Host Cell Protein Analysis using Enrichment Beads Coupled with Limited Digestion
Summary
A protocol is presented for enriching host cell proteins (HCPs) from drug products (DP) and detecting peptides using proteome enrichment beads. The method is demonstrated using an in-house manufactured monoclonal antibody (mAb) drug substance (DS), which is a well-characterized reference material for evaluating and comparing different methods in terms of performance.
Abstract
Host cell proteins (HCPs) are impurities that can adversely affect therapeutic proteins, even in small quantities. To evaluate the potential risks associated with drug products, methods have been developed to identify low-abundance HCPs. A crucial approach for developing a sensitive HCP detection method involves enriching HCPs while simultaneously removing monoclonal antibodies (mAbs) before analysis, utilizing liquid chromatography-mass spectrometry (LC-MS).
This protocol offers detailed instructions for enriching host cell proteins using commercially available proteome enrichment beads. These beads contain a diverse library of hexapeptide ligands with specific affinities for different proteins. The protocol also incorporates limited digestion and subsequent peptide detection using nano LC-MS/MS. By employing these techniques, HCPs with low abundance can be enriched over 7000-fold, resulting in an impressive detection limit as low as 0.002 ppm. Significantly, this protocol enables the detection of 850 HCPs with a high level of confidence using a NIST mAb. Moreover, it is designed to be user-friendly and includes a video demonstration to assist with its implementation. By following these steps, researchers can effectively enrich and detect HCPs, enhancing the sensitivity and accuracy of risk assessment for drug products.
Introduction
Host cell proteins (HCPs) are impurities that are released from the cell culture of the host organism and co-purified with monoclonal antibody (mAb)1,2,3,4. Trace levels of HCPs can negatively impact the quality of the drug product5,6,7,8,9,10,11,12,13,14,15, and therefore, a sensitive HCP analysis method is desired to detect HCPs in sub-ppm to ppm levels.
Orthogonal methods can be applied to detect HCPs in low abundance. Enzyme-linked immunosorbent assay (ELISA) is generally used to quantitate overall HCPs, and it can also detect and quantitate individual HCPs if the corresponding antibodies are available16. However, the production of HCP-specific antibodies is time-consuming and labor-intensive. In contrast, liquid chromatography coupled with mass spectrometry (LC-MS) can provide comprehensive information about individual HCPs in mAb drug products and is widely applied for HCP identification4,7,9,10,12,13,14,15,17,18,19,20,21,22,23,24,25,26,27.
Several methods have been developed to detect HCPs with LC-MS/MS, including limited digestion20, filtration17, Protein A deletion21, immunoprecipitation (IP), and ProteoMiner enrichment (PM)18. Most methods aim to reduce the amount of mAb and enrich HCPs prior to LC-MS/MS analysis, thereby decreasing the dynamic range between mAb peptides and HCP peptides. This protocol presents a proteomic sample enrichment method that combines ProteoMiner technology and limited digestion (PMLD)28. The ProteoMiner enrichment principle involves using commercially available proteome enrichment beads containing a diverse library of combinatorial peptide ligands. These ligands specifically bind to proteins on antibody-drug products, allowing for the removal of excess molecules while concentrating low-abundance host cell proteins (HCPs) on their respective affinity ligands. On the other hand, the principle of limited digestion involves using a low concentration of trypsin. This concentration is sufficient to digest low-abundance HCPs but not enough to digest all antibody drug products. This approach enables the recovery and enrichment of digested HCP peptides from the solution.
Compared to filtration methods, the PMLD technique is not limited by the size of the detected HCPs17. Protein A deletion methods are specific to detecting HCPs associated with antibodies21, while immunoprecipitation is restricted to predefined HCPs from a particular cell line (such as the Chinese Hamster Ovary (CHO) cell line), where an anti-HCP antibody was generated4. In contrast, PMLD can be applied to detect HCPs from any drug modules and host cell proteins co-purified with drug products from various cell lines. Additionally, PMLD exhibits better sensitivity compared to the mentioned methods17,18,20,21,24.
This approach can enrich the HCP concentration by 7000-fold and lower the detection limit to 0.002 ppm28. The experimental setup is illustrated in Figure 1.
Protocol
Abbreviations used in the protocol are listed in Supplementary Table 1.
1. Preparation of solutions and buffers
NOTE: The commercial details of all the reagents are listed in the Table of Materials.
- Prepare 0.1 M Tris-HCl, pH 8.0 solution by adding 1 mL of 1 M Tris-HCl, pH 8.0 into 9 mL of deionized water in a glass vial, and mix well by vortexing. Store at 4 °C for up to 3 months.
- Prepare 24 mM sodium deoxycholate (SDC) by dissolving 10 mg of SDC in 1 mL of 0.1 M Tris-HCl, pH 8.0.
- Prepare 24 mM sodium lauroyl sarcosinate (SLS) by dissolving 7 mg of SLS in 1 mL of 0.1 M Tris-HCl, pH 8.0.
- Prepare elution buffer by mixing 24 mM SDC and 24 mM SLS at a 1:1 (v/v) ratio.
- Prepare 50 ng/µL of trypsin solution by adding 400 µL of deionized water into 20 µg trypsin.
- Prepare 25 mg/mL dithiothreitol (DTT) by adding 308 µL of 0.1 M Tris-HCl, pH 8.0, into 7.7 mg of DTT.
- Prepare 0.25 M iodoacetamide (IAM) by adding 1.2 mL of 0.1 M Tris-HCl, pH 8.0, into 56 mg of IAM.
- Prepare 10% TFA by adding 1 mL of trifluoroacetic acid (TFA) into 9 mL of deionized water. Vortex for 4 s and spin down. Store at 4 °C for up to 3 months.
- Prepare Buffer A by adding 1 mL of 10% TFA into 99 mL of deionized water.
- Prepare Buffer B by adding 1 mL of 10% TFA, and 49 mL of deionized water into 50 mL of acetonitrile (ACN).
- Prepare 10 mM Histidine buffer by adding 37 mg of L-histidine and 54.8 mg of L-Histidine monohydrochloride monohydrate into 50 mL of water.
2. Preparation of monoclonal antibody (mAb) solutions
- For drug products with concentrations above 50 mg/mL, dilute 15 mg of the monoclonal antibody (mAb) (see Table of Materials) with deionized water in a 2 mL microcentrifuge tube, resulting in a total volume of 300 µL and a final pH of ~6. If necessary, adjust the pH using acetic acid or Tris-HCl.
- For drug products with concentrations below 50 mg/mL, perform buffer exchange following steps 2.2.1 to 2.2.5.
- Transfer 20 mg of each sample to a 10k centrifugal filter device (see Table of Materials) and centrifuge at 14,000 x g for 25 min (at room temperature, RT) until approximately 100 µL volume remains.
- Add 350 µL of 10 mM Histidine (see Table of Materials), pH 6.0 buffer into the filter, vortex, and centrifuge at 14,000 x g for 25 min at RT. Repeat once.
- Invert the filter and place it into the sample collection tube, then centrifuge the samples at 1000 x g for 5 min at RT to retrieve the entire volume in the collection tube. Wash the filter with 200 µLof 10 mM Histidine buffer and pipet up and down to recover any remaining protein samples. Transfer the entire solution into the same collection tube, vortex, and spin down.
- Measure the sample concentration by NanoDrop. Adjust the concentration of each protein sample to 50 mg/mL by adding the Histidine buffer prior to incubation with enrichment beads.
- Transfer 300 µL of the sample into a 2 mL microcentrifuge tube.
3. Preparation of proteome enrichment beads
- Remove the top and bottom cap from each spin column obtained from the commercial protein enrichment kit (see Table of Materials). Do not discard the caps, as they will be used later.
- Take the spin column and position it in a 2 mL microcentrifuge tube without a cap. Centrifuge the setup at room temperature and 1,000 x g for 30-60 s at RT in order to eliminate the storage solution. Dispose of the material collected during this step.
- Add 200 µL wash buffer to the commercially available enrichment beads (see Table of Materials) and pipet up and down several times. Place the spin column in a 2 mL microcentrifuge tube and centrifuge at 1,000 x g for 30-60 s at RT to remove the buffer. Discard collected material.
NOTE: The wash buffer is included in the commercial protein enrichment kit. - Repeat steps 3.3 twice.
- Replace the bottom cap and add 200 µL water, then replace the top cap.
4. Protein enrichment
- Pipet up and down the slurry (from step 3.5) and transfer 40 µL of slurry to the sample prepared in step 2. Incubate and rotate the tube at room temperature for 2 h.
- Make a tip with frit (see Table of Materials) using the 16 G needle to get the appropriate frit size, then insert it into the tip end of the 200 µL tip.
- Transfer the sample with beads to the tip prepared in step 4.2. Centrifuge the tip with 2 mL microcentrifuge tube at 200 x g for 3 min at RT to remove the solution.
- Wash the beads by adding 200 µL wash buffer to the tip and centrifuge the tip with a 2 mL microcentrifuge tube at 200 x g for 2 min at RT to remove the washing solution. Repeat the step twice.
- Wash the beads by adding 200 µL water to the tip and centrifuge the tip with a 2 mL microcentrifuge tube at 200 x g for 2 min at RT to remove the water.
- Elute proteins from beads by adding 10 µL of elution buffer (step 1.4) into the tip, and pipet the slurry ten times in the tip to ensure the beads are soaked with elution buffer.
- Centrifuge the tip with a new 0.5 mL microcentrifuge tube at 200 x g for 30 s at RT to collect the eluent. Repeat steps 4.6-4.7 twice and combine all elution into one 0.5 mL microcentrifuge tube.
- Add 1.5 µL of trypsin solution (step 1.5) into eluent for overnight digestion at 28 °C. Add 1.5 µL of 25 mg/mL DTT (step 1.6), and heat the sample at 90 °C for 20 min.
- Add 1.5 µL of 0.25 M IAM (step 1.7) and incubate at room temperature in the dark for 20 min. Add 3.5 µL 10% TFA (step 1.8), vortex for 2 min, and ensure pH is at ~2-3.
- Centrifuge at 14,400 × g for 10 min at RT to precipitate SDC and SLS. Collect the supernatant for desalting.
- Perform desalting following the steps below.
- Add 50 µL of Buffer B (step 1.10) to the GC-desalting tip (see Table of Materials). Centrifuge the tip with a holder at 1000 x g for 3 min at RT. Discard the collected material.
- Add 50 µL of Buffer A (step 1.9) to the tip. Centrifuge the tip with a holder at 1000 x g for 3 min at RT. Discard the collected material.
- Add the acidifed sample to the tip. Centrifuge the tip with a holder at 500 x g for 6 min at RT. Discard the collected material.
- Wash the tip by adding 50 µL of Buffer A to the tip. Centrifuge the tip with the holder at 500 x g for 3 min at RT. Discard the collected material. Repeat once.
- Elute the peptide from the tip by adding 50 µL of Buffer B to the GC-desalting tip. Centrifuge the tip with a new tube at 500 x g for 3 min at RT. Collect the material. Repeat the step once and combine the eluent.
- Dry the eluent with a vacuum concentrator (see Table of Materials).
- Resuspend the dried eluent into 30 µL of 0.1% formic acid (FA) solution.
- Measure the UV of peptide mixtures at 214 nm by a spectrophotometer (see Table of Materials). The concentration of peptide mixtures should range between 0.1 and 0.5 mg/mL.
- Transfer 10 µL of each digested sample into an LC sample vial, then inject 1 µg of each digested sample onto nano LC-MS/MS (see Table of Materials). Perform the analysis following step 5. Store the rest of digested samples in a -80 °C freezer.
5. Nano LC-MS/MS analysis
- Inject the peptide mixture into a nano-LC system coupled to a mass spectrometer. Load the peptide mixtures (~1 µg) onto a C18 trap column with the gradient provided in Table 1A for desalting and then onto a C18 analytical column at 40 °C for separation.
NOTE: The mobile phase A buffer consisted of 0.1% FA in ultra-pure water, and the mobile phase B consisted of 0.1% FA in 80% ACN. - Separate the peptides and elute with the gradient provided in Table 1B with a flow rate of 250 nL/min.
NOTE: The mass spectrometer was operated in data-dependent mode (DDA) with a cycle time of 3 s. Ions underwent fragmentation through higher-energy collisional dissociation (HCD) with a normalized collision energy of 30% for each full MS scan. The scans were performed at a resolution of 60,000, with an automatic gain control (AGC) target of 3e6, a maximum injection time of 20 ms, and an m/z range of 380-1600. MS/MS events were conducted at a resolution of 15,000, with an AGC target of 1e5, a maximum injection time of 60 ms, and an m/z range of 200-2000. The exclusion duration was set to 45 s. The ion source properties are provided in Table 1C, and the specific mass spectrometry parameters can be found in Table 2A-F.
6. Data analysis
- Perform a search of the NISTmAb mass spectrometry raw files against the UniProt mus+musculus database29. Additionally, search for the recombinant protein spiked-in mAb DS mass spectrometry raw files against the UniProt Cricetulus Griseus database30.
NOTE: These searches were conducted in Proteome Discoverer software (see Table of Materials) using the Sequest HT and Mascot search engines. The databases used were free of redundant entries. - For the mass spectrometry searches, apply a mass tolerance of 10 ppm, and set the fragment mass tolerance to 0.02 Da.
NOTE: The search criteria encompassed static carbamidomethylation of cysteine residues (+57.0214 Da) and variable modification of oxidation (+15.9949 Da) on methionine residues. Additionally, a variable modification of deamination (+0.984 Da) was applied. - Perform the database search using trypsin digestion, allowing for a maximum of two missed cleavages. Set the false-discovery rates for both proteins and peptides at 0.01.
NOTE: To positively identify host cell proteins (HCPs), a minimum requirement of at least two unique peptides was applied. Table 3 provides the representative summary of identified HCPs associated with NIST mAb analyzed by Proteome Discoverer software.
Representative Results
This protocol presented a sample preparation workflow, termed protein enrichment coupled with limited digestion (PMLD), for the analysis of host cell proteins (HCPs) in a monoclonal antibody (mAb) sample. Figure 1 illustrates the step-by-step procedure of PMLD. The researchers compared the results of HCP analysis using direct digestion (shown in the top panel of Figure 2) and PMLD (shown in the bottom panel of Figure 2). The Total Ion Chromatogram (TIC) profiles indicated that PMLD significantly reduced or eliminated major mAb peptides while allowing the observation of some HCP peptides comparable to mAb peptides. Detailed parameters for nano LC (Table 1) and MS/MS (Table 2) analyses are provided. Additionally, Table 3 displays a summary of identified HCPs associated with the NIST mAb, including information such as accession number, HCP name, species, coverage percentage, PSMs, unique peptides, molecular weight, expected isoelectric point (pI), Mascot score, Sequest HT score, and the number of peptides identified by Mascot and Sequest HT.
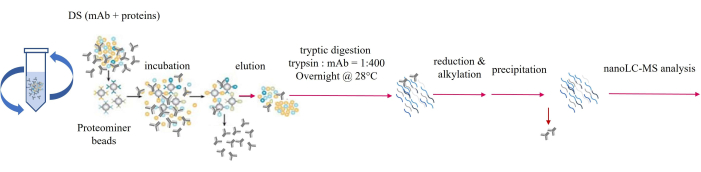
Figure 1: Sample preparation workflow of protein enrichment coupled with limited digestion (PMLD). Please click here to view a larger version of this figure.
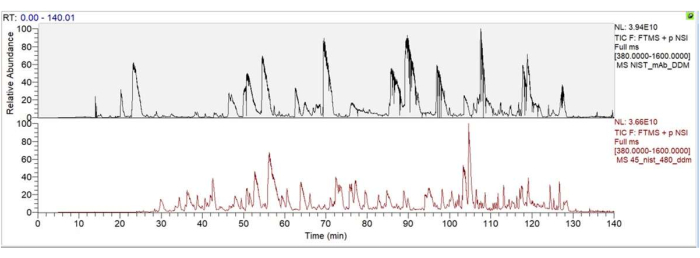
Figure 2: Total Ion Chromatogram (TIC) profile for HCP analysis of NIST mAb. Top panel: TIC profile for HCP analysis of NIST mAb using direct digestion. Bottom panel: TIC profile for HCP analysis of NIST mAb using PMLD. It is evident from the TIC profile that compared to direct digestion, most of the major mAb peptides have been reduced or eliminated after PMLD, while some peptides belonging to HCPs can be observed and are comparable to mAb peptides. Please click here to view a larger version of this figure.
Table 1: Parameters for nano LC. (A) Loading pump gradient. (B) NC pump gradient. (C) Ion source properties. Please click here to download this Table.
Table 2: Parameters for MS/MS. (A) Full scan properties. (B) MIPS properties. (C) Intensity properties. (D) Charge state properties. (E) Dynamic exclusion. (F) Data-dependent MS2 scan properties. Please click here to download this Table.
Table 3: Representative summary table of identified HCPs associated with NIST mAb analyzed by Proteome Discoverer software. The table includes information such as the accession number, HCP name, species, percentage of coverage, number of peptide spectrum matches (PSMs), number of unique peptides, molecular weight, expected isoelectric point (pI), Mascot score, Sequest HT score, and the number of peptides identified by Mascot and Sequest HT. Please click here to download this Table.
Supplementary Table 1: List of abbreviations used. Please click here to download this Table.
Discussion
There are two versions of commercially available protein enrichment beads: one with a smaller capacity and the other with a larger capacity (see Table of Materials). Both versions of the enrichment beads contain ten preps in the package. The manufacturer’s instructions suggest that each prep from the small capacity kit can be used to enrich 10 mg of total protein. However, for optimal performance of host cell protein (HCP) enrichment from DS, each prep is good for five DS samples. Therefore, each kit can be used to enrich HCPs from fifty samples. For each prep from the large capacity kit, it is good for the enrichment of HCPs from twenty-five DS samples, allowing the detection of HCPs from a total of two hundred and fifty samples.
Step 3.1 advises against discarding the top or bottom caps, as they will be reused throughout the entire protocol. If beads settle in the top cap, replace them after removing the bottom plug and centrifuging while keeping the top cap on the column. To utilize the bottom cap as a plug, invert it and securely position it at the bottom of the spin column.
A critical step in the procedure is step 4.1, where the bead slurry settles at the bottom of the tube. Therefore, it is crucial to maintain continuous pipetting up and down while transferring the slurry into the monoclonal antibody (mAb) samples. Another critical step is step 4.6, where it is essential to pipette the slurry ten times within the tip while the beads are saturated with elution buffer. The duration of centrifugation in steps 4.3-4.5, 4.7, and 4.10 may vary depending on the amount of accumulated host cell proteins (HCPs). Therefore, it is crucial to check and ensure that the liquids in the tip have been properly spun down before proceeding to the next step. Again, when starting with an initial quantity of 15 mg of antibody-drug product, approximately 30 µg of antibody and enriched host cell proteins (HCPs) can be eluted. To achieve a protein-to-enzyme ratio of 400:1 for limited digestion, 75 ng of trypsin was added. When a lower amount of input material is used, measuring the concentration of the eluted protein sample is necessary. This measurement helps adjust the amount of trypsin that must be added accordingly. Step 4.9 shows a white precipitate after adding the 10% TFA. To prevent any interference from surfactants with the MS signal, it is crucial to check the pH after adding trifluoroacetic acid (TFA) to ensure the complete removal of the detergent from the supernatant.
Following the drying and resuspension of the eluent in water, it is necessary to perform a UV measurement of the peptide mixture. This measurement is crucial because the quantity of peptides loaded onto the column can influence the detection using MS. After evaluation, it was found that approximately 1 µg of the peptide mixture exhibited the best performance in MS. Consequently, this optimal amount needs to be injected into nano LC-MS/MS for further analysis.
The PMLD approach offers several advantages in sample preparation, including sufficient monoclonal antibody (mAb) DS reduction while preserving the majority of low-abundance host cell proteins (HCPs). Unlike immunoprecipitation (IP), this technique does not rely on anti-HCP antibodies, thus eliminating the bias associated with predefined antibodies and reducing the labor and time required for producing anti-HCP antibodies. Furthermore, unlike filtration methods based on molecular weight cut-off17, PMLD enriches HCPs regardless of their size. It can be applied to enrich HCPs from various drug modules, such as antibodies, fusion proteins, ScFv, and more, making it a versatile approach compared to Protein A deletion methods21.
In addition to these advantages, PMLD exhibits a lower detection limit and enables the detection of a greater number of HCPs from NISTmAb, a widely characterized reference standard, compared to other methods. However, it is worth noting that PMLD requires homemade equipment, which limits its application for automation. To expand its usability, replacing certain equipment, such as the tip with frit, with commercially available alternatives is possible. Additionally, performing desalting on 96-well plates can enable higher throughput using this approach. Exploring automation or semi-automation in future experiments can be a logical next step to expand the wider application of PMLD.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| 16 G, Metal Hub Needle, 2 in, point style 3 | Hamilton | 91016 | |
| Acclaim PepMap 100 C18 trap column (20 cm × 0.075 mm) | Thermo Fisher | 164535 | |
| Acetonitrile | Fisher-Scientific | A955 | |
| Acetonitrile with 0.1% Formic Acid (v/v), Optima LC/MS Grade | Fisher-Scientific | LS120-4 | |
| Amicon Ultra-0.5 Centrifugal Filter Unit | Millipore Sigma | UFC5010 | |
| C18 analytical column (0.075 mm × 1.7 μm × 30 cm, 100 Å) | CoAnn Technologies | HEB07503001718I | |
| Centrifuge 5424 | Eppendorf | 5405000646 | |
| Dithiothreitol (DTT) | Thermo Fisher | A39255 | |
| Frit for SPE cartridges, 9.5 mm, 3 mL, 100/pk | Agilent | 12131020 | |
| GL-Tip GC | GL Sciences Inc | 7820-11201 | |
| in-house mAb | Regeneron | concentration 200 mg/mL | |
| Iodoacetamide (30 x 9.3 mg) | Thermo Fisher | A39271 | |
| Isopropanol | Fisher-Scientific | 149320025 | |
| L-Histidine | Sigma Aldrich | H6034 | |
| L-Histidine monohydrochloride monohydrate | Sigma Aldrich | 53370 | |
| Methanol | Fisher-Scientific | A456-4 | |
| Milli-Q | Millpore | 30035 | |
| NanoDrop 2000 | Thermo Scientific | ND-2000 | |
| Orbitrap Exploris 480 | Thermo Fisher | BRE725539 | |
| Protein LoBind Tube 0.5 mL | Eppendorf (VWR) | 22431064 | |
| Protein LoBind Tube 2.0 mL | Eppendorf (VWR) | 22431102 | |
| Proteome Discoverer software 2.4 | Thermo Scientific | ||
| ProteoMiner Protein Enrichment Large-Capacity Kit | Bio-Rad | 1633007 | |
| ProteoMiner Protein Enrichment Small-Capacity Kit | Bio-Rad | 1633006 | |
| Sodium deoxycholate (SDC) | Sigma Aldrich | D6750 | |
| Sodium lauroyl sarcosinate (SLS) | Sigma Aldrich | L5777 | |
| SpeedVac | Labconco | 7970010 | |
| Thermomixer R | Eppendorf | 22670107 | |
| Trifluoracetic acid (TFA) | Fisher-Scientific | 28904 | |
| Trypsin (Sequencing Grade Modified) (5 x 20 ug) | Promega | V5111 | |
| Tube Revolver Rotator | Thermo Fisher | 88881001 | |
| UltiMate 3000 RSLC nano system | Thermo Fisher | ULTIM3000RSLCNANO | |
| UltraPure 1 M Tris-HCl pH 8.0 | Thermo Fisher | 15568-025 | |
| Vortex Genie 2 | VWR | 102091-234 | |
| Water with 0.1% Formic Acid (v/v), Optima LC/MS Grade | Fisher-Scientific | LS118-4 |
References
- Aboulaich, N. A novel approach to monitor clearance of host cell proteins associated with monoclonal antibodies. Biotechnology Progress. 30 (5), 1114-1124 (2014).
- Goey, C. H., Alhuthali, S., Kontoravdi, C. Host cell protein removal from biopharmaceutical preparations: Towards the implementation of quality by design. Biotechnology Advances. 36 (4), 1223-1237 (2018).
- Levy, N. E., Valente, K. N., Choe, L. H., Lee, K. H., Lenhoff, A. M. Identification and characterization of host cell protein product-associated impurities in monoclonal antibody bioprocessing. Biotechnology and Bioengineering. 111 (5), 904-912 (2014).
- Molden, R. Host cell protein profiling of commercial therapeutic protein drugs as a benchmark for monoclonal antibody-based therapeutic protein development. MAbs. 13 (1), 1955811 (2021).
- Bee, J. S. Trace levels of the CHO host cell protease cathepsin D caused particle formation in a monoclonal antibody product. Biotechnology Progress. 31 (5), 1360-1369 (2015).
- Bracewell, D. G., Francis, R., Smales, C. M. The future of host cell protein (HCP) identification during process development and manufacturing linked to a risk-based management for their control. Biotechnology and Bioengineering. 112 (9), 1727-1737 (2015).
- Chiu, J., et al. Knockout of a difficult-to-remove CHO host cell protein, lipoprotein lipase, for improved polysorbate stability in monoclonal antibody formulations. Biotechnology and Bioengineering. 114 (5), 1006-1015 (2017).
- Gilgunn, S., et al. Identification and tracking of problematic host cell proteins removed by a synthetic, highly functionalized nonwoven media in downstream bioprocessing of monoclonal antibodies. Journal of Chromatography A. 1595, 28-38 (2019).
- Graf, T. Identification and characterization of polysorbate-degrading enzymes in a monoclonal antibody formulation. Journal of Pharmaceutical Sciences. 110 (11), 3558-3567 (2021).
- Hall, T., Sandefur, S. L., Frye, C. C., Tuley, T. L., Huang, L. Polysorbates 20 and 80 degradation by group XV lysosomal phospholipase A2 isomer X1 in monoclonal antibody formulations. Journal of Pharmaceutical Sciences. 105 (5), 1633-1642 (2016).
- Jones, M. 34;High-risk" host cell proteins (HCPs): A multi-company collaborative view. Biotechnology and Bioengineering. 118 (8), 2870-2885 (2021).
- Li, X., et al. Identification and characterization of a residual host cell protein hexosaminidase B associated with N-glycan degradation during the stability study of a therapeutic recombinant monoclonal antibody product. Biotechnology Progress. 37 (3), e3128 (2021).
- Zhang, S. Identification of the specific causes of polysorbate 20 degradation in monoclonal antibody formulations containing multiple lipases. Pharmaceutical Research. 39 (1), 75-87 (2022).
- Zhang, S., Xiao, H., Li, N. Degradation of polysorbate 20 by Sialate O-Acetylesterase in monoclonal antibody formulations. Journal of Pharmaceutical Sciences. 110 (12), 3866-3873 (2021).
- Zhang, S., Xiao, H., Molden, R., Qiu, H., Li, N. Rapid polysorbate 80 degradation by liver carboxylesterase in a monoclonal antibody formulated drug substance at early stage development. Journal of Pharmaceutical Sciences. 109 (11), 3300-3307 (2020).
- Gunawan, F. Comparison of platform host cell protein ELISA to process-specific host cell protein ELISA. Biotechnology and Bioengineering. 115 (2), 382-389 (2018).
- Chen, I. H., Xiao, H., Daly, T., Li, N. Improved host cell protein analysis in monoclonal antibody products through molecular weight cutoff enrichment. Analytical Chemistry. 92 (5), 3751-3757 (2020).
- Chen, I. H., Xiao, H., Li, N. Improved host cell protein analysis in monoclonal antibody products through ProteoMiner. Analytical Biochemistry. 610, 113972 (2020).
- Doneanu, C. E., et al. Enhanced detection of low-abundance host cell protein impurities in high-purity monoclonal antibodies down to 1 ppm using ion mobility mass spectrometry coupled with multidimensional liquid chromatography. Analytical Chemistry. 87 (20), 10283-10291 (2015).
- Huang, L., et al. A Novel sample preparation for shotgun proteomics characterization of HCPs in antibodies. Analytical Chemistry. 89 (10), 5436-5444 (2017).
- Johnson, R. O., Greer, T., Cejkov, M., Zheng, X., Li, N. Combination of FAIMS, Protein A depletion, and native digest conditions enables deep proteomic profiling of host cell proteins in monoclonal antibodies. Analytical Chemistry. 92 (15), 10478-10484 (2020).
- Kreimer, S. Host cell protein profiling by targeted and untargeted analysis of data independent acquisition mass spectrometry data with parallel reaction monitoring verification. Analytical Chemistry. 89 (10), 5294-5302 (2017).
- Madsen, J. A., et al. Toward the complete characterization of host cell proteins in biotherapeutics via affinity depletions, LC-MS/MS, and multivariate analysis. MAbs. 7 (6), 1128-1137 (2015).
- Nie, S. Simple and sensitive method for deep profiling of host cell proteins in therapeutic antibodies by combining ultra-low trypsin concentration digestion, long chromatographic gradients, and boxcar mass spectrometry acquisition. Analytical Chemistry. 93 (10), 4383-4390 (2021).
- Yang, F. Versatile LC-MS-Based workflow with robust 0.1 ppm sensitivity for identifying residual HCPs in biotherapeutic products. Analytical Chemistry. 94 (2), 723-731 (2022).
- Zhang, Q. Comprehensive tracking of host cell proteins during monoclonal antibody purifications using mass spectrometry. MAbs. 6 (3), 659-670 (2014).
- Zhang, S., et al. Putative phospholipase B-Like 2 is not responsible for polysorbate degradation in monoclonal antibody drug products. Journal of Pharmaceutical Sciences. 109 (9), 2710-2718 (2020).
- Zhang, J., He, J., Smith, K. J. Fatty acids can induce the formation of proteinaceous particles in monoclonal antibody formulations. Journal of Pharmaceutical Sciences. 111 (3), 655-662 (2022).
- Uniprot1. . , (2023).
- Uniprot2. . , (2023).

