Larval Ring Gland Dissection: Isolation of Developmental Endocrine Organs from Drosophila
Abstract
Source: Imura, E., et al. Protocols for Visualizing Steroidogenic Organs and Their Interactive Organs with Immunostaining in the Fruit Fly Drosophila melanogaster. J. Vis. Exp. (2017).
This video describes the Drosophila ring gland, a complex endocrine structure important for biosynthesis and secretion of ecdysteroids and other hormones. The featured protocol demonstrates its dissection, fixation, and immunostaining.
Protocol
This protocol is an excerpt from Imura et al., Protocols for Visualizing Steroidogenic Organs and Their Interactive Organs with Immunostaining in the Fruit Fly Drosophila melanogaster, J. Vis. Exp. (2017).
NOTE: The overall scheme of protocols is shown in Figure 1.
1. The Dissection of the Larval Ring Gland (RG)
NOTE: In D. melanogaster, which belongs to cyclorrhaphous Diptera, the PG is within a composite endocrine organ called the ring gland (RG, Figure 2D). Since it is unfeasible that the PG is surgically separated from other types of cells (discussed later), a practical target is to isolate an intact, undamaged RG by dissection.
- Preparation of larvae at the appropriate developmental stages
NOTE: To synchronize the developmental stages of D. melanogaster larvae, it is necessary to collect eggs within a narrow time window and to collect larvae at the appropriate times.- Collect eggs for 2 h on a grape-juice agar plate at 25 °C.
- Collect newly-hatched 1st instar larvae under a dissecting microscope with forceps and transfer them to a small vial containing mashed standard yeast/cornmeal fly food.
NOTE: The age of larvae is represented by "hours after egg laying (hAEL)", "hours after hatch (hAH)", or "hours after L3 ecdysis (hAL3E)". - At the appropriate time point, collect the staged larvae with a disposable plastic loop to avoid undesirable injury. To minimize a difference in developmental progression of the 3rd instar larvae, collect the 2nd instar larvae at 70 hAEL (48 hAH) and allow them to molt in 2 h intervals. Then, collect the 3rd instar larvae within 2 h after the L3 ecdysis; this procedure gives 0-2 hAL3E larvae.
- Transfer the staged larvae to a dish filled with phosphate buffered saline (PBS).
- Rinse the larvae with PBS to remove residual food from the body.
- Coarse dissection of larvae under a dissecting microscope
- Hold the mouth hook of a larva with forceps. Sever the larval body at the anterior one-third of the body length using micro scissors.
- Hold the cut end of the anterior larval body with one pair of forceps. Using the other pair of forceps, gently push the tip of the head toward the inside of the body; this procedure turns the larval body inside out.
NOTE: This step can be skipped for the dissection of 1st instar larvae. - Repeat steps 1.2.1-1.2.2 with additional larvae for 5-10 min.
NOTE: The number of larvae should be equal to or less than 20 to save time for dissection.
- Fixation and staining of tissue with immunohistochemistry
- Put the anterior one-third of the larval bodies (step 1.2.2) into a 1.5 mL microtube filled with 500 µL the fix solution (4% paraformaldehyde in PBS). Fix less than 20 samples at one time, otherwise PBS attached to the fixed samples may cause an unfavorable dilution of the fixative. For fillet dissection, remove a drop of PBS and then add a drop of fix solution.
NOTE: For fix solution, either 3.7% formaldehyde or 4% paraformaldehyde may be used. - Incubate the dissected tissues in the fix solution for 30 min at room temperature (RT) or alternatively for 2 h at 4 °C.
- Replace the fix solution with 500 µL PBS and quickly rinse the samples 3 times. Wash the samples with 500 µL 0.3% PBT (PBS + 0.3% octylphenol ethoxylate) for 15 min on a rocker at RT. For fillet dissection, remove the insect pins and transfer the samples into a 1.5 mL microtube.
NOTE: For increasing the permeability of antibodies into tissues, the samples are treated with 500 µL 2.0% PBT for 1-2 h on a rocker at RT. - Replace PBT with 500 µL blocking solution (2% bovine serum albumin in PBT). Incubate the samples for 1.5 h on a rocker at RT.
- Replace the blocking solution with 50 µL the primary antibody solution, i.e. antibodies diluted in the blocking solution.
- Incubate the samples overnight on a rocker at 4 °C.
- Replace the primary antibody solution with 500 µL 0.3% PBT and quickly rinse the samples 5 times. Wash the samples 3 times with 500 µL 0.3% PBT for 15 min each on a rocker at RT.
- Replace 0.3% PBT with 50 µL the secondary antibody solution (dye-conjugated antibodies diluted in the blocking solution). For nuclear staining, 0.5 µL 4',6-diamidino-2-phenylindole (DAPI, 0.1 mg/mL stock solution) is added to 50 µL solution. Incubate the samples on a rocker for 2 h at RT or alternatively at 4 °C overnight.
NOTE: Keep the samples in the dark. - Replace the antibody solution with 500 µL 0.3% PBT and rinse 5 times. Wash the samples 3 times with 500 µL 0.3% PBT for 15 min each at RT.
- Put the anterior one-third of the larval bodies (step 1.2.2) into a 1.5 mL microtube filled with 500 µL the fix solution (4% paraformaldehyde in PBS). Fix less than 20 samples at one time, otherwise PBS attached to the fixed samples may cause an unfavorable dilution of the fixative. For fillet dissection, remove a drop of PBS and then add a drop of fix solution.
- Fine dissection of the brain-RG complex containing the PG and mounting
- Use a disposable pipet to transfer the immunostained samples to a dish filled with 0.3% PBT.
NOTE: To avoid inconvenient bubbles during dissection and mounting, 0.3% PBT can be replaced with PBS. - Under a dissecting microscope, hold the cuticle or mouth hook with one pair of forceps. Using a 27 G needle attached to a 1 mL syringe as a "knife", cut the anterior tip of the esophagus and eye discs to remove the brain-RG-eye disc complex from body cuticle.
- Separate the esophagus and gut from the brain-RG-eye disc complex with forceps. Since the esophagus passes through the brain above the ventral nerve cord (VNC), pull out the gut to the posterior side.
NOTE: To remove extra imaginal discs from the samples, cut the connections between the brain, eye discs, and leg discs with a needle knife. - Repeat the steps 1.4.1-1.4.4 for other samples.
NOTE: To prevent tissue debris from sticking to the samples, transfer each completed sample to a different clean dish filled with 0.3% PBT or PBS. - Transfer the samples to the center of a clean glass slide with a micropipette.
NOTE: For 2nd or 3rd instar larvae, the end of a micropipette tip should be truncated with a razor blade. - Under a dissecting microscope, align individual samples with their dorsal side up by using forceps.
NOTE: The RG is located on the dorsal side of the brain (Figure 2A). This alignment facilitates the specification of individual samples during imaging. - Tilt a glass slide and wipe off as much excess PBT as possible. Put a drop of mounting reagent on a side of the slide. Place the edge of a cover slip from the other side of the drop and put the cover slip on the samples slowly with forceps.
NOTE: This procedure prevents the samples from moving outside the cover slip. - Store the samples at 4 °C. Keep the samples in the dark.
- Use a disposable pipet to transfer the immunostained samples to a dish filled with 0.3% PBT.
Representative Results
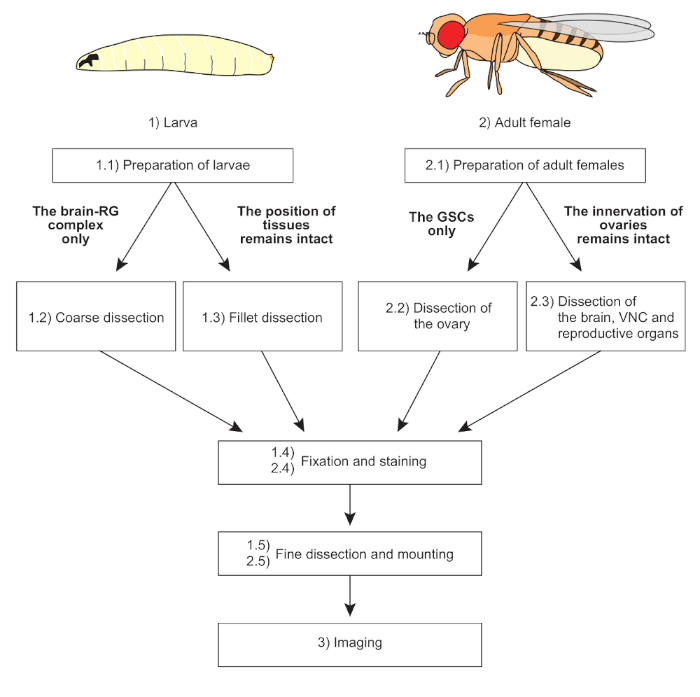
Figure 1: The overall scheme of protocols. Two distinct dissection methods are applicable to larvae and adult females, depending on the purpose of the experiments. The mounting methods are also devised according to sample conditions. Fixation, staining, and imaging techniques are basically common to all samples. Please click here to view a larger version of this figure.
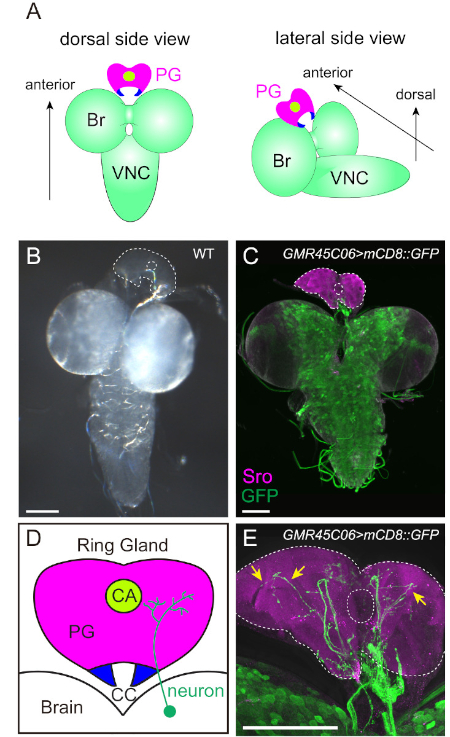
Figure 2: Visualization of the PG and the PG-projecting Neurons. (A and B) The brain-ring gland (RG) complex of the wild-type 3rd instar larva. In (B), the PG is outlined by dotted lines. The filamentous structure between the brain and the RG is the trachea. (C-E) The innervation of the PG was visualized with mCD8::GFP driven by GMR45C06-GAL4 in FlyLight collection. The PG and GFP-positive neurons were labeled with anti-Sro antibody (magenta) and anti-GFP antibody (green), respectively. The fluorescent image is merged with the transmitted-light image to specify the outline of tissues. In (D), the PG, the corpora allata (CA) and the corpora cardiaca (CC) are illustrated. The PG-projecting neurons are more prominent in the high-power image with 40X objective lens (arrows in E) than the low-power image with 10X objective lens (C). Scale bar = 100 µm. Please click here to view a larger version of this figure.
Materials
| Egg collection | |||
| tissue culture dish (55 mm) | AS ONE | 1-8549-02 | for grape-juice agar plates |
| collection cup | HIKARI KAGAKU | ||
| yeast paste | Oriental dry yeast, Tokyo | ||
| 100% grape juice | Welch Food Inc. | ||
| rearing larvae | |||
| small vials (12ml, 40×23.5 mm, PS) | SARSTEDT | 58.487 | |
| disposable loop | AS ONE | 6-488-01 | |
| standard fly food | The recipe is on the website of Bloomington stock center. | ||
| Dissection | |||
| dissecting microscope | Carl Zeiss | Stemi 2000-C | |
| dissecting microscope | Leica | S8 AP0 | |
| tissue culture dish (35 x 10 mm, non-treated) | IWAKI | 1000-035 | |
| Sylgard | TORAY | coarting silicon inside dishes | |
| Terumo needle (27G, 0.40 x 19 mm) | TERUMO | NN-2719S | A "knife" to cut the tissue |
| forceps, Inox, #5 | Dumont, Switzerland | ||
| insect pin (0.18 mm in diameter) | Shiga Brand | for fillet dissection | |
| micro scissors | NATSUME SEISAKUSHO CO LTD. | MB-50-10 | |
| Fixation | |||
| ultrapure water | Merck Millipore | ||
| phosphate buffered saline (PBS) | |||
| Formaldehyde | Nacalai tesque | 16222-65 | |
| Paraformaldehyde | Nacalai tesque | 02890-45 | |
| Triton-X100 | Nacalai tesque | 35501-15 | |
| microtubes (1.5 ml) | INA OPTIKA | CF-0150 | |
| Incubation | |||
| As one swist mixer TM-300 (rocker) | As one | TM-300 | rocker |
| Bovine Serum Albumin | SIGMA | 9048-46-8 | |
| Primary antibody | |||
| anti-Sro (guinea pig), 1:1000 | |||
| anti-GFP (rabbit), 1:1000 | Molecular Probes | A6455 | Shimada-Niwa ans Niwa, 2014 |
| anti-GFP (mouse mAb, GF200), 1:100 | Nakarai tesque | 04363-66 | |
| anti-5HT (rabbit), 1:500 | SIGMA | S5545 | |
| anti-Hts 1B1 (mouse) | Developmental Studies Hybridoma Bank (DSHB) | 1B1 | |
| anti-DE-cadherin (rat), 1:20 | DSHB | DCAD2 | |
| anti-nc82 (mouse), 1:50 | DSHB | nc82 | |
| Secondary antibody | |||
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugate | Life Technologies | A-11008 | |
| Goat anti-Mouse IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugate | Life Technologies | A-11001 | |
| Goat anti-Rat IgG (H+L) Secondary Antibody, Alexa Fluor 546 conjugate | Life Technologies | A-11081 | |
| Goat anti-Guinea Pig IgG (H+L) Secondary Antibody, Alexa Fluor 555 conjugate | Life Technologies | A-21435 | |
| Alexa Fluor 546 dye-conjugated phalloidin | Life Technologies | A-22283 | |
| Mounting reagents | |||
| Micro slide glass | Matsunami Glass Ind.,Ltd. | SS7213 | |
| Square microscope cover glass | Matsunami Glass Ind.,Ltd. | C218181 | |
| FluorSave reagent (Mounting reagent) | Calbiochem | 345789 | |
| Transfer pipette 1 ml (Disposable dropper) | WATSON | 5660-222-1S | |
| Fly stocks | |||
| w; GMR45C06-GAL4 | from Bloomington Drosophila Stock Center. (#46260) | ||
| UAS–GFP; UAS–mCD8::GFP | gifts from K. Ito, The University of Tokyo. | ||
| w[1118] | |||
| w; phantom-GAL4#22/UAS-turboRFP | |||
| w; UAS-mCD8::GFP; TRH-GAL4 | Li, H. H. (2014) and Alekseyenko, O. V, Lee, C. & Kravitz, E. A. (2010) | ||
| w; UAS-mCD8::GFP | from Bloomington Drosophila Stock Center. (#32188) | ||
| yw;; nSyb-GAL4 | from Bloomington Drosophila Stock Center. (#51941) |

