Embedding Adult Drosophila Heads in Paraffin: Preparing Tissue for Histology
Abstract
Source: Sunderhaus, E. R., Kretzschmar, D. Mass Histology to Quantify Neurodegeneration in Drosophila .J. Vis. Exp. (2016).
This video describes how to embed Drosophila heads in paraffin using the collar method, a technique that prepares fly heads for serial sectioning. Paraffin sections are used for histological examinations of the brain's anatomy and for the assessment of neurodegeneration. The featured protocol demonstrates how multiple flies can be processed in one preparation, thereby increasing time-efficiency and minimizing experimental artifacts.
Protocol
This protocol is an excerpt from Sunderhaus and Kretzschmar, Mass Histology to Quantify Neurodegeneration in Drosophila, J. Vis. Exp. (2016).
1. Fixing the Head on Collars and Embedding in Paraffin
NOTE: All of the steps in the fixation process should be done in a fume hood. Methylbenzoate, while not posing a health risk, has a highly distinct odor, which can be overwhelming if not handled in a fume hood.
- Before anesthetizing the flies, make up 50 mL of Carnoy solution by adding 15 mL of chloroform and 5 mL of glacial acetic acid to 30 mL of 99% ethanol (do not mix the chloroform and acetic acid). Pour it in a glass container with a flat bottom, such as a crystalizing dish, to ensure that the collars can lay flat and are completely covered by the solution.
- Anesthetize the flies with CO2 or ether.
- Thread flies (up to 20 with most collars) by their necks into the collars using forceps. Remember to align all of the heads in the same orientation, as seen in Figure 1A, and be gentle to ensure that no damage occurs to the head or eyes.
- Include sine oculis flies (arrows, Figure 1A) at random positions so that the order of the flies can be easily identified in the sections. In addition, if the experimental flies have a light or white eye color, thread some red-eyed flies, such as wild type, between them to ensure that sufficient pigment is present to stain the slide. Record the order of the flies on a protocol sheet together with the collar number if using more than one collar.
- Once a collar has been finished, place it in the prepared Carnoy solution for 3.5 – 4 h.
- Dump out the Carnoy solution into the appropriate disposal canister and begin the ethanol washes. Make sure to pour slowly so as not to disturb the placement of the collars in the container.
- Wash the collars for 30 min in 99% ethanol twice.
- Wash the collars in 100% ethanol for 1 h. Be sure to change the washes on time to prevent overdehydration.
- Put the collars in methylbenzoate O/N at RT. Seal the container with parafilm to prevent the evaporation of the methylbenzoate.
- Pour the methylbenozate into the proper disposable container in the fume hood. Add a previously-prepared mixture of 1:1 low melting point (56 – 57 °C) paraffin wax and methylbenzoate. From this point on, the collars need to be kept in an incubator at 65 °C to make sure that the paraffin does not harden.
- Pour out the methylbenozate and paraffin mixture into the proper disposable container, and pour molten pure paraffin wax, kept at 65 °C, onto the collars.
- Change the paraffin after 30 min and repeat this at least 5 times. At least 6 – 8 washes should be performed.
- Once the washes are complete, place the collars into a rubber ice cube tray with slots approximately the size of the collars. Pour molten paraffin over them until completely covered and allow it to harden O/N (try to avoid air bubbles).
- Remove the paraffin blocks containing the collars from the ice cube tray. Separate the paraffin block from the collar using a razor blade, gently breaking off the collar. The heads will be in the paraffin block while the bodies will stay in the collar. The blocks can be kept at room temperature.
- To clean the collars, soak them in a deparafinization agent at 65 °C to remove the paraffin, clean with light scrubbing, and wash in ethanol before reusing.
Representative Results
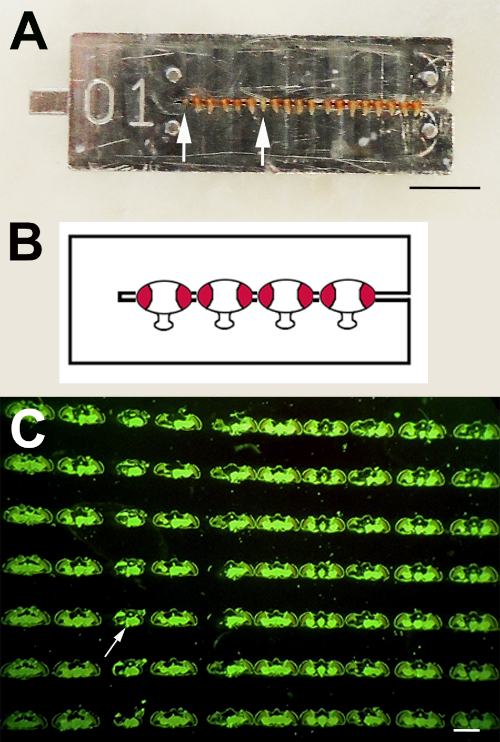
Figure 1: Paraffin Serial Sections. A) Using the collar method, experimental and control flies can be processed as one sample by threading them onto one collar. Eyeless sine oculis flies are inserted for orientation (arrows). B) Schematic showing the orientation of the fly heads in the collar. C) In this image, sections from different fly heads are oriented left to right on the slide. From top to bottom on the slide, serial sections from the same fly head can be seen. In this case, a sine oculis fly was inserted at position three (arrow). The sections are stained by the fluorescent eye pigment that washes over the sections after cutting. Scale bar in A = 5 mm and in C = 0.5 mm. Please click here to view a larger version of this figure.
Materials
| Collar | Genesee Scientific | TS 48-100 | We are using custom made collars that are made from one piece of metal instead of layers as the ones by Genesee. |
| Rubber ice cube tray for embedding | Household store | The size can be made-to-fit by glueing in additional walls | |
| Crystallizing dish | Fisher Scientific Company | 08-762-3 | |
| Ether | Fisher Scientific Company | E138-1 | |
| Ethanol | Decon Laboratories Inc. | 2701 | |
| Choloroform | Fisher Scientific Company | C298-500 | |
| Glacial Acetic Acid | Fisher Scientific Company | A38-212 | |
| Methylbenzoate | Fisher Scientific Company | M205-500 | Distinct Odor – Use in fume hood! |
| Low Melting Point Paraffin Wax | Fisher Scientific Company | T565 | Make sure to keep extra melted in a 65 °C waterbath |

