Extracellularly Identifying Motor Neurons for a Muscle Motor Pool in Aplysia californica
Summary
In animals with large identified neurons (e.g. mollusks), analysis of motor pools is done using intracellular techniques1,2,3,4. Recently, we developed a technique to extracellularly stimulate and record individual neurons in Aplysia californica5. We now describe a protocol for using this technique to uniquely identify and characterize motor neurons within a motor pool.
Abstract
In animals with large identified neurons (e.g. mollusks), analysis of motor pools is done using intracellular techniques1,2,3,4. Recently, we developed a technique to extracellularly stimulate and record individual neurons in Aplysia californica5. We now describe a protocol for using this technique to uniquely identify and characterize motor neurons within a motor pool.
This extracellular technique has advantages. First, extracellular electrodes can stimulate and record neurons through the sheath5, so it does not need to be removed. Thus, neurons will be healthier in extracellular experiments than in intracellular ones. Second, if ganglia are rotated by appropriate pinning of the sheath, extracellular electrodes can access neurons on both sides of the ganglion, which makes it easier and more efficient to identify multiple neurons in the same preparation. Third, extracellular electrodes do not need to penetrate cells, and thus can be easily moved back and forth among neurons, causing less damage to them. This is especially useful when one tries to record multiple neurons during repeating motor patterns that may only persist for minutes. Fourth, extracellular electrodes are more flexible than intracellular ones during muscle movements. Intracellular electrodes may pull out and damage neurons during muscle contractions. In contrast, since extracellular electrodes are gently pressed onto the sheath above neurons, they usually stay above the same neuron during muscle contractions, and thus can be used in more intact preparations.
To uniquely identify motor neurons for a motor pool (in particular, the I1/I3 muscle in Aplysia) using extracellular electrodes, one can use features that do not require intracellular measurements as criteria: soma size and location, axonal projection, and muscle innervation4,6,7. For the particular motor pool used to illustrate the technique, we recorded from buccal nerves 2 and 3 to measure axonal projections, and measured the contraction forces of the I1/I3 muscle to determine the pattern of muscle innervation for the individual motor neurons.
We demonstrate the complete process of first identifying motor neurons using muscle innervation, then characterizing their timing during motor patterns, creating a simplified diagnostic method for rapid identification. The simplified and more rapid diagnostic method is superior for more intact preparations, e.g. in the suspended buccal mass preparation8 or in vivo9. This process can also be applied in other motor pools10,11,12 in Aplysia or in other animal systems2,3,13,14.
Protocol
1. Preparation of Recording Dish
- During the force transducer experiments, the buccal ganglia, cerebral ganglion, and buccal mass are placed in a round Pyrex dish that is specialized for force studies.
- To induce ingestive-like patterns in the experiments, we need to apply the non-hydrolyzable cholinergic agonist carbachol to the cerebral ganglion15. To avoid direct contact from carbachol onto the buccal ganglia and buccal mass, separate chambers are needed to isolate the cerebral ganglion from the buccal ganglia and the buccal mass (Figure 1).
- Since the buccal mass is much thicker than the buccal ganglia, they will not be placed on the same level. Therefore, this dish should have a back chamber for the cerebral ganglion (area A in Figure 1), a middle platform for the buccal ganglia (area C in Figure 1), and a much deeper front chamber for the buccal mass (area D in Figure 1).
- To create this dish, begin with a round 100×15 Pyrex dish (15 mm high, 100 mm in diameter). Construction of the dish will require several pours of Sylgard. Follow the instructions provided with the Sylgard product. Sylgard must be allowed to set between different pours.
- The first pour is to create the highest level of Sylgard in the dish (area B in Figure 1), which is the wall between the middle platform and back chamber.
- Use two modeling clay backings to isolate the area for Sylgard wall (area B in Figure 1). Coat the modeling clay backings with plastic wrap where they will contact the Sylgard for easier removal. Ensure tight seals at the edges, where modeling clay will contact the dish, to minimize leakage.
- Pour Sylgard into the portion between the two modeling clay backings nearly up to the top of the dish. Let the Sylgard fully set overnight. Keeping the dish in a warm place will induce faster setting. Remove the modeling clay backings and clean up any clay residue on the Sylgard.
- Next, the back chamber (area A in Figure 1) and the middle platform (area C in Figure 1) should be poured.
- Place a modeling clay backing about 5 mm away from the front surface of the Sylgard for the section of the middle platform (area C).
- Pour Sylgard into the sections for the back chamber (area A) and middle platform (area C) up to a height of approximately 3-5 mm below the top level of the first Sylgard wall (area B). The Sylgard surface of the back chamber should be slightly lower than that of the middle platform to avoid leakage from the back chamber containing carbachol to the middle platform. Again, let the Sylgard fully set overnight and then remove the modeling clay backing.
- The final step is to cut a notch in the middle of the Sylgard wall to provide a channel for the cerebral-buccal connectives (CBCs) to go through between the middle platform and back chamber. The width of this notch should be approximately 3-4 mm, which is wide enough for the CBCs. The bottom of the notch should not be lower than the Sylgard surface of the middle platform to prevent leakage. A scalpel blade can be used to cut the notch.
2. Electrode Preparation
- Pull extracellular glass electrodes from single-barreled capillary glass using a Flaming-Brown micropipette puller as described by McManus et al.8 in section 3.1. With the FT345B filament in the puller, our typical program settings are Heat 480, Pull 50, Velocity 13, and Time 20, but note that settings will be different for different filaments. This program creates the electrodes in a single pull with no fire-polishing stage. The size of the electrode tip should be smaller than the size of the cell bodies. For the motor neurons ranging from 50 μm to 400 μm in soma diameter, the inner diameters of the extracellular glass electrodes should be about 40 μm and their resistances should be about 0.1 MΩ when they are filled with Aplysia saline.
- Pull suction electrodes from polyethylene tubing using a Bunsen burner. Cut a piece of polyethylene tubing about 10 cm long. Hold the tubing on both ends and place it very close to the flame generated by the Bunsen burner while rotating the tube until it becomes soft from the heat. Stretch the tubing carefully along its length while moving it away from the flame. The middle part of the tubing will elongate and narrow as the tubing is pulled.
- Cut the tubing in half to form two suction electrodes. Suction electrodes are generally applied to the cut ends of nerves or muscles, though they can sometimes be applied en passant.
- Create hook electrodes for nerve recordings following the protocol described by McManus et al8 in sections 3.2-3.13. These electrodes are especially useful when a nerve or muscle is not cut.
3. Hook Electrode Attachment
- Dissect the animal and remove the buccal mass following the protocol described in McManus et al.8 section 4.
- For recording and stimulation, hook electrodes can be attached to a number of different nerves.
- To characterize patterns as was done in vivo by Cullins and Chiel9, recordings must be obtained from the I2 nerve and muscle that indicates the protraction phase of feeding16, the radular nerve (RN) that indicates the closure of the food grasper17, buccal nerve 2 (BN2) and buccal nerve 3 (BN3) that indicate the retraction phase17,18. Attachment of the hook electrodes follows a procedure similar to that described by McManus et al8, section 5.
- The locations of these nerves refer to the schematic of the Aplysia feeding apparatus shown in Figure 2 of McManus et al8. Note that the BN2 trifurcates into branches a, b, and c before going beneath the I1 muscle at the lateral groove. Branch a is the first branch to separate from the main trunk, and is adjacent to the BN3.
- The nomenclature of branches a, b, and c was used by Warman and Chiel18. Branches a, b, and c correspond to branches 3, 2, and 1, respectively, in the nomenclature used by Nargeot et al.19. Furthermore, the RN, the BN1, the BN2, and the BN3 correspond to nerves 1, 6, 5, and 4, respectively, in the nomenclature used by Kandel20 and Scott et al.21
- To study the muscle innervation of the I1/I3 muscle, all the nerves except buccal nerves 2 will be severed from the buccal mass during experiments. Thus, we used a hook electrode to record from the BN2.
- Since the I2 nerve and the RN will not be attached to the buccal mass, and they are very difficult to access using hook electrodes, it is preferable to apply suction electrodes to record from them instead. We will describe the application of suction electrodes in section 7.
- Use either a hook electrode or a suction electrode to record from the BN3, because it is easy to access using either kind of electrode. We chose to use a hook electrode for the BN3 recordings to minimize the number of manipulators for holding the suction electrodes, and to save space for other manipulators or equipment.
- Attach a hook electrode to branch a of BN2 (BN2-a) to initiate rejection-like patterns during experiments. It is useful to attach an additional hook electrode to the BN2-a on the other side, because some neurons respond differently to the ipsilateral vs. contralateral BN2-a stimulation.
- To help distinguish neurons with unilateral vs. bilateral projections, it is also useful to attach hook electrodes to BN2 and the BN3 on the other side of the buccal ganglia.
4. Ganglia and Muscle Preparation
- The buccal ganglia, cerebral ganglion and buccal mass will be prepared for the force transducer experiments, in which the cerebral ganglion is attached to the buccal ganglia via the CBCs and the buccal mass is attached to the buccal ganglia via the BN2s only.
- After attaching the hook electrodes, cut buccal nerve 1 (BN1) and the esophageal nerve (EN) bilaterally, cutting at the attachment point to the buccal mass.
- Pull the cerebral ganglion forward to move it out of the way of the I2 muscle. Make a cut into the I2 muscle over the radular sac, extend the cut laterally and anteriorly in both directions, and pull the flap of the I2 muscle forward to expose the radular nerve. Cut the two RN branches and make sure that the branches are long enough for suction electrode attachment.
- Continue the I2 cut in a wide circle around the buccal ganglia, being careful not to cut the BN2s or the BN3s, until the buccal ganglia and the attached part of the I2 muscle can be fully separated from the buccal mass. Cut the bilateral BN3s at the attachment point to the buccal mass, beyond the hook electrode attachment.
- Apply a thin layer of vacuum grease to the notch in the recording dish described above that connects the back chamber and middle platform, using a pipette tip to pick up a glob of vacuum grease and spread it over the notch.
- Apply a thin layer of Quick-Gel super glue to the glass bottom of the front chamber where the buccal mass will be placed, just in front of the Sylgard base of the middle platform.
- Carefully transfer the cerebral ganglion, buccal ganglia and buccal mass to the recording dish (Figure 1) described in section 2, making sure that none of the hook electrodes are pulled tightly, which could damage the nerves.
- Carefully place the buccal mass on the glue in the front chamber of the recording dish, to ensure that its ventral surface is glued to the bottom of the dish. Be sure to keep the ganglia and electrodes from touching the glue. Add Aplysia saline8 (460 mM NaCl, 10 mM KCl, 22 mM MgCl2, 33 mM MgSO4, 10 mM CaCl2, 10 mM glucose, 10 mM MOPS, pH 7.4-7.5) to the dish, which will induce the glue to set.
- If the dish must be transferred to another microscope for preparing the buccal ganglia for extracellular soma recordings, be very careful with the hook electrodes. Group the electrodes on one side of the buccal mass together, and also group the electrodes on the other side of the buccal mass together. Carefully hold the electrodes by grasping the lab tape that covers the connector pins, again making sure that none of the electrodes are pulled tightly.
- When the dish is positioned under the microscope, the electrodes should be draped gently over the sides of the dish and rest on the platform next to the dish.
- During breaks and between stages of the experiment, aerate the saline in the buccal mass chamber using an aquarium airstone.
- Use forceps to grab the sheath of the cerebral ganglion and pull it into the back chamber, ensuring that the CBCs run through the notch. Pin the cerebral ganglion using nerves other than the CBCs to avoid damage to the intact CBCs.
- Apply more vacuum grease over the CBCs, and then add more Aplysia saline to both chambers of the dish, so that the ganglia are completely submerged. Ensure that the top of the vacuum grease is slightly higher than the Sylgard wall so that no leakage will occur between the chambers.
- To stabilize the buccal ganglia, first pin the ends of the BN3s, then the BN1s and the ENs on the Sylgard base of the middle platform (Figure 2). Since the BN3s will be recorded using hook electrodes, the pins should be placed more distally than the attachment points of the hook electrodes.
- Use two pins, bent 90 degrees, as hooks to stretch and anchor the CBCs, so that the CBCs will not be damaged (Figure 2).
- Pin down the RN branches between the back chamber and the buccal ganglia. Then the I2 muscle will be on top of the RNs. To expose the I2 nerve, use forceps to grab the I2 muscle and pull it over the buccal ganglia. Pin two corners of the I2 muscle to avoid damage to the I2 nerve.
- Sever the I2 nerve distal to the point at which its two branches merge into the I2 muscle. Make sure the muscle is still innervated to be comparable to the in vivo recordings. Cut away the rest of the I2 muscle and flip the I2 nerve back and pin it down between the two RN branches (see Figure 2, inset).
- Adjust the locations of the pins to stretch and add tension if a nerve is too loose, or to release tension if a nerve is too tight. To further stabilize the buccal ganglia, add more pins on the sheath between nerves.
- Since the buccal ganglia are placed caudal side up, rotate the buccal ganglia if the neurons of interest are on the rostral side. To rotate one of the two buccal ganglia, use fine forceps to grab some excess sheath of the CBC where it is near to the buccal ganglia and pin it down between the BN2 and the BN3. In some ganglia, it may be more convenient to pin it down between CBC and BN3.
- Add an additional pin on the sheath of the buccal ganglion on the side close to the front chamber to minimize the movement of the buccal ganglion.
- To trim the sheath covering the buccal ganglia, use fine forceps to grab the sheath on the side close to the back chamber, and then cut away the excess sheath with fine scissors without exposing the cell bodies. In order to minimize damage, only remove the minimum amount of sheath necessary to see the cell bodies.
- After the sheath of the buccal ganglia is trimmed, pull the I2 nerve and the RNs over the buccal ganglia and pin them down between the buccal ganglia and front chamber to further rotate the buccal ganglia. (See Figure 2).
- To wash out any remaining magnesium chloride8 that was used to anesthetize the animal before dissection, replace the Aplysia saline in the dish with fresh Aplysia saline.
5. Electrically Connecting Hook Electrodes
- After the ganglia and muscle are prepared, carefully transfer the dish to the vibration isolation table for the experiments.
- Attach all electrode pins to their sockets on the BNC cables that connect to the amplifiers (A-M Systems model 1700 amplifier). Again, make sure that the electrodes are not pulled tightly while doing this. Make sure that the electrodes are correctly attached to their appropriate cables and that the polarities are correct.
6. Setting up the Extracellular Glass Electrodes for Soma Recordings
- Fill the electrode with Aplysia saline using a syringe attached to a piece of polyethylene tubing of around 15-20 cm. Attach the free end of the polyethylene tubing to the end of the glass electrode. Pull back on the plunger of the syringe to fill up the electrode with Aplysia saline.
- Place the filled extracellular glass electrode in the notch of the holder on the manipulator. Use the manipulator to place the electrode tip into the Aplysia saline containing the buccal ganglia.
- Insert a silver/silver chloride wire soldered to a male gold connector pin into the electrode to serve as the recording wire. Place another silver/silver chloride wire soldered to a male gold connector pin directly into the Aplysia saline within the section of the recording dish containing the buccal ganglia to act as the reference wire. Connect both the recording and reference wires to the BNC cable that connects to the amplifier.
- If there is enough room for more manipulators, additional extracellular glass electrodes can be added to record multiple neurons simultaneously.
7. Setting up the Suction Electrodes for the Nerve Recordings
- Trim the narrow end of the suction electrode tip to match the diameter of the nerve. The inner diameter of the electrode tip should be similar to or slightly smaller than the nerve’s diameter to ensure tight suction.
- Since the I2 nerve and the RN are very close to each other, their electrodes can be held by the same manipulator to save space. Place two electrodes in two notches of the same holder. Rotate the two electrodes and ensure that their tips are close to one other. Choose one of them for the I2 nerve recording, the other one for the RN recording.
- Place the electrode tip in the Aplysia saline within the recording dish containing the buccal ganglia. Attach the free end of the polyethylene tubing on the syringe to the suction electrode. Use the syringe to fill up the electrode with Aplysia saline. Move the electrode tip close to the end of the target nerve, i.e. the I2 nerve, and use the syringe to suck the nerve into the electrode. The length of the nerve within the electrode should be about 0.5-1.0 mm to ensure a tight seal.
- Repeat the suction for the electrode that will be attached to the RN.
- Connect the electrodes to the corresponding BNC cables as described in section 6.3.
8. Setting up the Force Transducer to Measure the I1/I3 Muscle Contraction
- To attach the force transducers to the muscle, use silk sutures. Bend the curved needle of each suture, and tie the suture to the force transducer. Gently grab and lift a small amount of muscle with forceps and, holding the needle in another set of forceps, insert the needle through the muscle up to the bent point in the needle (Figure 1).
- Transducers can be attached either dorsally or laterally on the I1/I3 muscle. Dorsal attachment allows measurement of contractions evoked by activation of either the left or right side of the muscle. Lateral attachment will show stronger forces for the majority of neurons, but will only allow measurement of contraction on the side to which the transducer is attached.
- To help identify neurons that may activate the anterior, posterior, or both regions of I1/I3, attach a force transducer to the posterior part of the muscle, just anterior to the pharyngeal tissue, and attach another force transducer to the anterior part of the muscle, at the jaws (Figure 1; note hooks).
- Lift the force transducers until the sutures are pulled taut, but do not overstretch. To check this, view the measurement from the force transducer when the suture has some slack in it, and then lift the transducer until the measurement is slightly above this baseline level.
9. Identifying Motor Neurons within a Motor Pool
- This protocol describes a process for extracellularly identifying motor neurons within a motor pool. We used AxoGraph software to monitor the activity of individual neurons, multiple nerves, and the muscle (EMG signal or contraction forces). In this protocol, we used the contraction forces of the muscle as an illustration for the process of identifying motor neurons; in other experiments, we used EMG as well, and the setup for such experiments is very similar (see Discussion).
- To locate a candidate neuron, use the manipulator to gently press the tip of the extracellular glass electrode down onto the sheath over the center of the neuron soma5 (Figure 3), which is the best location for stimulation and recording selectivity5. Since the threshold current for activating a neuron increases linearly with electrode-to-soma distance5, the stimulation selectivity will become worse when the electrode is moved away from the center of the target neuron toward a neighboring neuron.
- To identify a motor neuron, first directly stimulate the neuron using the extracellular glass electrode to ensure that only this neuron is firing, and examine whether it innervates the muscle. Then, extracellularly record from this neuron to establish a one-for-one relationship between the extracellular soma recording and the nerve recordings, which is also crucial for neuron identification.
- Since most extracellular amplifiers do not allow simultaneous stimulation and recording in a channel, set the channel used to stimulate and record the soma (the soma channel) to stimulation mode and apply a brief anodic current (e.g. 6 msec for Aplysia neurons5) to the soma (Figures 4A, 5A; note arrows 1 in both figures), starting from a low current (e.g. 200 μA), and gradually increasing the current until the neuron bursts.
- Once the neuron is activated to burst, one should immediately switch the soma channel from the stimulation mode to the recording mode (Figures 4A, 5A; note arrows 2 in both figures). However, there will still be delays between the soma stimulation and recording because of human response delays.
- If the neuron fires for a reasonable amount of time, it should be possible to observe one-for-one corresponding action potentials from the soma recording and on the nerve(s) through which the neuron projects (Figures 4B, 5B; note dashed lines), as well as the forces generated by the neuron (Figures 4A, 5A; note blue boxes). If the neuron stops firing before the soma recording begins, increase the current to activate it for a longer time.
- The signal-to-noise ratio of the extracellular recordings depends on the electrode location and soma size. The extracellular recording will become bigger as the soma size increase and electrode-to-soma distance decreases. Since the noise only varies in a narrow range, the signal-to-noise ratio will also increase as the soma size increases and the electrode-to-soma distance decreases. The most common range of the signal-to-noise ratio is from 4:1 to 8:1.
- The motor neurons can be identified based on their characteristics, such as soma location, nerve projection and muscle innervation4,6,7. Since only the two BN2s are attached to the buccal mass, by monitoring the activity on the BN2s, one can ensure that the muscle contraction force is only caused by the neuron activated by the extracellular stimulation.
- For example, B3 is a large motor neuron for the I1/I3 muscle (300-400 μm in soma diameter in animals weighing 200 to 350 grams), located on the rostral side of the buccal ganglion (Figure 3). It only projects through the ipsilateral BN2, and innervates both the anterior and posterior parts of the I1/I3 muscle. Most of the time, activating it generates a larger anterior than posterior force (8 out of 9 experiments).
- After a neuron is identified, its activity can be recorded in different feeding-like behaviors via the extracellular glass electrode (Figures 4 and 5), which can be elicited as described below. The extracellular recording on the soma will be much more specific than the nerve recordings, which include the activity of many different neurons.
- To induce egestive-like motor programs, stimulate BN2-a with 1-2 min of pulses19 (2Hz, each pulse is 1 msec). This stimulation reliably generates egestive patterns in this setting. With sufficient current (e.g. 300 μA), patterns may persist for the duration of the stimulation. Sometimes there will be one more pattern that occurs shortly after the stimulation ends.
- To induce ingestive-like motor programs, place a few crystals of solid carbachol directly onto the sheath of the cerebral ganglion15. If one wants to control the level of carbachol exposure, use a solution of 1 to 10 mM carbachol in Aplysia saline. Higher concentrations are more likely to induce responses. Repetitive patterns generally begin within five minutes, and may last for approximately ten to fifteen minutes before beginning to run down.
- After washing out carbachol several times and waiting for at least 30 min, a subsequent application of carbachol can be added to the cerebral ganglion to induce more ingestive-like motor patterns.
- After multiple motor neurons for the particular motor pool have been identified and characterized during motor programs, one may develop a greatly simplified diagnostic method that requires minimal information for quickly identifying these neurons in future work (Figure 6), e.g. in the suspended buccal mass preparations or in vivo. The criteria may include soma size and location, nerve projection, unit size on the nerves, and timing of activity during motor patterns.
Representative Results
Figures 4 and 5 show typical results used to identify two I1/I3 motor neurons. Figure 4 shows the soma recordings of a large motor neuron, B3, during egestive-like and ingestive-like patterns (Figures 4C, 4D). The one-for-one corresponding spikes on the soma channel and the ipsilateral BN2 channel (Figure 4E) show that the specificity of B3 soma recording was maintained during patterns. B3 fires during the middle-to-late retraction phase of the patterns. From Figure 4 and other results (not shown), we found that the BN2 unit of B3 is always the largest BN2 unit. Thus, it can also be detected directly from BN2 recordings.
Figure 5 shows the soma recordings of a small neuron, B43, during egestive-like and ingestive-like patterns (Figures 5C, 5D). The one-for-one corresponding spikes on the soma channel and the ipsilateral BN2 channel (Figure 5E) also show that the specificity of B43 soma recording was maintained during patterns. Neuron B43 bursts at the end of the retraction phase during patterns. Since the BN2 unit of B43 is small, it would be difficult to identify it from the BN2 recordings without the soma recording; however, because it fires most intensely at the end of the BN2 motor pattern, the end of B43’s burst can still be identified from BN2 recordings alone.
Figure 6 shows an optimized diagnostic tree that does not require muscle innervation as a criterion, which makes it much easier to extracellularly identify the I1/I3 motor neurons in the suspended buccal mass preparation or in vivo. The diagnostic tree was developed, however, by using measures of force and EMG, and thus illustrates how the techniques in this protocol can lead to streamlined motor neuron identification.
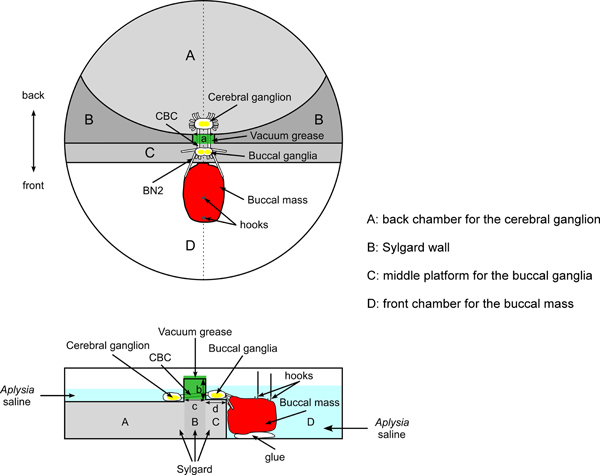
Figure 1. Schematic of overall setup and the dish for the force studies. The top image shows a top view. The bottom image shows a side view (corresponding to the dashed line in the middle of the top view). The cerebral ganglion is pinned to Sylgard in the back chamber (area A). The buccal ganglia are pinned to Sylgard on the middle platform (area C). The back chamber and middle platform are separated by an elevated Sylgard wall (area B). The cerebral-buccal connectives (CBCs) pass through a notch in the Sylgard wall, sealed with vacuum grease. The buccal mass is glued to the glass bottom of the front chamber (area D). The buccal nerves 2 (BN2s) are attached to the buccal mass. Two hooks attached to silk sutures are inserted into the anterior and posterior regions of the I1/I3 muscle. The silk sutures are then tied to the force transducer. The figure uses dark gray, light gray, and white to indicate the surfaces of areas A, B, C, and D. The darker the color, the higher the corresponding surface. The figure uses a, b, c, and d to indicate important dimensions of the dish. Length a is 3-4 mm, the width of the notch that connects the back chamber and middle platform. Length b is about 3-5 mm, the height difference between the surfaces of the middle platform (area C) and the Sylgard wall (area B). Length c indicates the length of the notch, which is about 5 mm. Length d shows the width of the middle platform (area C), which is about 5 mm.
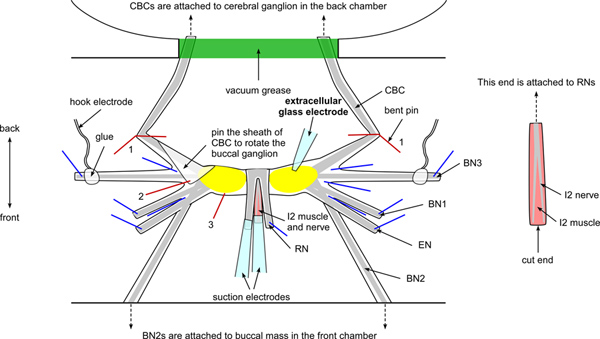
Figure 2. Schematic of the buccal ganglia and electrodes setup. The figure shows the locations of key nerves, including buccal nerves 1, 2, and 3 (BN1, BN2, and BN3), the esophageal nerve (EN), the radular nerve (RN), the I2 nerve and muscle, and the cerebral buccal connective (CBC). Note that the BN2s are attached to the buccal mass (see Figure 1). The CBCs are attached to the cerebral ganglion, passing through the notch of the Sylgard wall and are sealed with vacuum grease (see Figure 1). The RN and the I2 nerve and muscle are pulled above the ganglia and pinned proximal to the buccal mass (front direction). Blue lines indicate the location of pins. Two bent pins (red lines labeled 1) are used to anchor the CBCs. Note that a flap of sheath of the CBC on the left side is folded and pinned down between BN2 and BN3 to rotate the left buccal ganglion (red line labeled 2). In some ganglia, it may be more convenient to pin the sheath down between CBC and BN3. An additional pin is added to the side of the ganglion that is proximal to the EN (red line labeled 3) for further rotation and stabilization. The extracellular glass electrode is placed on top of the sheath above the soma for extracellular stimulation and recording. The hook electrodes are attached to the BN3s and the pins holding those nerves in place should be placed more distally than the attachment points of these hook electrodes. Two suction electrodes are attached to the RN and the I2 nerve and muscle (see inset for a clearer view of the I2 nerve and muscle). Click here to view larger figure.
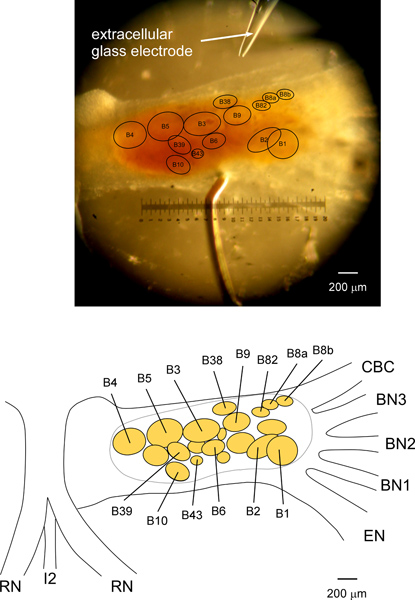
Figure 3. A picture and schematic of the neuron map for extracellular identification of the I1/I3 motor neurons in the Aplysia buccal ganglion. The top picture shows a right side buccal ganglion, pinned caudal side up. To rotate the buccal ganglia, the RN and the I2 nerve/muscle are pulled above the buccal ganglia and pinned proximal to the side of the EN. A flap of the CBC sheath is also folded and pinned for rotation (see Figure 2), so that the neurons at the rostral side or at the caudal/rostral border can be seen. The bottom schematic is drawn based on the top picture. The picture and schematic together indicate the locations of the I1/I3 motor neurons B3, B6, B9, B10, B38, B39, B436,7 and B8222,23, as well as some other neurons. Neurons B8a and B8b are responsible for the largest unit on the RN, and innervate the muscle I4 controlling the grasper6,17. Neurons B4 and B5 are responsible for the largest unit on the BN318. Although the sizes and locations of the I1/I3 motor neurons are variable from animal to animal, the relative sizes and locations are quite reliable for most neurons: B3, B6, B9, B38, B43, and B82. See Discussion for more details about the I1/I3 motor neurons, especially some of the difficulties of uniquely identifying B10 and B39.
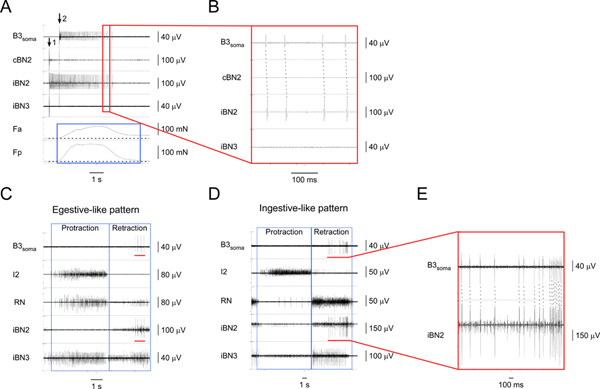
Figure 4. Identifying and characterizing the I1/I3 motor neuron B3. A) Extracellular stimulation of B3 (at arrow 1) and recording from the B3 soma (starting at arrow 2) as well as from the corresponding nerves and muscle regions. From top to bottom, the channels are recordings from the B3 soma, the contralateral BN2, the ipsilateral BN2, the ipsilateral BN3, the contraction force of the anterior region of the I1/I3 muscle, and the contraction force of the posterior region of the I1/I3 muscle. The blue box highlights the duration of forces in the anterior and posterior regions of the I1/I3 muscle. In this particular case, the posterior force is greater than the anterior force. B) Expanded view of the area outlined by the red box in A1. The one-for-one corresponding action potentials in the B3soma and the iBN2 channels show that B3 only projects on the ipsilateral BN2. C) Extracellular recording from the B3 soma and nerves in an egestive-like motor pattern. D) Extracellular recording from the B3 soma and nerves in an ingestive-like motor pattern. In C and D, from top to bottom, the channels are recordings from the B3 soma, the I2 nerve, the RN, the ipsilateral BN2, and the ipsilateral BN3. The blue boxes indicate the protraction and retraction phases of the patterns. The red bars in the B3soma channel in both C and D highlight the action potentials recorded from the B3 soma. The red bars in the iBN2 channel in both C and D indicate the corresponding timing when B3 is firing in the ipsilateral BN2 during the feeding motor patterns. E) Expanded view of the B3soma and the iBN2 channels marked by the red bars. The dashed lines show the one-for-one relationship between the action potentials in the B3soma and the iBN2 channels. Note that the BN2 unit of B3 is the largest of all units. Thus, we can also detect the BN2 units of B3 directly from the BN2 recordings without soma recordings. Click here to view larger figure.
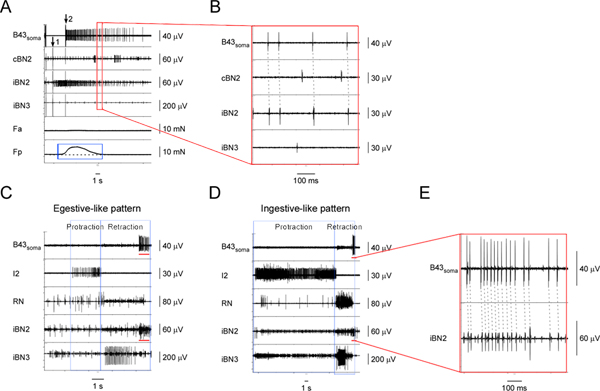
Figure 5. Identifying and characterizing the I1/I3 motor neuron, B43. A) Extracellular stimulation of B43 (at arrow 1) and recording from its soma (starting at arrow 2) as well as from the corresponding nerves and muscle regions. From top to bottom, the channels are recordings from the B43soma, the contralateral BN2, the ipsilateral BN2, the ipsilateral BN3, the contraction force of the anterior region of the I1/I3 muscle, and the contraction force of the posterior region of the I1/I3 muscle. The blue box highlights the force measurements of the I1/I3 muscle during B43 activity. Activating B43 generates a small posterior force, but no anterior force. B) Expanded view of the area outlined by the red box in A. The dashed lines show the one-for-one relationship between the action potentials in the B43soma and the iBN2 channels, which indicates that B43 projects on the ipsilateral BN2 only. C) Extracellular recording from the B43 soma and nerves in an egestive-like motor pattern. D) Extracellular recording from the B43 soma and nerves in an ingestive-like motor pattern. In C and D, from top to bottom, the channels are recordings from the B43 soma, the I2 nerve, the RN, the ipsilateral BN2, and the ipsilateral BN3. The blue boxes indicate the protraction and retraction phases of the patterns. The red bars in the B43soma channel in both C and D highlight the action potentials recorded from the B43 soma. The red bars in the iBN2 channel in both C and D indicate the corresponding timing when B43 is firing in the ipsilateral BN2 in these patterns. E) Expanded view of the B43soma and the iBN2 channels marked by the red bar in D. The dashed lines show the one-for-one relationship between the action potentials in the B43soma and the iBN2 channels. Note that the BN2 units of B43 are small and very difficult to detect without soma recordings, but fire consistently at the end of the BN2 motor program, providing another way to identify them. Note also that the larger unit shown in the bottom panel in E is a collision of a B43soma unit with another extracellular unit. Click here to view larger figure.
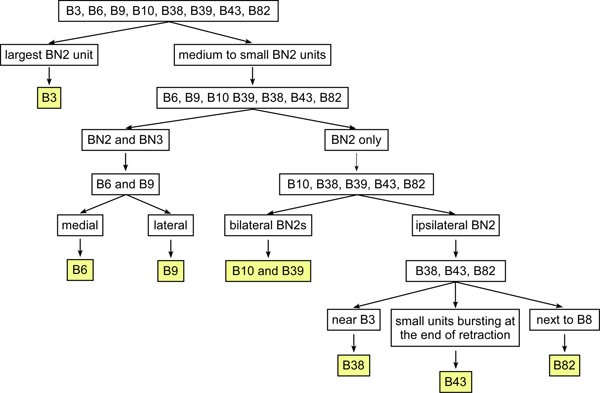
Figure 6. The optimized diagnostic tree for identifying some of the I1/I3 motor neurons using extracellular soma and nerve recordings. This diagnostic method requires the minimal information for identifying the I1/I3 motor neurons, making it much easier to identify motor neurons in the suspended buccal mass preparation or in vivo. B3 has the largest BN2 unit among the identified I1/I3 motor neurons. In the rest of the motor neurons, B6 and B9 are the only two neurons that project on both BN2 and BN3. B9 is more lateral than B6. The rest of the neurons projecting only on BN2 can also be divided into two groups. One group of neurons projects bilaterally through the BN2s, which includes B10 and B39 and some unknown neurons. The other group of neurons projects ipsilaterally on BN2 only, which includes B38, B43, and B82. B38 is near B3 and B9. B82 is near B8 (see Figure 3). B43 is near B6. Its BN2 unit is small and bursts at the end of feeding patterns.
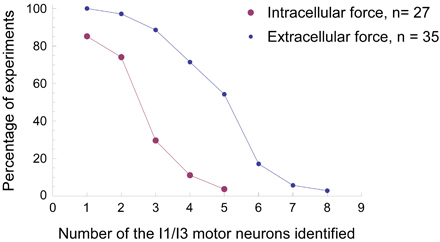
Figure 7. Comparison of success rates of neuron identification during force experiments using either the extracellular technique or the intracellular technique. With the same force transducer setup, we did 35 experiments using the extracellular technique (small blue dots) and 27 experiments using the conventional intracellular technique (large purple dots) to identify the I1/I3 motor neurons. The x-axis indicates the least number of motor neurons for the I1/I3 muscle that were identified in each type of experiment. The y-axis indicates the percentage success rate of each type of experiment. For example, in 19 out of 35 (54%) of the extracellular experiments, we were able identify at least five different I1/I3 motor neurons. In only 1 out of 27 (4%) of the intracellular experiments, we were able to identify at least five I1/I3 motor neurons. It is clear that the success rate in identifying neurons is much higher for any given number of neurons using the extracellular technique.
Discussion
In animals with large identified neurons, such as mollusks (for example, Lymnaea, Helix, and Aplysia), analysis of motor pools is typically done using intracellular recording1,2,3,4. In this protocol, we describe a process for uniquely identifying the motor neurons for a motor pool using an extracellular technique. We used the force measurements as an illustration of this process. One could also use EMG to measure muscle innervations. Briefly, to do so, the protocol needs to be altered to attach hook electrodes to different regions of the I1/I3 muscle for EMG recordings.
The extracellular technique has certain advantages over intracellular techniques, some of which have already been described above. First, the extracellular technique requires less time and effort to prepare ganglia for experiments and will cause less damage to neurons. Usually, it will take 20-30 min to prepare the buccal ganglia for extracellular experiments and approximately 1.5 hr to prepare the buccal ganglia that are attached to the buccal mass for intracellular experiments. Since muscles will become less active as the time passes, the time difference between the ganglia preparations for extracellular experiments and intracellular ones might be critical for the success of experiments. Figure 7 shows the comparison of success rates for identifying the motor neurons for the I1/I3 muscle using extracellular or intracellular technique in force studies. In all 35 extracellular force experiments (100%), we were able to identify at least one motor neuron for the I1/I3 muscle. In 31 out of 35 (89%) extracellular experiments, we were able to identify at least three I1/I3 motor neurons. In 19 out of 35 (54%) extracellular experiments, we were able identify at least five different I1/I3 motor neurons. In contrast, the success rates of the intracellular experiments with the same force transducer setup were lower. In 23 out of 27 (85%) intracellular experiments, we were able to identify at least one I1/I3 motor neuron. In 8 out of 27 (30%) intracellular experiments, we were able to identify at least three I1/I3 motor neurons. In only 1 out of 27 (4%) intracellular experiments, we were able to identify five I1/I3 motor neurons. Thus, the likelihood of identifying multiple neurons in the same ganglion is higher using extracellular techniques in contrast to intracellular techniques.
In addition, the extracellular technique can access many neurons on both sides of ganglia during the same experiment. Usually, after desheathing, intracellular electrodes can only access neurons on the side of the ganglion that has been desheathed. For example, when one of the two buccal ganglia (e.g. the hemiganglion on the left side) is pinned caudal side up, it will be easy for intracellular electrodes to access the neurons on that side of the ganglion, e.g. B6, B9, B10, B39, and B43, but difficult to access the neurons on the rostral side of the ganglion, such as B4, B5, B8a, B8b, B38 and B82. In contrast, extracellular electrodes can access many neurons on both sides of the same buccal ganglion with appropriate rotation of the ganglion. The degree of rotation is adjustable and reversible. This also increases the likelihood of identifying multiple neurons in the same ganglion.
Since extracellular electrodes are gently pressed onto the sheath covering the neurons, these electrodes will not be pulled out of neurons, which may create large holes in the membrane and cause damage, as occurs with intracellular electrodes during muscle movements. The signal size will vary as the ganglia move during the muscle movements. Note that sometimes during large muscle movements, the extracellular soma recording signals will be decreased or even lost. However, we can easily move the extracellular electrode back onto the neuron and recover the original signals. This makes it feasible to apply the extracellular technique to the suspended buccal mass preparation8 for behavioral studies, during which muscles generate large contractions as the preparation generates different behavioral responses. For example, in 47 out of 48 suspended buccal mass experiments (98%), we were able to identify at least one motor neuron for the I1/I3 muscle. In 23 out of 48 (48%) suspended buccal mass experiments, we were able to identify at least three I1/I3 motor neurons. In 11 out of 48 (23%) suspended buccal mass experiments, we were able to identify at least five motor neurons for the I1/I3 muscle and record from them during motor patterns as the buccal mass was performing feeding-like behaviors. The extracellular technique is also applicable to other more complicated semi-intact preparations, such as the isolated head feeding preparations that include the tentacles, lips, jaws, buccal mass, buccal ganglia, and cerebral ganglion12,24,25,26. Since the sensory input is very important for eliciting feeding behaviors in such preparations, the extracellular technique will be particularly useful because of its simplicity and less damaging features. Previous studies also show that it is possible to identify and chronically record B4/B5 in vivo18. In these earlier experiments, the investigators used low current (10-20 μA) BN2-a stimulation to selectively activate B4/B5 and glued a short polyethylene tube to the sheath above B4/B5 for recording, into which were inserted a pair of twisted stainless steel wires. Thus, it is also possible to identify and record from motor neurons in vivo using the polyethylene tube electrode that is glued onto the sheath covering the ganglia (Chestek and Chiel, unpublished results).
The extracellular technique also has some limitations. First, it will be difficult for extracellular electrodes to stimulate or record neurons that are too small or too deep within the ganglion. Note that it is still possible to activate neurons that are not at the surface via extracellular stimulation. However, our model5 has showed that the stimulation may lose specificity when the target neuron is deeper than the neighboring neurons. When the neuron is deeper, the electrode-to-soma distance will be greater and higher current will be needed to activate this neuron, which may be high enough to activate other surface neurons nearby. Second, if a neuron is stimulated extracellularly with too much current, it may be damaged and no longer respond; much smaller currents are used in intracellular stimulation, though too much current intracellularly can also damage neurons. Sometimes the soma recording will include multiple units from both the target neuron and adjacent neurons, which is less specific than intracellular recording. In addition, it may be more difficult to precisely control and monitor the firing frequency of an individual neuron using the extracellular rather than the intracellular technique, because the extracellular electrode cannot stimulate and record the same neuron simultaneously. Moreover, the extracellular technique will not be able to record the synaptic input from premotor neurons. In addition, it may be difficult to apply neurotransmitters iontophoretically to a specific neuron unless the ganglion is desheathed, although we have shown that it is possible to stimulate a ganglion using carbachol without removing the sheath27.
The limitations of extracellular identification techniques made some neurons in the motor pool difficult to identify. In this particular example, the extracellular technique reliably identified most of the motor neurons for the I1/I3 muscle in Aplysia: B3, B6, B9, B38, B43, and B82, based on soma size and location, nerve projection, and muscle innervation. However, we have not been able to reliably identify B10 and B39. Previous intracellular work6,7 showed that B10 and B39 are two adjacent neurons on the caudal side of the buccal ganglia, between the B4/B5 region and the B6 region. Both neurons project bilaterally onto the BN2s. B10 innervates the middle and posterior region of the I1/I3 muscle, whereas B39 innervates the anterior region of the I1/I3 muscle. Based on the soma location and nerve projection criteria, we found more than two motor neurons that project bilaterally onto the BN2s in four different experiments. Since their soma locations, muscle innervations, and timing of activity during motor patterns were variable from animal to animal, we were not sure if they were the same neurons. Thus, we were not able to reliably identify B10 and B39 using the extracellular technique because of the lack of consistency. To uniquely identify them, we need to do a more thorough survey of neurons in the buccal ganglia, and may need additional criteria, such as the synaptic input from premotor neurons B4/B5, and the responses to transmitters, which require intracellular techniques.
With appropriate modifications, this technique is also applicable to other motor pools, e.g. the I5 muscle10, the I2 muscle11, and the I4 muscle12 in Aplysia or to other systems, e.g. Lymnaea stagnalis 2, Helix pomatia3, cockroach13, and zebrafish14. For example, if one wants to apply this technique to the motor neurons for the I5 muscle (also known as the accessory radular closer muscle or ARC10,28) in Aplysia, one should keep the BN3s attached to the buccal mass instead of the BN2s, because the I5 motor neurons B15 and B16 project on the ipsilateral BN36,7. Then the buccal mass should be prepared to expose the I5 muscle for EMG or force studies. After the neurons have been reliably identified in the reduced preparation, an optimized diagnostic method could also be created for future behavioral studies.
The technique we have described compares favorably with other extracellular techniques such as multi-electrode arrays and voltage-sensitive dyes. The voltage-sensitive dye29 technique is only used for recording, whereas our extracellular electrodes and multi-electrode arrays30 can be used for both stimulation and recording. Both the multi-electrode array29 and voltage-sensitive dyes30 can record signals from many neurons simultaneously. Although one single extracellular electrode may only record from one or two neurons depending on its tip size and the electrode location, it is certainly possible to position several on a ganglion simultaneously, and we have done this successfully. The standard in vitro multi-electrode array has 8 x 8 or 6 x 10 electrodes29. Since the electrodes are evenly distributed in the array, it is often challenging to determine the identity of the underlying neurons from which recordings are obtained, since the neurons are not evenly distributed, and significant post-processing of the signals, some of which is still manual, must be done to resolve this ambiguity. In contrast, because the extracellular electrodes are positioned over single somata, the identity of the underlying neuron is clear. Thus, it seems that multi-electrode arrays and voltage-sensitive dyes may be more efficient for multiple simultaneous recordings. However, our extracellular electrode technique may provide better selectivity for both stimulation and recording.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by NIH grant NS047073 and NSF grant DMS1010434.
Materials
| Name | Company | Catalog Number | Comments |
| Sodium chloride | Fisher Scientific | S671 | Biological, Certified |
| Potassium chloride | Fisher Scientific | P217 | Certified ACS |
| Magnesium chloride hexahydrate | Acros Organics | 19753 | 99% |
| Magnesium sulfate heptahydrate | Fisher Scientific | M63 | Certified ACS |
| Calcium chloride dihydrate | Fisher Scientifc | C79 | Certified ACS |
| Glucose (dextrose) | Sigma-Aldrich | G7528 | BioXtra |
| MOPS buffer | Acros Organics | 17263 | 99% |
| Carbachol | Acros Organics | 10824 | 99% |
| Sodium hydroxide | Fisher Scientific | SS255 | Certified |
| Hydrochloric acid | Fisher Scientific | SA49 | Certified |
| Single-barreled capillary glass | A-M Systems | 6150 | |
| Flaming-Brown micropipette puller model P-80/PC | Sutter Instruments | Filament used: FT345B | |
| Enamel coated stainless steel wire | California Fine Wire | 0.001D, coating h | |
| Household Silicone II Glue | GE | ||
| Duro Quick-Gel superglue | Henkel corp. | ||
| A-M Systems model 1700 amplifier | A-M Systems | Filter settings: 10-500 Hz for the I2 nerve/muscle; 300-500 Hz for all the other nerves | |
| Pulsemaster Multi-Channel Stimulator | World Precision Instruments | A300 | |
| Stimulus Isolator | World Precision Instruments | A360 | |
| AxoGraph X | AxoGraph Scientific | Software for recordings | |
| Gold Connector Pins | Bulgin | SA3148/1 | |
| Gold Connector Sockets | Bulgin | SA3149/1 | |
| Sylgard 184 Silicone Elastomer | Dow Corning | ||
| 100 x 15 mm Crystalizing Dish | Pyrex | ||
| High Vacuum Grease | Dow Corning | ||
| Pipet Tips | Fisher Scientific | 21-375D | |
| Minutien Pins | Fine Science Tools | 26002-10 | |
| Modeling Clay | Sargent Art | 22-4400 | |
| Whisper Air Pump | Tetra | 77849 | |
| Aquarium Tubing | Eheim | 7783 | 12/16 mm |
| Elite Airstone | Hagen | A962 | |
| Vannas Spring Scissors | Fine Science Tools | 15000-08 | |
| Dumont #5 Fine Forceps | Fine Science Tools | 11254-20 | |
| Kimwipes | Kimberly-Clark | 34155 |
References
- McCrohan, C. R., Benjamin, P. R. Synaptic relationships of the cerebral giant cells with motoneurones in the feeding system of Lymnaea stagnalis. J. Exp. Biol. 85, 169-186 (1980).
- Benjamin, P. R., Rose, R. M. Central generation of bursting in the feeding system of the snail, Lymnaea stagnalis. J. Exp. Biol. 80, 93-118 (1979).
- Peters, M., Altrup, U. Motor organization in pharynx of Helix pomatia. J. Neurophysiol. 52 (3), 389-409 (1984).
- Church, P. J., Cohen, K. P., Scott, M. L., Kirk, M. D. Peptidergic motoneurons in the buccal ganglia of Aplysia californica: immunocytochemical, morphological, and physiological characterizations. J. Comp. Physiol. A. 168 (3), 323-336 (1991).
- Lu, H., Chestek, C. A., Shaw, K. M., Chiel, H. J. Selective extracellular stimulation of individual neurons in ganglia. J. Neural. Eng. 5 (3), 287-309 (2008).
- Church, P. J., Lloyd, P. E. Expression of diverse neuropeptide cotransmitters by identified motor neurons in Aplysia. J. Neurosci. 11 (3), 618-625 (1991).
- Church, P. J., Lloyd, P. E. Activity of multiple identified motor neurons recorded intracellularly during evoked feedinglike motor programs in Aplysia. J. Neurophys. 72 (4), 1794-1809 (1994).
- McManus, J. M., Lu, H., Chiel, H. J. An In Vitro Preparation for Eliciting and Recording Feeding Motor Programs with Physiological Movements in Aplysia californica. J. Vis. Exp. (70), e4320 (2012).
- Cullins, M. J., Chiel, H. J. Electrode fabrication and implantation in Aplysia californica for multi-channel neural and muscular recordings in intact, freely behaving animals. J Vis. Exp. (40), e1791 (2010).
- Zhurov, Y., Weiss, K. R., Brezina, V. Tight or loose coupling between components of the feeding neuromusculature of Aplysia. J. Neurophysiol. 94 (1), 531-549 (2005).
- Hurwitz, I., Goldstein, R. S., Susswein, A. J. Compartmentalization of pattern-initiation and motor functions in the B31 and B32 neurons of the buccal ganglia of Aplysia californica. J. Neurophysiol. 71 (4), 1514-1527 (1994).
- Morton, D. W., Chiel, H. J. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J. Comp. Physiol. A. 173 (5), 519-536 (1993).
- Iles, J. F. Structure and synaptic activation of the fast coxal depressor motoneurone of the cockroach. Periplaneta americana. J. Exp. Biol. 56 (3), 647-656 (1972).
- Westerfield, M., McMurray, J. V., Eisen, J. S. Identified motoneurons and their innervation of axial muscles in the zebrafish. J. Neurosci. 6 (8), 2267-2277 (1986).
- Susswein, A. J., Rosen, S. C., Gapon, S., Kupfermann, I. Characterization of buccal motor programs elicited by a cholinergic agonist applied to the cerebral ganglion of Aplysia californica. J. Comp. Physiol. A. 179 (4), 509-524 (1996).
- Hurwitz, I., Neustadter, D., Morton, D. W., Chiel, H. J., Susswein, A. J. Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J. Neurophys. 75 (4), 1309-1326 (1996).
- Morton, D. W., Chiel, H. J. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J. Comp. Physiol. A. 172 (1), 17-32 (1993).
- Warman, E. N., Chiel, H. J. A new technique for chronic single-unit extracellular recording in freely behaving animals using pipette electrodes. J. Neurosci. Methods. 57 (2), 161-169 (1995).
- Nargeot, R. N., Baxter, D. A., Byrne, J. H. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J. Neurosci. 17 (21), 8093-8105 (1997).
- Kandel, E. R. . Behavioral biology of Aplysia. , (1979).
- Scott, M. L., Govind, C. K., Kirk, M. D. Neuromuscular organization of the buccal system in Aplysia californica. J. Comp. Neurol. 312 (2), 207-222 (1991).
- Rosen, S. C., Miller, M. W., Cropper, E. C., Kupfermann, I. Outputs of radula mechanoafferent neurons in Aplysia are modulated by motor neurons, interneurons, and sensory neurons. J. Neurophysiol. 83 (3), 1621-1636 (2000).
- Rosen, S. C., Miller, M. W., Evans, C. G., Cropper, E. C., Kupfermann, I. Diverse synaptic connections between peptidergic radula mechanoafferent neurons and neurons in the feeding system of Aplysia. J. Neurophysiol. 83 (3), 1605-1620 (2000).
- Weiss, K. R., Chiel, H. J., Koch, U., Kupfermann, I. Activity of an identified histaminergic neuron, and its possible role in arousal of feeding behavior in semi-intact Aplysia. J. Neurosci. 6 (8), 2403-2415 (1986).
- Rosen, S. C., Teyke, T., Miller, M. W., Weiss, K. R., Kupfermann, I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J. Neurosci. 11 (11), 3630-3655 (1991).
- Jing, J., Weiss, K. R. Generation of variants of a motor act in a modular and hierarchical motor network. Curr. Biol. 15 (19), 1712-1721 (2005).
- Azizi, F., Lu, H., Chiel, H. J., Mastrangelo, C. H. Chemical neurostimulation using pulse code modulation (PCM) microfluidic chips. J. Neurosci. Methods. 192 (2), 193-198 (2010).
- Zhurov, Y., Proekt, A., Weiss, K. R., Brezina, V. Changes of internal state are expressed in coherent shifts of neuromuscular activity in Aplysia feeding behavior. J. Neurosci. 25 (5), 1268-1280 (2005).
- Baker, B. J., Kosmidis, E. K., Vucinic, D., Falk, C. X., Cohen, L. B., Djurisic, M., Zecevic, D. Imaging brain activity with voltage- and calcium-sensitive dyes. Cell. Mol. Neurobiol. 25 (2), 245-282 (2005).
- Fejtl, M., Stett, A., Nisch, W., Boven, K. -. H., Möller, A., Baudry, M., Taketani, M. On Micro-Electrode Array Revival. Advances in Network Electrophysiology Using Multi-Electrode Arrays. , 24-37 (2006).

