Author Spotlight: Analyzing Maxillary Bone Remodeling and Dental Root Resorption for Improved Dental Treatments
Summary
Here, we present a protocol for studying orthodontic tooth movement (OTM), serving as a suitable model for investigating the mechanisms of bone adaptation, root resorption, and the response of bone cells to mechanical stimuli. This comprehensive guide provides detailed information on the OTM model, micro-computed tomography acquisition, and subsequent analysis.
Abstract
Orthodontic tooth movement (OTM) represents a dynamic process in which the alveolar bone undergoes resorption at compression sites and deposition at tension sites, orchestrated by osteoclasts and osteoblasts, respectively. This mechanism serves as a valuable model for studying various aspects of bone adaptation, including root resorption and the cellular response to mechanical force stimuli. The protocol outlined here offers a straightforward approach to investigate OTM, establishing 0.35 N as the optimal force in a mouse model employing a nickel-titanium (NiTi) coil spring. Utilizing micro-computed tomography analysis, we quantified OTM by assessing the discrepancy in the linear distance at the cement-enamel junction. The evaluation also included an analysis of orthodontic-induced inflammatory root resorption, assessing parameters such as root mineral density and the percentage of root volume per total volume. This comprehensive protocol contributes to advancing our understanding of bone remodeling processes and enhancing the ability to develop effective orthodontic treatment strategies.
Introduction
Bone remodeling is an ongoing process orchestrated by osteoclasts, osteoblasts, bone lining cells, and osteocytes, essential for maintaining the integrity of the adult skeleton1,2. Primarily driven by the differentiation and activity of osteoclasts and osteoblasts, this dynamic process involves the resorption and deposition of bone, triggered by mechanical stress and loading3,4,5.
Animal experiments play a pivotal role in elucidating the intricate biological and cellular mechanisms underpinning orthodontic tooth movement (OTM)6,7. This process involves a diverse array of cell types, such as osteoblasts, osteoclasts, osteocytes, fibroblasts, and immune cells like macrophages and T cells, situated within the jawbone and periodontal ligament7,8. These cells dynamically respond to mechanical stimuli and changes in the local milieu, influencing the composition and architecture of the surrounding bone7,8. Moreover, they also trigger an inflammatory response at a cellular level, even though there are no pathogens present. This inflammatory response plays a role in increasing the turnover of bone tissue9.
Various animal models, including mice, rats, rabbits, dogs, and monkeys, have been utilized in experimental studies of OTM7,8,10. Among these, rodents, particularly mice, are favored for investigating the initial phases of tooth movement and bone remodeling6. Previous research has emphasized the advantages of using mouse models over rat models, primarily due to the widespread availability of genetically modified strains, enabling detailed exploration of genetic influences in OTM7,11. Currently, two main models are employed to induce tooth movement in mice. The first method entails inserting a nickel-titanium (NiTi) coil spring between the first upper molar and upper incisors4,12. The second approach involves placing an elastic band within the interdental space between the first and second upper molars13. The primary outcomes analyzed typically include the magnitude of the tooth movement and bone microarchitecture, preferably evaluated using micro-computed tomography (micro-CT)14. Ideally, assessing the integrity of dental roots is important to ensure that appropriate forces are employed to produce OTM4.
While micro-CT is widely acknowledged as the gold standard for evaluating the microarchitecture of mineralized tissues14, the absence of standardized methodologies and protocols for scanning, analyzing, and reporting data often presents challenges in discerning the precise procedures employed, interpreting results, and facilitating comparisons between different OTM models14,15.
Here, we present a step-by-step guide to the OTM mouse model, including micro-CT acquisition and analysis of OTM, bone microstructure, and dental roots. This method entails applying controlled mechanical force to the first molar to induce movement within the jawbone. The selection of this method stems from several factors, including feasibility, relevance, and precision. Such an approach enables detailed quantitative analysis, providing valuable insights into the biological processes underlying orthodontic tooth movement and facilitating the development of improved orthodontic treatment strategies in the future.
Protocol
All procedures strictly adhered to the ethical standards established by the Universidade Federal de Minas Gerais Ethics Committee (No. 166/2022). Before each experiment, a sample size calculation is mandatory. Use 8-10-week-old male C57BL6/J wild-type mice weighing approximately 20-30 g. The mice must be housed in a cage within a room maintained at 25 °C, adhering to a 12 h light/12 h dark cycle. Following coil attachment, the animal should be fed with a soft diet. Daily monitoring should include assessments of body weight and overall health.
1. Mechanically-induced alveolar bone remodeling
- Using distal cut pliers, cut the 0.25 x 0.76 inches NiTi open-coil spring to the following dimensions of six loops and two loop-shaped ends positioned perpendicular to the spring using orthodontic Weingart pliers.
- Shape the 0.20 mm diameter round chrome-nickel (CrNi) wire to the desired configuration with loop-shaped ends using Mathieu tweezers and a round-shaped instrument as a size reference.
- Put together the loop-shaped ends of the coil and the 0.20 mm round CrNi wire.
- Anesthetize the animal with an intraperitoneal injection of 0.2 mL of solution containing xylazine (10 mg/Kg) and ketamine (100 mg/Kg). Before starting the procedure, evaluate the depth of anesthesia using the pedal reflex. Carefully pinch one of the animal's toes using tweezers. The absence of a reflex indicates an adequate plane of general anesthesia. To avoid corneal injuries and post-operative pain, apply an ocular lubricant after the animal is anesthetized.
- Position the animal in dorsal decubitus on a surgical table, immobilizing its limbs to restrict movement and enable intraoral access.
- Utilize a mouth-opener, fashioned from 0.50 mm diameter wire and secured with a 0.08 mm wire, to facilitate full visualization while preventing head movement. Use the right side as the experimental side (OTM side) and the left side as the control without an orthodontic coil (Control side).
NOTE: Enhanced visualization of intraoral structures must be achieved using a stereomicroscope and an optical light system. - Clean and etch the right first molar and incisor surfaces using acetone and a self-etching primer, respectively. The system is self-etching, meaning it does not require prior acid conditioning.
- Apply the primer in a single step, which also functions simultaneously as an acid and an adhesive. Using a microbrush, collect a small amount of self-etching primer and apply it to the occlusal surface of the upper first molar and incisors. Care must be taken in this step to ensure that the self-etching primer does not reach the proximal surface between the first and second molars, as this could cause the dental elements to stick together, preventing tooth movement. Light cure the primer at the occlusal surface of the molars and incisors for 30 s.
- Bond the distal end of a six-loop NiTi open-coil spring to the occlusal surface of the right first maxillary molar with light-cured resin and light cure for 30 s. Add additional resin increment to the edge of the wire to avoid harm to the mice and light cure for 30 s.
- Activate the coil using a specifically designed apparatus consisting of a rail and crank mechanism attached to the surgical table. This enables longitudinal movement to slide back and forth.
- Connect the free loop-shaped end of the 0.20 mm round wire to the hook of the tension gauge.
- Upon activation of the crank, move the surgical table along the rail until the dynamometer registers a force of 0.35 N.
- Bond the 0.20 mm round wire to both upper incisors to anchor the coil. No further reactivation is performed during the experimental period. Cut the wire to detach the animal from the dynamometer. Add another increment of resin so the metal edge of the device is not exposed and does not hurt the animal. Light cure for 30 s. Disassemble the animal from the table.
- The upper right first molar with a device imposing a force of 0.35 N in the mesial direction consists of the experimental side. Use the left side of the maxilla (without an orthodontic appliance) as a control4,16.
- Maintain this device for a period of 12 days without any activation being required. Do not use any pain control medication. Orthodontic movement occurs through an inflammatory process, and pain-relieving medication may negatively affect the arachidonic acid cascade, impacting the rate of bone remodeling and potentially invalidating the results.
- After the end of the surgery, treat the animals with a subcutaneous injection of saline solution to avoid dehydration during the adaptation period with the device. Keep the animal in an individual cage with heating until it is fully recovered and only after this period place the animal in collective cages.
- Euthanize the mouse on the 12th day performed by sedation with intraperitoneal injection of a 0.2 mL solution containing xylazine (10 mg/Kg) and ketamine (100 mg/Kg) followed by decapitation with sharp scissors.
2. Micro-CT measurements
- Harvest the maxillary bone with sharp scissors cutting all soft tissue, the zygomatic bone in the sagittal plane, and the frontonasal suture and the spheno-occipital synchondrosis in the coronal plane. Immerse the maxillary bone in 10% neutral buffered formalin (pH=7.4) for a fixation period of 48 h. After this period, change the formaldehyde solution to 70% alcohol.
- Perform micro-CT scanning of the maxillary bone with the following parameters for high-resolution scans: isotropic voxel size of 9 to 18 µm, X-ray settings of 50 kV, 0.5 mm aluminum filter, and rotation angle of 0.5°. More than one jaw can be fit during the microCT scanning.
- Reconstruct the acquired images using the microtomography reconstruction program indicated by the manufacturer of the micro-computed tomography (microCT) used17.
- Position the reconstructed images using the 3D Inspection program indicated by the manufacturer of the microtomography used.
- Quantify OTM by measuring the difference in linear distance between the cement-enamel junction (CEJ) of the first and second molars of the right hemi-maxilla (OTM side) relative to the left hemi-maxilla (control side). Utilize the proper microCT analyzer software with the line tool for this measurement17,18,19,20.
- Check the samples for the presence of orthodontic-induced inflammatory root resorption (OIIRR). Select the region of interest (ROI) of the disto-vestibular root of the first maxillary molar by using an irregular, anatomic region of interest drawn manually contouring method. Measure the following parameters: root mineral density (RMD; g/cm3) and percentage of root volume per total volume (RV/TV; %). Utilize the proper microCT analyzer software with the 3D volumetric tool for this measurement16,21.
- Conduct the reconstruction of the first maxillary molar using Mimics software and analyze the obtained data to draw conclusions regarding OTM and OIIRR in the experimental model16,21.
Representative Results
This protocol enables the investigation of an OTM mouse model using a NiTi coil spring. With a force of 0.35 N applied, the mean CEJ distance on the control side between the first and second molars was 243.69 µm (Figure 1A, line A), whereas on the OTM side was measured at 284.66 µm (Figure 1A, line B). The difference between the OTM and control sides was 40.97 µm (Figure 1B). The linear distance between the CEJ between the first and second molars of the right hemi-maxilla (OTM side) and the left hemi-maxilla (control side) was measured in at least six different sections with the line tool using the proper microCT analyzer software. The mean value of the six measurements of the OTM side was subtracted from the mean value of the control side to generate the OTM result (Figure 1).
Regarding OIIRR, values of dental RV/TV and RMD were demonstrated on both the control side (71.02 ± 10.11% and 1.071 ± 0.1536 g/cm2, respectively) and the OTM side (73.38 ± 6.162% and 1.048 ± 0.2119 g/cm2 µm, respectively; Figure 2).
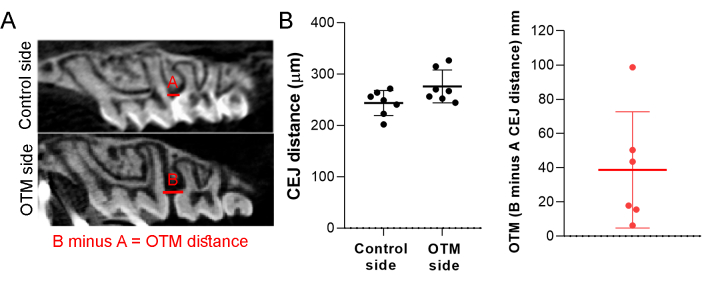
Figure 1: Orthodontic tooth movement (OTM). (A) Representative image of the control and OTM sides. (B) Results of the CEJ distance of the control and OTM sides and the difference between the distance resulting in the OTM distance. A force of 0.35 N was applied in the mesial direction of the upper right first molar using a NiTi coil spring, and the endpoint of the study was the 12th day. Mean ± standard deviation of the OTM results generated from 10 mice. Please click here to view a larger version of this figure.
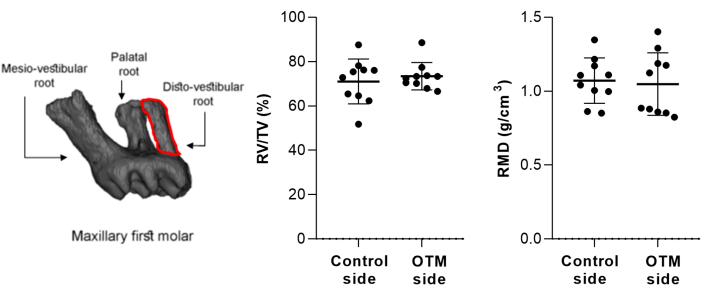
Figure 2: Representative image of the selected region of interest (ROI). The ROI of the disto-vestibular root of the first maxillary molar is shown, which was selected by using an irregular, anatomic region of interest drawn using the manual contouring method to analyze root mineral density (RMD; g/cm3) and percentage of root volume per total volume (RV/TV; %). Mean ± standard deviation results were generated from 10 mice. Please click here to view a larger version of this figure.
Discussion
Here, we describe a standardized protocol designed to elucidate the cellular and molecular mechanisms underlying bone remodeling during OTM. A thorough understanding of these mechanisms in mice requires a meticulously planned protocol to ensure accuracy and reliability7,11. Studies conducted by our research group have shown that this protocol effectively reduces operator variability by incorporating a tension gauge and a specially designed apparatus, establishing 0.35 N as the optimal force for OTM in a mouse model4,16,17,18,19,20. To improve the efficacy of experimental procedures and maximize the use of animal samples, specimens utilized for micro-CT analysis can also undergo processing for routine histology. Subsequently, OTM can be evaluated in 5 µm sections, utilizing the CEJ as a reference point for measurements16,19. Hematoxylin and eosin staining, alongside specialized techniques such as tartrate-resistant acid phosphatase (TRAP) and Masson's trichrome, also serve as effective methods for assessing root integrity and quantifying osteocytes, osteoclast, and osteoblasts within the regions of interest4,5,16.
The dynamic alterations in the microstructure of alveolar bone during OTM in rodents have been investigated using micro-CT systems. These evaluations aim to offer valuable insights for clinical orthodontic treatment14,15. Micro-CT is an analytical technique capable of capturing internal structures with high resolution and micron-level precision. This method allows for the reconstruction of small-scale specimens into detailed 3D images, enabling accurate qualitative and quantitative analysis of samples22. Consistent with previous studies, OTM was assessed by quantifying the linear discrepancy between the CEJ of the first and second molars of the right hemi-maxilla compared to the left hemi-maxilla17,18,19,20. In the context of dental imaging, micro-CT presents several advantages. It can identify small defects on root surfaces, precisely measure linear dental changes, and assess the trabecular and cortical bone morphology22,23. It is noteworthy that the current guideline for assessing bone microstructure in rodents using micro-CT emphasizes the minimal set of variables recommended for describing trabecular and cortical bone morphometry23. Additionally, it is crucial to analyze the occurrence of OIIRR, a serious complication during orthodontic treatment16,21. Researchers should evaluate the intensity of root resorption, as the presence of OIIRR indicates that the applied force may be excessive, necessitating adjustments16,21.
Adhering to critical steps in OTM research in animal models is essential for obtaining reliable results. This includes careful selection of animals, considering factors such as strain, age, bone metabolism, growth rate, and genetic background. The choice of animal models significantly influences study outcomes, as variations in these factors affect bone physiology and response to orthodontic forces6. Timing the euthanasia of animals is also critical because it captures specific stages of bone remodeling in response to orthodontic forces. For instance, Taddei et al.4 conducted molecular analysis at 0, 12, and 72 hours, with histopathological analysis at 6 days, enabling the assessment of temporal changes in bone remodeling markers during OTM. In addition, age-related variations in OTM have been investigated, shedding light on how aging impacts bone remodeling processes23.
The use of a NiTi coil spring, while effective in inducing tooth movement, presents certain drawbacks that may affect animal welfare and experimental outcomes. Inserting a NiTi coil spring is noted to be more time-consuming and technically demanding compared to alternative methods, such as using elastic bands7. This increased complexity may lead to a higher risk of injury during insertion, which in turn can result in adverse effects such as a higher loss of body weight after the procedure and an elevated mortality rate among the animals7. In our experience, daily monitoring of animals and the implementation of supportive measures, such as softened feed, have proven instrumental in minimizing adverse effects associated with the use of NiTi coil springs for OTM in animal models. These measures not only contribute to the welfare of the animals but also enhance the reliability and validity of experimental outcomes by reducing confounding factors and ensuring consistency in research protocols4,16,17,18,19,20.
Employing OTM in mice, combined with micro-CT analysis, offers a suitable model for probing mechanisms of bone adaptation, root resorption, and cellular responses to mechanical force stimuli. This integrated approach provides valuable insights into the intricate processes underlying orthodontic treatment and facilitates the development of novel therapeutic strategies and interventions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We wish to express our sincere appreciation to Miss Beatriz M. Szawka for her contribution to the schematic diagram and to Mrs. Ilma Marçal de Souza for her technical support. J.A.A.A. is the recipient of a fellowship granted by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, E-26/200.331/2024), Brazil. This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (406928/2023-1), Fundação de Amparo a Pesquisa do Estado de Minas Gerais and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance code 001), Brazil. The authors thank Prof. Dr. Eduardo H. M. Nunes from LabBio/UFMG for the X-ray microtomography analysis.
Materials
| Acetone | Sigma-Aldrich | 67-64-1 | |
| Distal cut pliers | Quinelato | QO.700.00 | |
| Dynamometer | SHIMPO | FGE-5XY | |
| Fiber Optic Illuminator | Cole-Parmer | N/A | |
| ketamine | Syntec | 100477-72-3 | |
| NiTi open-coil spring 0.25 x 0.76 | Lancer Orthodontics | ||
| Ø 0.20 mm round chrome-nickel (CrNi) | Morelli | 55.01.208 | |
| Round CrNi Hard Elastic Orthodontic Wire Ø0.50 mm (.020 inch) | Morelli | 55.01.050 | |
| Round CrNi Tie Wire Ø0.20 mm (.008 inch) | Morelli | 55.01.208 | |
| Stereomicroscope | Quimis | Q7740SZ | |
| Transbond Plus Self Etching Primer | 3M | LE-Q100-1004-7 | |
| Weingart Plier | Quinelato | QO.120.00 | |
| Xylazine | Syntec | 23076-35-9 | |
| MicroCT Analysis | |||
| Skyscan 1174v2 | Bruker | 1174v2 | |
| Software | |||
| NRecon | Skyscan | N/A | |
| DataViewer | Skyscan | N/A | |
| CTAn | Skyscan | N/A | |
| Mimics | Materialise | N/A |
References
- Kohli, N., et al. Bone remodelling in vitro: Where are we headed?: -A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone. 110, 38-46 (2018).
- Epsley, S., et al. The effect of inflammation on bone. Front Physiol. 11, 511799 (2021).
- Bolamperti, S., Villa, I., Rubinacci, A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 10 (1), 48 (2022).
- Taddei, S. R., et al. Experimental model of tooth movement in mice: a standardized protocol for studying bone remodeling under compression and tensile strains. J Biomech. 45 (16), 2729-2735 (2012).
- Guerrero, J. A., et al. Maxillary suture expansion: A mouse model to explore the molecular effects of mechanically-induced bone remodeling. J Biomech. 108, 109880 (2020).
- Ibrahim, A. Y., Gudhimella, S., Pandruvada, S. N., Huja, S. S. Resolving differences between animal models for expedited orthodontic tooth movement. Orthod Craniofac Res. 20 (Suppl 1), 72-76 (2017).
- Kirschneck, C., Bauer, M., Gubernator, J., Proff, P., Schröder, A. Comparative assessment of mouse models for experimental orthodontic tooth movement. Sci Rep. 10 (1), 12154 (2020).
- Huang, H., Williams, R. C., Kyrkanides, S. Accelerated orthodontic tooth movement: molecular mechanisms. Am J Orthod Dentofac Ortho. 146 (5), 620-632 (2014).
- Ariffin, S. H. Z., Yamamoto, Z., Abidin, I. Z., Wahab, R. A. M., Ariffin, Z. Cellular and molecular changes in orthodontic tooth movement. Sci World J. 11, 1788-1803 (2011).
- Alhasyimi, A. A., Pudyani, P. P., Asmara, W., Ana, I. D. Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod Craniofac Res. 21, 112-118 (2018).
- Klein, Y., et al. Immunorthodontics: in vivo gene expression of orthodontic tooth movement. Sci Rep. 10 (1), 8172 (2020).
- Fujimura, Y., et al. Influence of bisphosphonates on orthodontic tooth movement in mice. Eur J Orthod. 31 (6), 572-577 (2009).
- Waldo, C. M., Rothblatt, J. M. Histologic response to tooth movement in the laboratory rat; procedure and preliminary observations. J Dental Res. 33 (4), 481-486 (1954).
- Chavez, M. B., et al. Guidelines for micro-computed tomography analysis of rodent dentoalveolar tissues. JBMR Plus. 5 (3), e10474 (2021).
- Trelenberg-Stoll, V., Wolf, M., Busch, C., Drescher, D., Becker, K. Standardized assessment of bone micromorphometry around teeth following orthodontic tooth movement: A µCT split-mouth study in mice. J Orofac Ortho. 83 (6), 403-411 (2022).
- Santos, M. S., et al. Targeting phosphatidylinositol-3-kinase for inhibiting maxillary bone resorption. J Cell Physiol. 238 (11), 2651-2667 (2023).
- Macari, S., et al. Oestrogen regulates bone resorption and cytokine production in the maxillae of female mice. Arch Oral Biol. 60 (2), 333-341 (2015).
- Macari, S., et al. Lactation induces increases in the RANK/RANKL/OPG system in maxillary bone. Bone. 110, 160-169 (2018).
- Pereira, L. J., et al. Aerobic and resistance training improve alveolar bone quality and interferes with bone-remodeling during orthodontic tooth movement in mice. Bone. 138, 115496 (2020).
- Silva, F. R. F., et al. Protective effect of bovine milk extracellular vesicles on alveolar bone loss. Mol Nutri Food Res. 68 (3), e2300445 (2024).
- Amaro, E. R. S., et al. Estrogen protects dental roots from orthodontic-induced inflammatory resorption. Arch Oral Biol. 117, 104820 (2020).
- Xu, X., Zhou, J., Yang, F., Wei, S., Dai, H. Using micro-computed tomography to evaluate the dynamics of orthodontically induced root resorption repair in a rat model. PLoS One. 11 (3), e0150135 (2016).
- Bouxsein, M. L., et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Mineral Res. 25 (7), 1468-1486 (2010).
- Kanou, K., et al. Effect of age on orthodontic tooth movement in mice. J Dent Sci. 19 (2), 828-836 (2024).

.
