induction. Another female began to decline several days after recovery, having never pulled the handle after stroke. After her euthanasia, it was found that she had probably experienced a brain hemorrhage sometime after her stroke. These two animals were eliminated from the data set entirely, including the pre-stroke assessment.
After stroke, animals did not immediately reinitiate regular lever pulling, though they continued to enter the chamber via the ORT and had to be encouraged via short sessions of manual shaping (i.e., reduced lever distance and delivered rewards contingent upon approaching or attempting to pull the lever). Female rats did not pull during days 4-7 after stroke and so were given supplementary lever baiting (i.e., a dab of peanut butter on the lever) and manual shaping on days 8-11. They began pulling autonomously on day 11. Males were allowed to recover until day 6 based on previous experience with the females. They did not pull during day 6 after the stroke. They were given supplementary baiting on day 7. They began autonomously pulling on the 8th day after the stroke. Once the animals again contacted reinforcement for pull attempts, supplementary baiting or shaping was ceased and post-stroke data was gathered. Males did not pull enough on day 8 for complete analysis of the more complex dependent variables (circadian measures and post-bout pausing), so they were allowed to continue to pull on the 9th, 10th, and 11th day under the same criterion. The 8th, 10th, and 11th day were complete days. The first day of pulling after stroke was used for all analyses except the circadian analysis and the analysis of pauses between bouts; for this analysis the one day for females and the three complete days for the males were used. For post-stroke analysis, the two female rats provided 55 and 844 pulls in one day, and the three male rats provided 536, 153, and 190 pulls in three days.
Data were organized according to pulls and bouts. To avoid registering tremors arising from the equipment itself, pulls were measured using a 5 g threshold with a +/- 1 g hysteresis. A pull was registered when the animal exerted pressure above 6 g and stopped when the handle registered a force below 4 g. Animals tended to pull in bouts of several rapid pulls. Once any single pull reached 120 g, reinforcement was delivered. A bout was considered a cluster of pulls whose peaks were all separated by less than 1 s. This threshold was selected based on previous data, which indicated that an inter-peak interval cluster under 1 s naturally developed, and other inter-peak intervals were reliably much longer. Rats would generally pull many times in a row before visiting the feeder, even when earlier pulls in the bout activated the feeder.
A total of 7 dependent variables were analyzed. A paired t-test was performed between baseline averages and post-stroke measures, which are reported in Figure 1, Figure 2, and Figure 3. These figures also display data by individual animals to provide an impression of the variation across days and between individuals for each measure that might be expected.
Figure 1 shows pre- and post-stroke performance along several performance measures typical of skilled reach assessments7,8,10. All post-stroke data was aggregated into a single data point even if it took several days to collect sufficient trials. The protocol and automated self-initiated system successfully assessed success rate per bout, mean force per pull, and pulls per bout, which all showed sensitivity to the stroke with varying degrees of statistical significance.
Figure 2 depicts two novel variables that arise from the colony cage-ORT setup: session initiations and cumulative session duration. Surprisingly, stroke did not affect session initiations. Females reliably initiated sessions more than males both before and after stroke, however neither changed their rate after stroke. Conversely, duration spent in the chamber increased for most rats, perhaps due to the decreased success rate of bouts (the result of which is a decreased rate of rewards)
Session initiation (which represents a choice between enrichment and social rewards available in the colony cage and food reinforcement) and duration of time in the chamber (in the case of a conditioned place preference with the value of reward) could also be taken as indices of motivation24,25,26,27. Additional motivation-based measures were included, such as "effort" as quantified by pulls per session minute28 and pauses between bouts29, which can be seen in Figure 3. These variables were impacted by stroke. As anticipated, the number of pulls per session minute decreased, and the duration of pauses between bouts increased. However, changes in the latter measure were complex. The distribution of bout pauses seemed to become more chaotic, including more long pauses, a few very long pauses, and more short pauses as well. This may indicate a breakdown in the original skilled motor unit; if so, it might be an easily measurable index of the same.
Despite the small group size, an investigation was conducted to determine if any of the measured variables exhibited correlations with the success rate, potentially implying their functional significance. Shapiro Wilk tests were performed to assess for equal distributions of the data for the variables success rate, mean peak pull, bout per minute, cumulative session duration, pauses between bouts, and pulls per bout. The Shapiro Wilk test indicated that the distribution of some variables departed significantly from normality. Therefore, Spearman's rank-order correlations were performed to determine the relationship between pre- or post-stroke success rate and the following variables: mean peak, pull bout per minute, cumulative session duration, pauses between bouts, and pulls per bout. No variables before stroke other than mean pull force were meaningfully correlated with success rate (see Table 1). After stroke, most variables also showed no meaningful correlation with success rate, except mean pull force (Table 1).
Finally, the ORT permits an analysis not only of skilled motor behavior, but also of the circadian patterning. Figure 4 shows the proportion of each hour for each cage in which the ORT was occupied, presented as an average over baseline and post-stroke days. The blue line in the figure indicates an averaged count of the number of entries per hour made throughout the day. Before stroke, animals would engage with the skilled reach task at high levels in the morning, diminishing their durations throughout the day. A few hours before lights-on, engagement either spiked again (females) or increased very slightly (males) before discontinuing shortly after the lights came on. This bimodal circadian distribution completely changed after stroke. Animals engaged less in the mornings, and their time in the chamber peaked later in the day. Such patterning may be reflective of the general sleep and circadian disturbances often observed after stroke30,31,32,33.
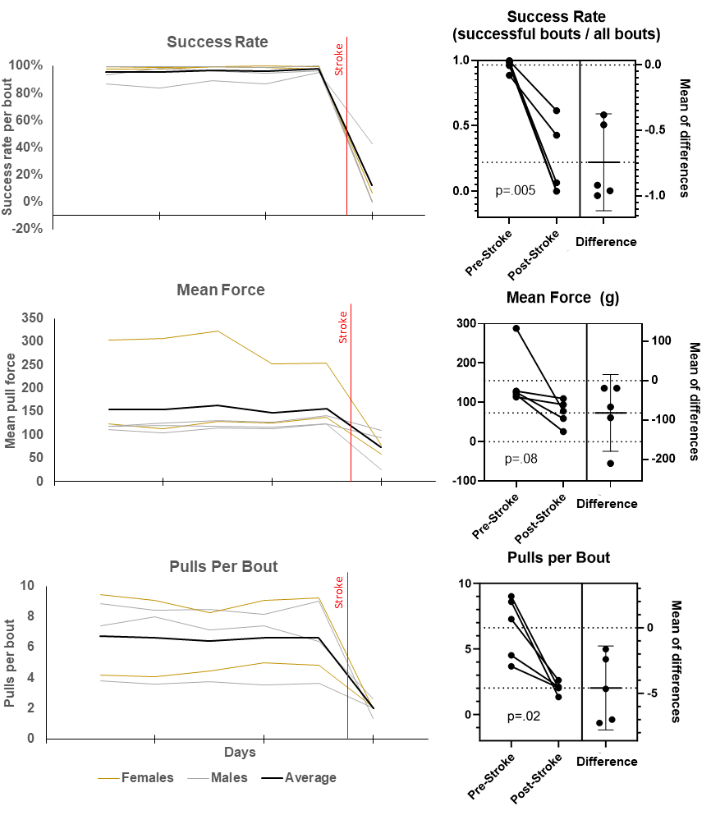
Figure 1: Measuring the typical performance changes in skilled reach after stroke using the ORT procedure. The performance of skilled reach before and after stroke was measured. Daily averages by animal for success rate per bout, mean force per pull, and number of pulls per bout are displayed over 5 days of baseline and one day post-stroke (left) and between an average of baseline and one day post-stroke (post-stroke) with reported paired t-tests. Please click here to view a larger version of this figure.
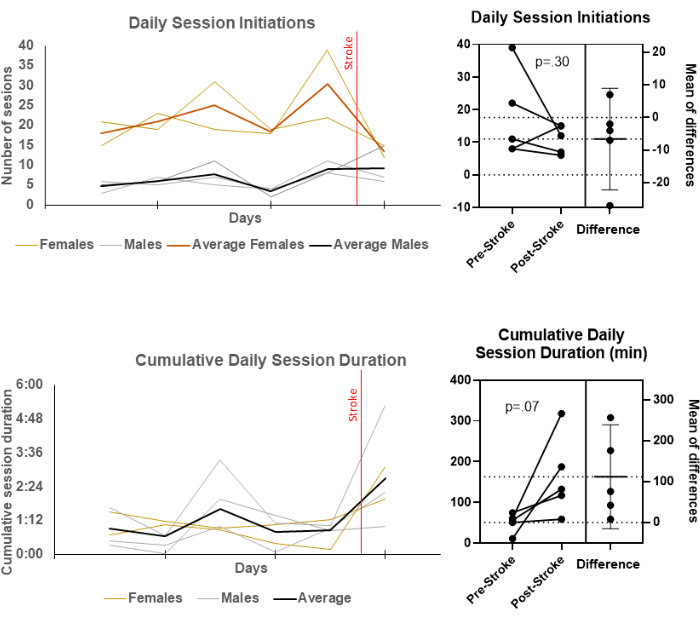
Figure 2: Different self-initiated session parameters show different tendencies to change after stroke. The measures of self-initiation for skilled reach behavior sessions before and after stroke was made possible by the ORT procedure. Daily averages by animal for session initiations and cumulative daily session durations are displayed over 5 days of baseline and one day post-stroke (left) and between an average of baseline and one day post-stroke (post-stroke) with reported paired t-tests. Please click here to view a larger version of this figure.
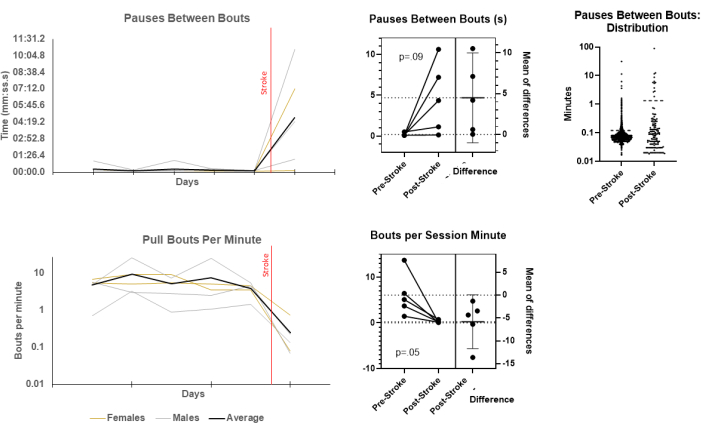
Figure 3: Motivation-related variables change after stroke. The performance measures of skilled reach behavior sessions before and after stroke related to motivation was determined. Daily averages by animal for pause duration between bouts and daily rate of bouts per session minute are displayed over 5 days of baseline and one day post-stroke (left) and between an average of baseline and one day post-stroke (post-stroke) with reported paired t-tests. Pauses between bouts changed in terms of these daily averages, but even more strikingly, the distribution of individual pause lengths after stroke also changed on both sides of the average. Individual pause lengths are pooled for all animals and displayed as distributions on a log axis (far right). Please click here to view a larger version of this figure.
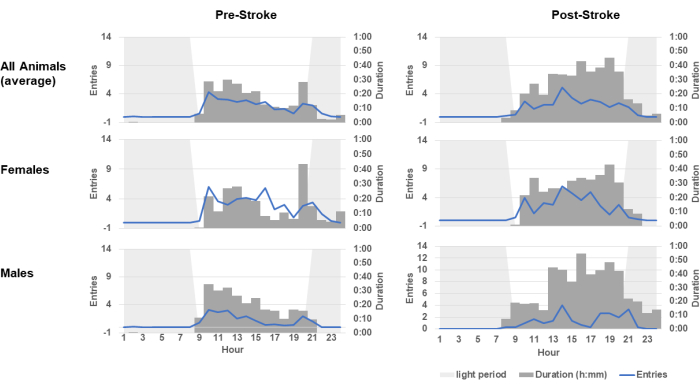
Figure 4: Circadian patterning of self-initiated sessions changes after stroke. The measures of circadian patterning of self-initiated skilled reach behavior sessions before (left) and after (right) stroke for all animals, females, and males were determined. These data include all entries and all times of chamber occupancy by cage, averaged across pre-stroke and post-stroke days. The two cages are then averaged again to show total distributions (top row). Pre-stroke patterns included high engagement in the morning, which diminished throughout the waking period with a new peak just before sleep phase. Post-stroke patterns show session durations increasing through the day and peaking before the sleep phase. Rats were on reverse light cycles in their housing room. The lights-on period is shown shaded in grey to indicate the rat's normal inactive period. Please click here to view a larger version of this figure.
| Correlations | ||||||
| Variables | Comparison Variable | Spearman | ||||
| n | Before Stroke rs | p | After Stroke rs | p | ||
| Mean Pull Force | Success Rate | 5 | 1 | <0.001 | -0.975 | 0.005 |
| Pull Bout per Minute | Success Rate | 5 | 0.3 | 0.624 | -0.154 | 0.805 |
| Cumulative Session Duration | Success Rate | 5 | -0.1 | 0.873 | 0.564 | 0.322 |
| Pauses Between Bouts | Success Rate | 5 | -0.6 | 0.285 | 0.205 | 0.741 |
| Pulls per Bout | Success Rate | 5 | 0.1 | 0.873 | -0.821 | 0.089 |
Table 1: Spearman correlation coefficients between variables. Spearman's rank-order correlations were performed to determine the relationship between pre- or post-stroke success rate and the following variables were determined: mean peak, pull bout per minute, cumulative session duration, pauses between bouts, and pulls per bout. Prior to the correlation, Shapiro Wilk tests were performed to assess for equal distributions of all test variables success rate, and indicated some variables departed significantly from normality. No variables before stroke other than mean pull force were meaningfully correlated with success rate. This table depicts the results of Spearman correlation coefficients (ρ) assessed if there was a relationship between success rate and five test variables.
Supplementary File 1: Steps to building the ORT. Instructions for printing and constructing a "One Rat Turnstile." Included in the instructions are a list of all needed materials as well as step-by-step instructions (with images). The file also includes directions for attaching a microswitch to register entries and exits, as well as the wiring and programming for attaching an RFID-reader. Please click here to download this File.
Supplementary Coding File 1: This includes all the components needed for 3D-printing the "One Rat Turnstile." This file can either be used directly or accessed using the instructions in Supplementary File 1. All components in this file must be scaled using the "ruler" piece that is included (see Supplementary File 1 for further details). Please click here to download this File.
Discussion
This protocol has multiple uses. First, and most broadly, the ORT was developed for the purpose of enabling automated single-subject behavioral training and data collection in the context of social, enriched housing. While this study tested the idea of collecting typical behavioral measures and elaborating upon them in the context of stroke, the same can be done for other applications and behavioral tasks. Even the measures gathered in this validation can also be adjusted as needed to include alternative reinforcement schedules, alternative behaviors, etc. Second, this study evaluated the system’s ability to collect data related to the impairment of skilled reach following a stroke. The current protocol has been validated previously for teaching and measuring basic lever pressing16. The current data show that it is a valid approach to gathering data on stroke-related motor deficits, and that measures typical of traditional skilled reach assessments as well as novel measures are possible. While troubleshooting the colony cage ORT system, a large question was whether the animals would self-initiate behavioral sessions with enough frequency, and whether they would do so in various experimental contexts, particularly after stroke. It was discovered that all or almost all animals can be encouraged to participate sufficiently in simple operant procedures and now for post-stroke behavioral testing. Post-stroke periods required some manual shaping, but animals quickly returned to engagement in the behavioral task without experimenter presence.
During troubleshooting, several procedural points were discovered that enhance the likelihood of success, and these have been integrated into the protocol. These included the need to scale the ORT according to rat size and to assure that animals are similarly sized and raised in a social context. Additionally, it was discovered that a single ORT is the most effective for accommodating 4-6 rats due to potential high competition for the chamber. It is worth noting that this competition does not appear to induce greater stress compared to traditional methods; a previous experiment recorded reduced cortisol levels in the colony caging setup as opposed to traditional behavioral testing16. Furthermore, it was found that, for timid animals, removing the turnstile or leaving it unlocked during the initial training phase aids in allowing multiple rats to explore the chamber together.
Although this approach is effective in assessing post-stroke motor deficits, it is essential to acknowledge a few limitations. First, post-stroke animals may not immediately reinitiate pulling and may require a few sessions of manual shaping and/or baiting. With this and other specific approaches, full automation of training and testing may be more difficult. However, this is not a limitation unique to the ORT-colony cage setup, and animals in experimenter-initiated behavioral sessions often have the same problem.
Another limitation is the potential for use of the chambers for other than the intended purpose. Anecdotally, several instances were observed where animals entered the behavioral chambers and spent a significant amount of time inside without actively participating in the behavioral task. This occasionally happened with all animals, but especially with the males. This may have been simply because the ORT provided a bit of privacy for animals when they did not wish to engage socially. That said, adding “alone” rooms to the colony cage using other ORTs mitigates this problem.
It was also observed that animals would occasionally stop in the tunnel with the ORT half-open and stay there for periods of time. This is the reason that it is best to have the RFID reader close to the behavioral chamber so that the animal is not registered until they fully enter. It might be speculated that lingering in the ORT is pleasant in the same manner as a squeeze chute. In any case, animals do not linger there in ways that would prevent data collection, particularly when alternate ORT-attached isolation chambers are available.
Another limitation is that self-initiated session can be more difficult to equalize behavioral session time or behavioral response opportunities between individuals in groups. This limitation may be especially relevant to stroke-related studies, given the relation between cumulative training time and recovery. However, this limitation can be at least partially addressed. If an experiment requires, it is possible to program many behavioral apparatuses to terminate sessions when a target duration or target number of responses are reached (i.e., through termination of rewards or retraction of the manipulandum). This solution does not address any tendency for individuals not to reach their targets. But, again, that limitation is not unique to this setup.
This setup also holds an advantage that behavioral training or exposure can occur throughout the day rather than at a few restricted times. This could be useful in several contexts; specifically in stroke it could be utilized to investigate the dosage effect of behavioral rehabilitation approaches.
Finally, the ORT likely cannot be used for aversive procedures such as social defeat, forced swim, conditioned fear, etc. The ORT requires the behavioral paradigm to be appetitive; otherwise, the animals are likely to simply avoid the behavioral chambers.
Despite limitations, this procedure adds significant advantages to the currently available behavioral paradigms for rats. First, the setup allows for continual high-throughput data collection while freeing up experimenter time. While it is important to continually monitor the equipment and the animals, experimenters need be present only for short periods of time throughout the day. Running behavioral sessions for 8 animals would have required 4-10 h of daily sessions using the traditional approach. The ORT-colony cage approach not only reduces that time to practically zero (assuming a working and automated apparatus), but also permits data collection over weekends with little need for additional presence; such continued availability of behavioral activities over weekends also increases the total enrichment available to animals.
The ORT-colony cage protocol provides data advantages as well as logistical advantages. Many dependent variables are available that are not traditionally possible, including pacing, duration, or other parameters of self-initiated sessions such as those explored here. Circadian variables are often measured using running wheels; however, this protocol makes it possible to look at patterning as well as choice parameters in other kinds of behavior. The ORT could even be used to connect colony cages and provide visual or olfactory access to the opposite sex, making it possible not only to assess motivation but circadian patterning of mate pursuit. This study has characterized several new dependent variables that could be useful in stroke research, including circadian variables and initiation-related variables that may help with exploring translational questions related to or in the context of the loss of motivation and depression that can occur after stroke34,35. Though not addressed here, other important stroke-relevant variables, like qualitative analyses of reach kinematics, should also be easily addressable with this ORT-colony cage protocol via motion-activated cameras.
Finally, a significant advantage of this protocol is its ability to allow animals to remain in large, enriched, and social environments for most of their lives. Progress in animal welfare is important in basic laboratories not only for ethical reasons but for scientific reasons. Animals who can move around and socialize should serve as better models for disease states that don’t naturally involve restriction or deprivation. Further, the self-sufficient nature of the behavioral sessions in this protocol means that humans need not be present while behavioral data are collected. The presence of humans in a laboratory can affect behavioral data, sometimes differentially depending on the human in question, how well they are trained, and how they handle animals2,19. Such uncontrolled and variable impacts on the data can be minimized with the current protocol.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded in part by the Beatrice H. Barrett endowment for research on neuro-operant relations to the University of North Texas (UNT). We are grateful for the input and assistance of all members of the Neuroplasticity and Repair Laboratory, especially Valerie Rojas, Mary Kate Moore, Cameron Scallon, and Hannah McGee.
Materials
| 3D printer | Consult with local makerspace | ||
| bolt | Boltdepot | 1346 | 6-32 or 8-32 by 0.5" |
| bolt | Boltdepot | 1348 | 6-32 or 8-32 by 0.75" |
| door hinge | XJS (Amazon) | 43398-16234 | 1" cabinet stainless steel door hinge set; Optional (if "perfect hinge" is not printed) |
| drill | Any electric drill works | ||
| extension spring | Nieko (Amazon) | 50456A | Choose and adjust spring based on ORT sized and desired tension |
| granulated sugar | |||
| lock nuts | Boltdepot | 2551 | 6-32 or 8-32 |
| measuring tape | |||
| microcontroller | Arduino | A000066 | Arduino Uno |
| microswitch | Sparkfun | KW4-Z5F | mini microswitch (SPDT-roller lever) |
| One Rat Turnstile (ORT) | Vulintus | Contact company to request quote if not self-assembling | |
| Operant Chambers as desired for behavioral assessment: For this experiment we used automated isometric pull chambers from Vulintus | Vulintus | No cat #: contact Vulintus | Contact Vulintus for quote |
| PLA filament | OVERTURE (Amazon) | UK-MATTEPLA17511 | |
| plexiglass | Lesnlok (Amazon) | B09P74K7BR | clear, 1/8" thickness, Cut to size |
| plexiglass cutter | |||
| python program | Python Software Foundation | software available on request | |
| RFID reader | Priority 1 Design | RFIDRW-E-USB | With antenna |
| RFID tag | Unified Information Devices | UC-1485-10 | |
| rod | Boltdepot | 23632 | cut to > 3.5" |
| Rotary tool | Used to bore hole in apparatus and colony caging for ORT; any hardware usable | ||
| sand paper | HSYMQ (Amazon) | TOMPOL-1118-1915-11 | |
| socket wrench set | Any socket wrench set works | ||
| soldering iron | |||
| super glue | 234790 | ||
| wire | Plusivo (Amazon) | EAN0721248989789 |
References
- Whishaw, I. Q., Kolb, B. . The behavior of the laboratory rat: A handbook with tests. , (2004).
- Sorge, R. E., et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature Methods. 11 (6), 629-632 (2014).
- Ottesen, J. L., Weber, A., Gürtler, H., Mikkelsen, L. F. New housing conditions: Improving the welfare of experimental animals. Alternatives to Laboratory Animals. 32 (Suppl 1B), 397-404 (2004).
- Arakawa, H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behavioural Brain Research. 341, 98-108 (2018).
- Simpson, J., Kelly, J. P. The impact of environmental enrichment in laboratory rats-behavioural and neurochemical aspects. Behavioural Brain Research. 222 (1), 246-264 (2011).
- Van Praag, H., Kempermann, G., Gage, F. H. Neural consequences of enviromental enrichment. Nature Reviews Neuroscience. 1 (3), 191-198 (2000).
- Hays, S. A., et al. The isometric pull task: A novel automated method for quantifying forelimb force generation in rats. Journal of Neuroscience Methods. 212 (2), 329-337 (2013).
- Wong, C. C., Ramanathan, D. S., Gulati, T., Won, S. J., Ganguly, K. An automated behavioral box to assess forelimb function in rats. Journal of Neuroscience Methods. 246, 30-37 (2015).
- Sindhurakar, A., Butensky, S. D., Carmel, J. B. Automated forelimb tasks for rodents: Current advantages and limitations, and future promise. Neurorehabilitation and Neural Repair. 33 (7), 503-512 (2019).
- Sindhurakar, A., et al. An automated test of rat forelimb supination quantifies motor function loss and recovery after corticospinal injury. Neurorehabilitation and Neural Repair. 31 (2), 122-132 (2017).
- Gallistel, C., et al. Screening for learning and memory mutations: A new approach. Acta psychologica Sinica. 42 (1), 138 (2010).
- Fenrich, K. K., et al. Improved single pellet grasping using automated ad libitum full-time training robot. Behavioural Brain Research. 281, 137-148 (2015).
- Brenneis, C., et al. Automated tracking of motion and body weight for objective monitoring of rats in colony housing. Journal of the American Association for Laboratory Animal Science. 56 (1), 18-31 (2017).
- Pereira, T. D., et al. Sleap: A deep learning system for multi-animal pose tracking. Nature Methods. 19 (4), 486-495 (2022).
- Lauer, J., et al. Multi-animal pose estimation, identification and tracking with deeplabcut. Nature Methods. 19 (4), 496-504 (2022).
- Butcher, G., et al. An apparatus for automatically training and collecting individualized behavioral data with socially housed rodents. Journal of Neuroscience Methods. 365, 109387 (2022).
- Winter, Y., Schaefers, A. T. A sorting system with automated gates permits individual operant experiments with mice from a social home cage. Journal of Neuroscience Methods. 196 (2), 276-280 (2011).
- Rivalan, M., Munawar, H., Fuchs, A., Winter, Y. An automated, experimenter-free method for the standardised, operant cognitive testing of rats. PLOS One. 12 (1), e0169476 (2017).
- Deacon, R. M. Housing, husbandry and handling of rodents for behavioral experiments. Nature Protocols. 1 (2), 936-946 (2006).
- Lang, C. E., Lohse, K. R., Birkenmeier, R. L. Dose and timing in neurorehabilitation: Prescribing motor therapy after stroke. Current Opinion in Neurology. 28 (6), 549 (2015).
- Butcher, G., Becker, A., Davidson, A., Baltazar, M., Armshaw, J., Cruz, S. Inventing a supercage for rats. , (2019).
- Davidson, A., et al. Engineering an enriched environment operant chamber and its implications. , (2019).
- Windle, V., et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Experimental Neurology. 201 (2), 324-334 (2006).
- Reppucci, C. J., Veenema, A. H. The social versus food preference test: A behavioral paradigm for studying competing motivated behaviors in rodents. MethodsX. 7, 101119 (2020).
- Borland, J. M., et al. A novel operant task to assess social reward and motivation in rodents. Journal of Neuroscience Methods. 287, 80-88 (2017).
- Tzschentke, T. M. Review on cpp: Measuring reward with the conditioned place preference (cpp) paradigm: Update of the last decade. Addiction Biology. 12 (3-4), 227-462 (2007).
- Salamone, J. D., Correa, M. Neurobiology and pharmacology of activational and effort-related aspects of motivation: Rodent studies. Current Opinion in Behavioral Sciences. 22, 114-120 (2018).
- Shull, R. L. Bouts, changeovers, and units of operant behavior. European Journal of Behavior Analysis. 12 (1), 49-72 (2011).
- Gottlieb, E., et al. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Medicine Reviews. 45, 54-69 (2019).
- Lo, E. H., et al. Circadian biology and stroke. Stroke. 52 (6), 2180-2190 (2021).
- Meng, H., Liu, T., Borjigin, J., Wang, M. M. Ischemic stroke destabilizes circadian rhythms. Journal of Circadian Rhythms. 6 (1), 1-13 (2008).
- Stern, R. A., Bachman, D. L. Depressive symptoms following stroke. The American Journal of Psychiatry. 148 (3), 351-356 (1991).
- Rapolienė, J., Endzelytė, E., Jasevičienė, I., Savickas, R. Stroke patients motivation influence on the effectiveness of occupational therapy. Rehabilitation Research and Practice. 2018, (2018).
- Robinson, R. G., Jorge, R. E. Post-stroke depression: A review. American Journal of Psychiatry. 173 (3), 221-231 (2016).
- Faraji, J., et al. Sex-specific stress and biobehavioral responses to human experimenters in rats. Frontiers in Neuroscience. 16, 965500 (2022).

