The Corneal Micropocket Assay: A Model of Angiogenesis in the Mouse Eye
Summary
The protocol describes the corneal micropocket assay as developed in mice.
Abstract
The mouse corneal micropocket assay is a robust and quantitative in vivo assay for evaluating angiogenesis. By using standardized slow-release pellets containing specific growth factors that trigger blood vessel growth throughout the naturally avascular cornea, angiogenesis can be measured and quantified. In this assay the angiogenic response is generated over the course of several days, depending on the type and dose of growth factor used. The induction of neovascularization is commonly triggered by either basic fibroblast growth factor (bFGF) or vascular endothelial growth factor (VEGF). By combining these growth factors with sucralfate and hydron (poly-HEMA (poly(2-hydroxyethyl methacrylate))) and casting the mixture into pellets, they can be surgically implanted in the mouse eye. These uniform pellets slowly-release the growth factors over five or six days (bFGF or VEGF respectively) enabling sufficient angiogenic response required for vessel area quantification using a slit lamp. This assay can be used for different applications, including the evaluation of angiogenic modulator drugs or treatments as well as comparison between different genetic backgrounds affecting angiogenesis. A skilled investigator after practicing this assay can implant a pellet in less than 5 min per eye.
Introduction
The process of angiogenesis, the formation of new blood vessels form preexisting ones, is highly complex and is regulated by several endogenous factors that control different steps of vessel sprouting and morphogenesis. Angiogenesis is triggered due to a shift in balance between pro- and anti-angiogenic factors, a balance that normally maintains the vasculature in a quiescent state. Angiogenesis in adults occurs in certain physiological conditions such as during the female ovarian cycle or in repair processes such as wound healing and tissue regeneration. However, it is also a hallmark of several pathologies including malignancies, autoimmune conditions and inflammatory diseases. The involvement of angiogenesis in these physiological and pathological conditions makes it an important subject for research and an attractive target for therapy.
Due to the complexity of angiogenesis and the involvement of several cells and factors in the process, including endothelial cells, pericytes, circulating cells and stromal cells, in vitro models remain limited and cannot recapitulate the unique in vivo microenvironment. The main in vitro assays of angiogenesis are largely focused on observing direct effects on endothelial cells and measuring certain steps in angiogenic process under controlled conditions. These assays include the quantification of endothelial cell proliferation1, migration2, network formation3, tube formation4 and sprouting from spheroids5. Ex vivo models, unlike the in vitro ones, are more complex and incorporate multiple tissue cell types, an example being the aortic ring assay6. Nevertheless, like other systems, it cannot capture the contribution of circulating cells and the natural stroma of endothelial cells as exists in vivo. Attempts to study angiogenesis under flow to mimic the in vivo setting are performed using microfluidic systems7, however even these assays, although much improved, are still unable to account for all the compartments existing in vivo.
Due to the limitations of the in vitro and ex vivo angiogenesis models, in vivo models remain the more reliable choices for angiogenesis studies. Examples of these models include the implantation of transparent chambers, "windows", that allow the visualization of growing blood vessels under microscope8, injectable subcutaneous implants such as matrigel and vessel formation in normal tissues such as mouse ear and the chicken chorioallantoic membrane (CAM). However, one of the most acceptable and quantitative in vivo angiogenesis models is the corneal micropocket neovascularization assay described here, which exploits the naturally avascular cornea as a "screen" to visualize and assess new angiogenic growth9.
Here we describe the corneal micropocket assay as developed in mice. Initially the model was used to measure nonspecific angiogenic stimuli in rabbit corneas. This was done by introducing tumor pieces into the aqueous humor of the anterior chamber of the rabbit eye and measured tumor-induced neovascularization11.
However, the assay was later evolved to study effects of specific growth factors10 to better specify and standardize the angiogenic effect. In order to release the growth factor in the eye, slow-release pellets containing known quantities of angiogenic growth factors were used instead of tissues. The availability of purified recombinant angiogenic proteins such as bFGF or VEGF enabled their use as specific targets of angiogenesis modulaters12. Initially, the assay was largely used in rabbits, which are easier to work with due to their size but later the model was translated into mice; a smaller and less expensive animal model. Shifting from rabbit to mice provided an important advantage of being able to use genetically manipulated animals, thereby creating a new area of research into the genetic components impacting angiogenesis13. In addition to the more acceptable usage of cornea assay in studying angiogenesis, other biological processes can also being investigated using modified assays. For example, studies of lymphangiogenesis were made possible through the implantation of low dose bFGF pellets which allowed the visualization of lymphatic vessels through specific molecular markers14. In addition, this assay has provided a means to evaluate the effects of radiation on angiogenesis15.
In summation, the corneal micropocket angiogenesis assay is a quantitative, reproducible, flexible assessment of angiogenesis in vivo. A major advantage of this assay is that the measurement of background vessels is unnecessary because the vessels grow on a naturally avascular tissue. We describe here the protocol of this assay in details and discuss different scenarios which can occur. The assay consists of 3 discrete segments. Here we will describe the preparation of growth factor-inclusive pellets, the subsequent surgical implantation and finally the method used to quantify the resulting neovascular growth.
Protocol
All protocols involving animals are presented to and approved by the Institutional Animal Care and Use Committee at Children's Hospital Boston and are conducted in accordance with the recommendations of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Be sure to use sterile instrumentation and aseptic techniqure while performing the procedure.
1. Preparation of Pellets
- Weigh out 10 mg of sucralfate and 60 mg of hydron with the sterile unbent spatula. Place in separate microcentrifuge tubes.
- Place a 1 cm square piece of mesh into a sterile 10 cm dish. Invert so mesh rests on shallow lid. Set aside.
- Add 500 μl of ethanol to the hydron and vortex for at least 10 min.
- Add the appropriate amount of growth factor (standard: 20 μg for bFGF, 50 μg for VEGF) to the sucralfate and vortex briefly. Store growth factor in a -80 °C freezer at a concentration of 1 mg/ml. Liquid volumes added should be a minimum of 20 μl to ensure the sucralfate is completely moistened but should not exceed 50 μl. Greater volume makes the evaporation of the liquid in the next step difficult.
- Place the sucralfate mixture into the centrifugal evaporator set on low until the mixture is completely dry, normally 30-50 min depending on the volume of liquid used.
- Check the mixture for residual moisture with the tapered end of a sterile spatula. The mixture should feel "crunchy".
- Use the tapered end of the spatula to break up the sucralfate mixture within the tube.
- Add 10 μl of the hydron to the sucralfate.
- Due to the viscous nature of hydron, cut the end from a 200 μl pipet tip and then draw and release the 10 μl before pipetting up again and adding to sucralfate.
- Quickly use bent spatula to mix the hydron and sucralfate and remove from tube.
- Gather the mixture along the bottom side of the tip of the bent spatula and then rotate the tube while drawing the spatula out along the wall of the tube. The mixture will dry rapidly so this step must be done efficiently.
- Spread the mixture onto the prepared mesh using forceps to hold it in place. The mixture should be an even layer and fill into the holes of the mesh.
- Immerse the bent spatula into the tube containing hydron and use it coat both sides of the mesh.
- Place the coated mesh leaning on side against edge of dish and allow drying at RT for 30-45 min.
- Place the mesh in -20 °C freezer or proceed immediately to next step. Keep mesh inside dish.
- Place the 35 mm dish inside of the larger petri dish, lid off. Use the pair of forceps to carefully pull the fibers of the mesh apart over the 35 mm dish. Stray pellets can be collected from the larger dish at the end.
- Check pellets for uniformity under scope. The process yields approximately 250 pellets thus the standard dose per pellet for bFGF and VEGF is 80 ng and 200 ng, respectively.
- Store pellets in 35 mm dish in -20 °C freezer for up to 3 months.
2. Surgical Implantation of Pellets
- Set up a surgical area under operating microscope.
- Anesthetize the mouse with avertin (400-500 mg/kg, intraperitoneal) and position the mouse under microscope so eye is visible. Perform toe pinch to ensure sufficient level of anesthesia.
- Anesthetize the mouse eye by placing 1 drop of proparacaine solution atop eyeball. Wait 20-30 sec and dab with a gauze pad.
- Proptose the mouse eye with the dulled #1 jewelers forceps being certain to leave skin between forceps and eye. It is important to judge the pressure applied correctly. Holding the eye too firmly will cause it to become red and irritated, while too loose a hold will allow eye to move during surgery.
- Use the 30° microknife to make an incision into the cornea approximately 1 mm from the limbus. The incision should be 1-2 mm in length. Incision should be deep enough to penetrate beyond the epithelial layer into midstroma but not so deep to rupture the eye.
- Use the von Graef knife to make a pocket perpendicular to the incision.
- Slide the knife under the corneal layer at the incision site and gently work knife in and move it along the incision to enlarge the space, thus forming the pocket. It helps to move the mouse as well to be able to work with the curvature of the eye. Be careful not to push downward with the tip to avoid rupturing the eye.
- Wet the #5 jeweler's forceps with proparacaine and pick a pellet up out of dish. Place a pellet on the eye.
- Moisten the von Graef knife with proparacaine and transfer some of liquid onto pellet to make it rubbery and pliable. Remove excess moisture with gauze pad or cotton swab.
- Insert the pellet into the pocket using the von Graef knife to push it inside beneath the cornea layer. Once the whole pellet is inside, run the flat side of von Graef knife over site to check that the pellet is secure.
- Coat the eye with triple antibiotic ointment.
- Turn the mouse over and repeat surgery in the opposite eye.
3. Quantification of Corneal Neovascularization.
Leave animals to develop vessels over a set period of time. The day of vessel grading depends on growth factor used. Grade bFGF on day 5 and the weaker VEGF on day 6. The day of implantation is day 0.
- Anesthetize the mouse with avertin (400-500 mg/kg, intraperitoneal).
- Use the slit lamp microscope for grading. One ocular has a reticule, lines within the eyepiece used as a measuring aid to quantify vessel length and distance around the eye's circumference that the vessels have sprouted.
- Hold the mouse in front of scope, positioning mouse so eye is seen in ocular with pellet directly ahead. Stabilize the head between thumb and forefinger so skin is tightened on face and eye is slightly proptosed.
- Place the y-axis of the reticule along the limbal vessel directly beneath pellet. Measure the length of vessels branching upward toward pellets. Record this measurement in tenths of millimeters and designate vessel length (VL) (Figure 1A).
- Turn either the mouse or the reticule so that the distance around the eye that these vessels have sprouted becomes clear. It is easiest to think of the eye as a clock face with intervals of 1 through 12. Designate this number clock hour (CH) (Figure 1B). It can be a whole number or fractions of 0.25 (e.g., 2, 2.25, 2.5 or 2.75). Note: CH is subjective. For example, some may measure the distance around the eye to include all vessels sprouted from the limbus while others may stop measuring at the point where vessel length is half maximal. The important factor is to be consistent with how one chooses to grade.
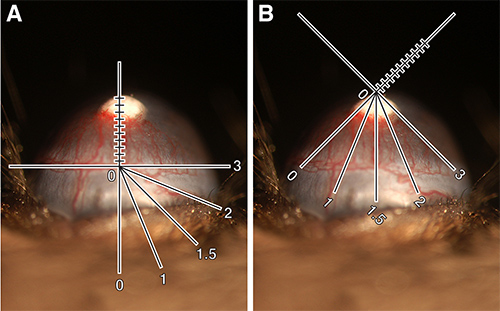
Figure 1. Quantification of corneal neovascularization. (A) 80 ng bFGF pellet implanted in C57BL/6J mouse showing slit lamp reticule oriented to measure vessel length. VL = 0.9 mm. (B) Same eye showing slit lamp reticule rotated to measure clock hour. CH = 3.25. Calculated vessel area = 1.84 mm2. These images have been digitally enhanced to allow for the addition of reticule schematic. Click here to view larger image.
- Calculate the vessel area (VA) using the formula below, which is based on the area of an oval (Note, it is helpful to keep this in mind when grading.) Express VA in millimeters squared. The formula is as follows:
Vessel length x Clock hour x 0.2 π = Vessel area
Representative Results
Typical results for bFGF and VEGF pellets in the normal low angiogenic C57BL/6J mice are shown in Figure 2A and B, respectively. Figure 2E shows the normal distribution of vessel area (VA) in the C57BL/6J strain with varying doses of these growth factors. 80 ng bFGF pellets normally result in a VA of approximately 2.0 mm2, though values in a range of 1.8-2.4 mm2 are acceptable. 200 ng VEGF pellets typically cause a response of approximately 0.7 mm2, with a range of acceptable values of 0.6-0.9 mm2. For experiments where an anti-angiogenic effect is suspected, larger vessel areas are desirable to be able to visualize a decrease. Figure 3 shows the effect of the anti-angiogenic drug thalidomide on bFGF-driven neovascularization. Treatment resulted in 38% inhibition of vessel growth. In experiments where an increase may be expected it is best to use lower doses as the standard pellet causes a near maximum response. Pellets consisting of 10-40 ng bFGF would be more appropriate (Figure 2C).
Strains known to have a high-angiogenic response to a particular growth factor can have a VA more than twice that of a typical low-angiogenic strain. Figure 2D shows the response of a 129S1/SvImJ mouse to a 20 ng FGF pellet. For comparison, the same dosage pellet is shown in a C57BL/6J in Figure 2C. An extensive table detailing the angiogenic potential of various strains to both bFGF- and VEGF-induced neovascularization has been previously published by our lab13.
Certain strains or pigmentations may exhibit growth or leakage of the iris vessels in response to a pellet. This can occur in one or both eyes of an individual mouse and may or may not be consistent over the entire group in which the assay is performed. This phenomenon can result in rupture of the iris vessels causing blood to fill the anterior chamber (hyphema). Hyphemas can make grading the corneal neovascularization difficult or impossible. Figure 4 illustrates a mild (4A) and severe (4B) form of this occurrence.
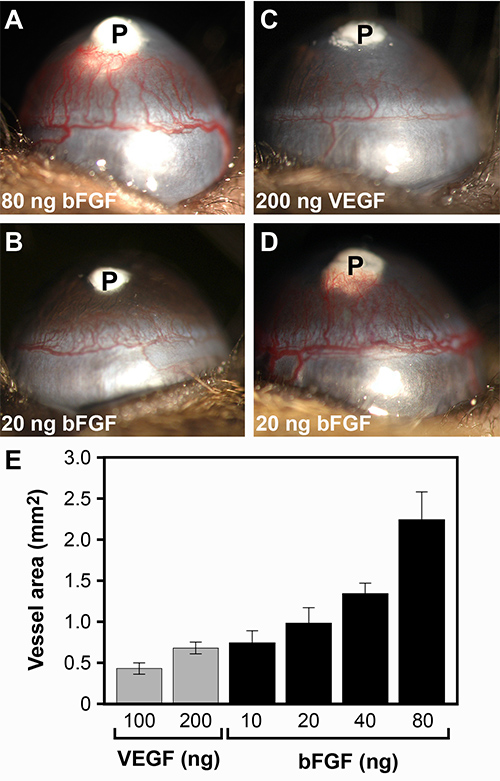
Figure 2. Examples of growth factor-induced corneal neovascularization. (A) 80 ng bFGF pellet implanted into a low angiogenic C57BL/6J mouse, resulting in a vessel area equal to 2.0 mm2. It is very common for this dose to cause vessels to reach the pellet and grow into it. (B) 20 ng bFGF pellet implanted into a C57BL/6J mouse, resulting in a vessel area equal to 1.04 mm2. (C) 200 ng VEGF pellet implanted into a C57BL/6J mouse, resulting in a vessel area equal to 0.71 mm2. (D) 20 ng bFGF pellet in the high angiogenic 129S1/SvImJ strain. Compared to (C), also a 20 ng FGF pellet, this strain has an angiogenic potential greater than twice that of C57BL/6J mice. (E) Corneal neovascular area (mean ± SD, n = 10) in C57BL/6J mice induced by increasing doses of either VEGF or bFGF. Click here to view larger image.
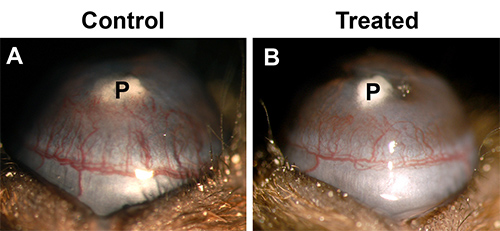
Figure 3. Inhibition of bFGF-induced vessel growth by anti-angiogenic agent. (A) Control vehicle treated C57BL/6J mouse. Implantation of 80 ng bFGF pellet resulting in a group vessel area equal to 2.0 mm2. (B) Thalidomide treated mouse (200 mg/kg, oral for 5 days). Group vessel area equals 1.25 mm2 and reflects 38% decrease in vessel growth. Click here to view larger image.
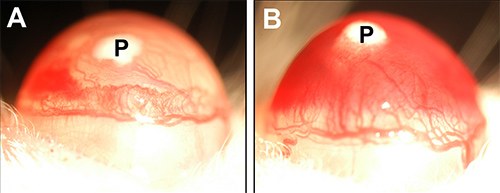
Figure 4. Growth factor-induced hyphemas in the murine iris. (A) Mild hyphema in a C57BL/6J-TyrC-2J/J. This strain exhibits the same corneal neovascularization as their pigmented counterparts but is more prone to iris bleeding. (B) Large scale hyphema in a 129P3/J mouse. The entire eye has a reddish tinge as well as more pronounced pools of blood closer to bottom of iris. Click here to view larger image.
Discussion
There are several critical steps in performing a successful corneal assay. The first is making uniform pellets capable of being implanted and stimulating vessels. The most important parts of pellet preparation are 1) using carrier-free growth factor; 2) ensuring a good mixture of growth factor with the sucralfate and hydron and 3) moving the final mixture swiftly but carefully from the eppendorf tube to the mesh where the pellets are cast. It is recommended that "dummy" pellets be made without growth factor to practice the technique. Pellets should appear white and chalky and be roughly square in shape. Any pellets that include clear areas should be excluded as these areas likely contain only hydron. It should also be noted that the pellets are formed with ethanol and that bFGF and VEGF are stabilized by the inclusion of sucralfate, which mimics heparin binding. Sucralfate also serves to provide a slow release platform for the growth factors. If other stimulators or inhibitors are added to the pellets, these factors should be tested first to determine stability in ethanol. It is recommended that "dummy" pellets be made without growth factor to initially practice the technique. "Dummy" or sham pellets contain only sucralfate and hydron with PBS substituted for the volume of growth factor or compound being tested. These pellets do not stimulate vessel growth or limbal inflammation. Sham pellets serve as an appropriate control in experiments where an agent is suspected of being pro-angiogenic and is tested without the addition of growth factor to the pellet. Compounds and growth factor can be combined in pellets for instances where an interaction is being investigated, in which case pellets of the growth factor alone would be the appropriate control.
Surgical implantation is the most challenging part of the assay. It is initially very difficult to gauge the appropriate depth for the incision. Too deep an incision causes the globe to rupture and the eye becomes inoperable. This is a dramatic effect and therefore one quickly learns not to "pop" the eye. It takes considerably longer to consistently make an incision deep enough to allow pocket formation. When the incision is too shallow, the uppermost layer of the cornea is easily damaged or ripped off with the von Graef knife as it is inserted to make the pocket. The final step of quantifying the new vessel growth requires consistency in the manner of grading. Vessel length is a straightforward measurement while the clock hour value depicting the growth around the circumference of the eye is more subjective. The method used should be the same from mouse to mouse and between experiments.
Another critical component of the assay is the choice of anesthesia, which can have an impact on the ease and success of the surgical procedure. Injectable avertin is the least complicated as it allows for easy manipulation of the mouse in contrast to vaporized anesthesia delivered via nose cone. It is advisable to avoid the combination of ketamine and xylazine as well due to its association with corneal lesions in rodents16,17.
It is important to note any change in the weight of mice during experiment. Weight loss can be indicative of poor housing conditions or illness and these can negatively affect a mouse's ability to grow vessels in the eye. Weights should be taken on days of pellet implantation and vessel grading to determine the percentage of weight lost, if any. Loss of 5% or less is acceptable and can result from the stress of handling the mice, especially during treatment experiments with frequent dosing. Greater than 5% weight loss is concerning and will nonspecifically suppress vessel growth. Thus results from such experiments should be considered invalid.
To account for inherent experimental variances from procedure technique and the efficacy of variables studied, a minimum number of mice should be used to achieve valid results. In our experience we advise no fewer than 5 mice per group yielding an n of 10 eyes.
Despite the many advantages of the corneal micropocket assay, there are also a few limitations. The main drawback is the need for a relatively long training period in order to master the technique. An individual with no prior technical experience working in the mouse eye would require approximately four months of practice before experiments of 20-25 mice could be regarded as reliable. Moreover, there are some variations between different people who perform the assay, thus limiting the possibility of comparing between experiments that are performed in different times by different people. Another limitation of this model is that experiments are relatively short (5-6 days) and are restricted by the release of the bFGF or VEGF from the pellet. Therefore, this assay is highly suitable for therapies that have a fast response in vivo, although this can be partially compensated for by pre-treating the mice for desired length of time prior to procedure. Standard drug testing assays typically begin administration on the day of pellet implantation (Day 0) continuing until day of grading (Day 5 or 6).
The significance of this technique is that it is one of the most reliable quantitative angiogenesis assays. An alternative in mice is the matrigel plug assay, however this assay is more qualitative and is less specific to a certain growth factor due to an extensive cell infiltration that occurs into the plug. The flexibility of the corneal assay allows one to tailor the amount of angiogenesis generated and to choose a specific stimulator. It is possible to substitute the standard growth factors with other known or suspected angiogenic factors. Additionally, it is possible to combine standard growth factors and possible inhibitors within the same pellet to evaluate anti-angiogenic activity without systemic delivery. Finally, this assay allows one to stimulate angiogenesis on different genetic backgrounds, which can be useful in mapping genetic effects and in establishing the impact of a particular gene on angiogenesis.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Kristin Johnson for the graphic work.
Materials
| Section 1: Pellet preparation | |||
| Sucralfate | Sigma | #S0652 | |
| Hydron (aka Poly(2-Hema)) | Sigma | #P3932-10G | |
| Ethanol | Pharmco Products Inc | #111000200CSGL | |
| Growth factors: | Must be carrier-free (no bovine serum albumin(BSA)) | ||
| Fibroblast growth factor (FGF) | PeproTech | #AF-100-18B | |
| Vascular endothelial growth factor (VEGF) | R & D Systems | #293-VE-050/CF | |
| 35 mm dish | Becton-Dickson | #353001 | Used for storage of pellets |
| 10 cm petri dish | VWR | #25384-342 | Used as work surface for preparing pellets |
| Mesh | Sefar America | #03–300/51 | 300 um nitex nylon, cut into cm square pieces and sterilzed in autoclave |
| Spatulas | Fisher Scientific | #21-401-10 | Use tapered end of one to break up pellet mixture. Bend tapered end of other to help remove mixture from microcentrifuge tube. |
| Microcentrifuge tubes | Fisher Scientific | #05-408-146 | One for hydron, one for sucralfate |
| Jewelers forceps, #5 | Ambler Surgical | #2315E | Need 2 for pulling mesh apart |
| Centrifugal evaporator | ThermoSavant | DNA110 SpeedVac | |
| Section 2: Surgical implantation of pellets | |||
| Operating microscope | Zeiss | ||
| 2.5% Avertin | General anesthetic | ||
| Proparacaine hydrochloride ophthalmic solution 0.5% | Falcon | NDC# 6131401601 | Eye anesthetic |
| Triple Antibiotic Ophthalmic Ointment | Bausch & Lomb | NDC# 2420878055 | Contains neomycin, polymixin and bacitracin |
| Ophthalmic microknife, 5 mm | Surgistar | #924501 | 30 degree angle |
| von Graef knife | Ambler Surgical | #3401E | |
| Jewelers forceps, #1 | Ambler Surgical | #2301E | Must be blunted with sharpening stone for proptosing eye |
| Jewelers forceps, #5 | Ambler Surgical | #2305E | For picking up pellets and placing on eye |
| Small curved scissors | Ambler Surgical | #5636E | For trimming whiskers |
| Gauze | For blotting eye after proparacaine | ||
| Section 3: Grading of Corneal Neovascularization | |||
| 2.5% Avertin | General anesthetic | ||
| Slit lamp | Nikon | FS-2 | Needs an ocular with a reticule to assist in measuring |
References
- Gospodarowicz, D., Moran, J., Braun, D., Birdwell, C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc. Natl. Acad. Sci. USA. 73, 4120-4124 (1976).
- Glaser, B. M., D’Amore, P. A., Seppa, H., Seppa, S., Schiffmann, E. Adult tissues contain chemoattractants for vascular endothelial cells. Nature. 288, 483-484 (1980).
- Kubota, Y., Kleinman, H. K., Martin, G. R., Lawley, T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 107, 1589-1598 (1988).
- Montesano, R., Orci, L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 42, 469-477 (1985).
- Korff, T., Augustin, H. G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 143, 1341-1352 (1998).
- Nicosia, R. F., Tchao, R., Leighton, J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro. 18, 538-549 (1982).
- Wong, K. H., Chan, J. M., Kamm, R. D., Tien, J. Microfluidic models of vascular functions. Annu Rev Biomed Eng. 14, 205-230 (2012).
- Sandison, J. C. A new method for the microscopic study of living growing tissues by the introduction of a transparent chamber in the rabbit’s ear. Anat. Rec. 28, 281-287 (1924).
- Jain, R. K., Schlenger, K., Hockel, M., Yuan, F. Quantitative angiogenesis assays: progress and problems. Nat. Med. 3, 1203-1208 (1997).
- Rogers, M. S., Birsner, A. E., D’Amato, R. J. The mouse cornea micropocket angiogenesis assay. Nat. Protoc. 2 (10), 2545-2550 (2007).
- Gimbrone, M. A., Leapman, S. B., Cotran, R. S., Folkman, J. Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med. 136, 261-276 (1972).
- Gimbrone, M. A., Cotran, R. S., Leapman, S. B., Folkman, J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J. Natl. Cancer Inst. 52, 413-427 (1974).
- Rohan, R. M., Fernandez, A., Udagawa, T., Yuan, J., D’Amato, R. J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 14 (7), 871-876 (2000).
- Chang, L. K., et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 101 (32), 11658-11663 (2004).
- Udagawa, T., Birsner, A. E., Wood, M., D’Amato, R. J. Chronic suppression of angiogenesis following radiation exposure is independent of hematopoietic reconstitution. Cancer Res. 67 (5), 2040-2045 (2007).
- Turner, P. V., Albassam, M. A. Susceptibility of rats to corneal lesions after injectable anesthesia. Med Comp. 55 (2), 175-182 (2005).
- Calderone, L., Grimes, P., Shalev, M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp. Eye Res. 42, 331-337 (1986).

