An Optogenetic Method to Control and Analyze Gene Expression Patterns in Cell-to-cell Interactions
Summary
Here, we present a protocol to analyze cell-to-cell transfer of oscillatory information by optogenetic control and live monitoring of gene expression. This approach provides a unique platform to test a functional significance of dynamic gene expression programs in multicellular systems.
Abstract
Cells should respond properly to temporally changing environments, which are influenced by various factors from surrounding cells. The Notch signaling pathway is one of such essential molecular machinery for cell-to-cell communications, which plays key roles in normal development of embryos. This pathway involves a cell-to-cell transfer of oscillatory information with ultradian rhythms, but despite the progress in molecular biology techniques, it has been challenging to elucidate the impact of multicellular interactions on oscillatory gene dynamics. Here, we present a protocol that permits optogenetic control and live monitoring of gene expression patterns in a precise temporal manner. This method successfully revealed that intracellular and intercellular periodic inputs of Notch signaling entrain intrinsic oscillations by frequency tuning and phase shifting at the single-cell resolution. This approach is applicable to the analysis of the dynamic features of various signaling pathways, providing a unique platform to test a functional significance of dynamic gene expression programs in multicellular systems.
Introduction
Cell-to-cell communications play critical roles in embryonic patterning in developmental processes. In vertebrate embryos, the metameric structures called somites are formed along the anterior-posterior body axis with a precise temporal accuracy under the control of a time-keeping clock, called the segmentation clock1. During this process, a group of presomitic mesoderm (PSM) cells are periodically converted into somites in a synchronous manner. This process involves synchronized oscillatory gene expression and PSM cells that oscillate in phase form the same somites. The period of the oscillatory gene expression is around 2 to 3 h in mice and about 30 min in zebrafish. When dissociated, PSM cells lose the synchrony2,3, but when they are re-aggregated, they can self-organize and recover the population synchrony4, suggesting that cell-cell coupling is a key for the synchronized oscillations.
Extensive efforts revealed that signaling molecules in the Delta-Notch pathway are tightly connected to the synchronized oscillations of the segmentation clock genes. Either pharmacological inhibitors or genetic mutations of Notch signaling desynchronize the population of the oscillators. In zebrafish, mutants of Notch signaling components, such as DeltaC, DeltaD, and Notch1a, display asynchronous oscillations5,6. In chick or mouse embryos, not only the Notch ligand Delta-like1 (Dll1) but also the Notch Modulator Lunatic fringe (Lfng) is required for synchronized oscillations7,8,9. However, it has been difficult to test the functional capability of such molecules for dynamic information transfer from cell to cell, because temporal resolutions of conventional perturbation of gene regulation dynamics were not sufficient to investigate the processes of timescales of 2–3 h (ultradian rhythms).
We have recently developed an integrated method to control and monitor gene expression patterns in mammalian cells10. This technology enables induction of gene expression pulses by periodic light illumination on ultradian time-scales. This protocol represents the methods to establish photosensitive cell-lines and observe dynamic responses of reporter cells by live-cell luminescence monitoring in the contexts of cell-to-cell communications. This method is applicable to the analysis of many other signaling pathways.
Protocol
1. Generation of Stable Cell Lines by the Tol2 System
- Transfect plasmid vectors (Figure 1A) of Tol2-based optogenetic modules together with the transposase (Tol2) expression vector (pCAGGS-mT2TP) into C2C12 cells. In all steps, culture cells with DMEM medium supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin at 37 °C (Table 1), in the presence of 5% CO2, otherwise denoted.
- Count trypsinized cells with a cell-counter, and plate 5 x 104 C2C12 cells per well in a 12-well plate one day before transfection.
- Co-transfect 0.375 μg of Tol2-based optogenetic vectors (pAI177 or pAI218), 0.125 μg of a drug selection vector (pAI170) and 0.5 μg of pCAGGS-mT2TP vector by using lipofection reagent.
- Trypsinize transfected cells, and plate them into 100 mm culture dishes one day after transfection.
- Exchange culture medium for transfected cells to culture medium supplemented with 100 mg/mL hygromycin.
- Culture cells for 3 days to eliminate un-transfected cells. Note that medium change is not necessary.
- Trypsinize transfected cells, and purify a population of cells expressing fluorescent proteins for selection. For receiver cells carrying pAI177, set the sorting gate to Green-PE channel for purification of mCherry-positive cell population. For photo-sensitive sender cells carrying pAI218, set the sorting gate to APC channel for purification of iRFP713-positive cell population.
- (optional) Establish a clonal cell population by standard methods, such as limited dilution or single-cell sorting by FACS.
2. Evaluation of Photo-sensitivity of Engineered Cells at the Population Level
NOTE: This part describes a protocol to check dynamic induction of Dll1 ligand proteins upon blue-light illumination by biochemical assays, such as qPCR and Western blotting.
- Set up two types of incubators with or without light sources for a light-induced condition or a dark condition, respectively. Set-up light-intensity of a blue-LED trans-illuminator, as shown in Figure 1B, by using a light meter. Program timings and duration of light illumination by loading a control-script to a single-board micro-controller that allows programmable schedules for illumination (Figure 1C).
- Count trypsinized cells with a cell-counter, and plate 1.0 x 105 photo-sensitive sender cells carrying pAI218 and pAI170 on 35-mm diameter plastic culture dishes (more than 12 dishes for light condition and 1 dish for dark condition) and set the dishes in separate incubators for dark/light conditions. After setting up dishes, keep doors of the incubators closed until collecting cell lysates.
- One and a half days after plating, start light illumination (cells are expected to be more than 80% confluent). Don't expose cells to light to avoid undesirable photo-stimulation.
NOTE: Schedules for illumination depends on the purpose of experiments. Note that a sustained illumination condition (e.g. 1-min duration and 10 min intervals with 40 µmol/m2/s light-intensity) is sufficient for a simple check of photo-induction, and that strong light illumination is harmful to cells. Thus, ensure that harmless parameters for illumination are selected. - About 2 days after plating, prepare cell-lysate with 30-min intervals. Move sample dishes from incubators to on-ice, and start preparing cell lysates for further analysis.
3. Dynamic Sender-receiver Assay at the Population Level: Real-time Monitoring of Cellular Responses upon Optical Stimulation by PMT
- Trypsinize and count the numbers of the sender and the receiver cells with standard methods. Prepare a 1-mL total volume of mixing suspension of 2.5 x 104 receiver and 1.25 x 105 sender cells (1:5 ratio).
NOTE: 2:1, 1:1, or 1:2 sender-receiver ratios also work with a total cell number of 1.5 x 105.10 - Plate mixed cells in each well of 24-well black plates with culture medium containing 1 mM luciferin.
- Analyze the cells on plate reader for luciferase assay, and confirm that the output signals are not too high for the recording system.
NOTE: If output signals are higher than 1 x 106 photon counts/s, they may be saturated. In that case, reduce the concentration of luciferin to optimal values so that output signals are lower than 1 x 106 photon counts/s. - Set the plate on the recording system, and start a recording program (Figure 1D). For example, start light illumination at 18 h after setting the recording.
NOTE: Temporal filtering, such as detrending signals with moving averaging and Savitzky-Golay filtering, are useful for visualization of wave-forms and peak detections.
4. Dynamic Sender-receiver Assay at the Single-cell Level: Real-time Imaging of Single-cell Responses under the Control of Optogenetic Perturbation
- Plate 0.5 x 105 receiver and 2.5 x 105 sender cells onto 27-mm-diameter-glass-base 35 mm diameter dishes. Ensure that mixing ratios and a total cell number (a cell density) are correctly adjusted.
- One day after plating, exchange medium with 2 mL of the recording medium (phenol-red free DMEM medium supplemented with 5% FBS, penicillin-streptomycin and 1 mM luciferin), and set the glass-base dish on the microscope. If necessary, wash out debris of dead cells with PBS (-).
NOTE: It is preferable to do this step in a dark condition. - Set up an inverted microscope equipped with an environmental chamber at 37 °C and 5% CO2.
- Set up a cooled CCD camera. Ensure that the temperature of CCD sensor is cooled down to the destination value (typically, -90 °C).
- Set parameters for "Multi-Dimensional Timelapse" window in automatic acquisition software to capture images with 5 min intervals and more than 288 times (more than one overnight recording).
- Open "Multi-Dimensional Timelapse" window, select a luminescence channel and a slow 50 kHz read-out mode from the pull-down menu and set 4 x 4 binning with 4-min exposure. Ensure that the read-out mode is set to the slow 50 kHz mode, which is critical for reducing read-out noises to detect weakly emitting bioluminescent light.
- Open "Multi-Dimensional Timelapse" window, select fluorescence channels and a fast 1 MHz read-out mode from the pull-down menu and set 2 x 2 binning with 400 ms exposure. Ensure that the read-out mode is set to the fast 1 MHz mode to reduce the time required for fluorescence imaging.
- Start time-lapse recording by clicking "Acquire" button.
- Extract single-cell traces of luminescence channels from time-lapse movies by image analysis software, as follows. First, make luminescence and fluorescence stack images by importing the image data to image analysis software. For luminescence images, remove hot pixels derived from cosmic rays by comparing the intensities of pixels in temporally adjacent images, replacing the pixel values to the temporal average of the previous and next frames (implemented as "SpikeNoise Filter"), apply these processed images to a spatially smoothing filter, and subtract background pixel values of out-field views from each frame. Then, determine a "region of interest" (ROI) for each cell on a fluorescent image at each time point, apply the ROI to luminescent images, and measure the luminescent intensity of each ROI.
Representative Results
We adapted the LightOn system11,12, which enables photo-induced gene expression in mammalian cells, to the study of genetic oscillators with 2- to 3-h periodicity. This system comprises of two parts: the photo-inducible transcriptional activator hGAVPO and a UAS-promoter cassette to drive transcription of arbitrary genes of interest. To accelerate the pulsatile kinetics of photo-induced gene expression, the polyA sequence in the UAS-promoter cassette was replaced by murine Hes1 3'UTR, which shortens the mRNA half-life to 20 min in murine fibroblasts.
To establish stable cell lines, tri-cistronic vectors based on the Tol2 transposase system was used13 (Figure 1A). The Tol2 expression vector is essential for enhancing the efficiency of chromosomal integration of plasmid cassettes14. Those vectors carry not only the expression cassettes of light-inducible genes driven by the UAS-promoter but also the expression cassettes of the photo-sensitive protein hGAVPO and fluorescent proteins (iRFP71315 or mCherry). This design allowed us to purify a population of cells that effectively integrated the plasmids into the host genomes.
We established photo-sensitive sender cells that produce Dll1 ligand proteins upon blue light illumination (Figure 2A). To this end, a Tol2 system-based bi-cistronic vector (pAI218) was used. To check induction of Dll1 ligand upon light illumination, cells were cultured in the incubators equipped with programmed LED blue-light sources shown in Figure 1B and 1C. Representative results of photo-induced Dll1 protein expression dynamics detected by Western blotting analysis are shown in Figure 2B and 2C.
To establish cells that respond to Notch signaling inputs, we used a Tol2 system-based bi-cistronic vector (pAI177) carrying the destabilized luciferase reporter under the control of the promoter of the Notch effector gene Hes1 and the phosphoglycerate kinase (PGK) promoter-driven nuclei-localized red fluorescent reporter H2B-mCherry. In this case, the fluorescent reporter is useful for both purification and single-cell tracking in time-lapse microscopy.
Once photo-inducible sender cells and receiver cells are successfully established, it is ready to perform dynamic sender-receiver assay by co-culturing these cells (Figure 2A). In this assay, the photo-sensitive sender cells that express the Notch ligand protein Dll1 upon blue light illumination were co-cultured with the photo-insensitive receiver cells that carry the endogenous Hes1 oscillator and a bioluminescence Hes1 reporter (pHes1-dLuc) (Figure 2D). The receiver cells endogenously express the Notch receptor, which is responsive to Dll1 presented by neighboring cells.
Representative results of dynamic sender-receiver assays are shown in Figure 2E and 2F. To detect the dynamic responses of receiver cells with bioluminescence recording, we first used a live-cell monitoring system equipped with a high-sensitive photo-multiplier tube (PMT) for photon-counting and a LED blue-light source for light stimulation (Figure 1D). This system allows real-time recording of bioluminescence signals at the population level with scheduled light illumination. When these two types of cells were co-cultured and exposed to repetitive light illumination (30 s duration, 2.5 h intervals), cyclic responses of receiver cells were detectable in various conditions of mixing ratios (Figure 2E). In this example, we applied Savitzky-Golay filtering (4th order and a 13 data-point window) to attenuate noisy signals. Time-lapse microscopy with repetitive light illumination (2 min duration, 2.75 h intervals) also revealed the synchronized responses of receiver cells at the single-cell level (grey lines in Figure 2F) and at the population level (red line in Figure 2F). These data suggest that cyclic expression of Dll1 protein in sender cells is sufficient to synchronize Hes1 oscillations in neighboring receiver cells.
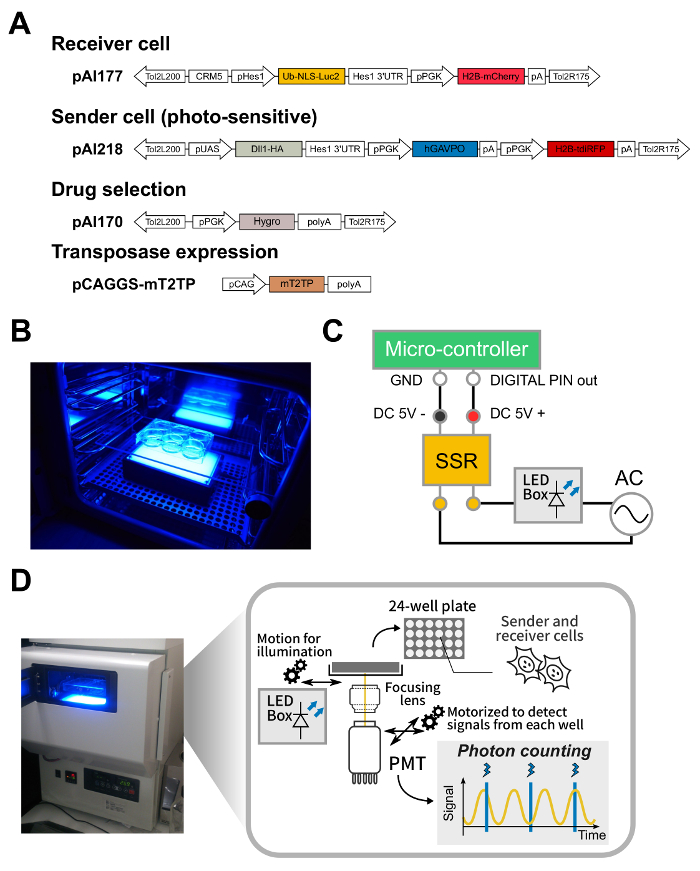
Figure 1: Strategy to analyze cellular responses upon optogenetic perturbations. (A) Schematic of representative plasmid vectors used for this protocol. These plasmids are available on request. (B) A photo of a CO2 incubator equipped with a blue LED device under a 6-well cell culture plate. (C) Schematic diagram of an electric circuit to control power supply for the LED light source by a programmable micro-computer. A solid-state relay (SSR) was set to relay on/off states of digital PIN-out of the micro-computer to on/off states of the LED light source. (D) A photo and schematic of a live-cell bioluminescence monitoring system. Note that the motorized stage can move unidirectionally from a recording position on the top of the PMT detector to an illumination position on the top of the LED light source. Also, note that the PMT detector can move from well to well to monitor signals from individual wells. Please click here to view a larger version of this figure.
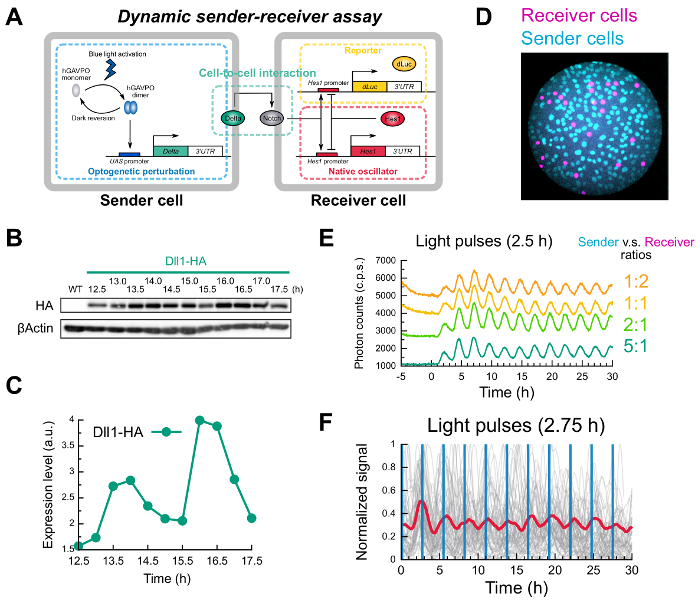
Figure 2: Examples of optogenetic perturbation experiments. In these examples, murine C2C12 myoblast cell lines were used. (A) Schematic of the dynamic sender-receiver assay. (B, C) Representative results of light-induced Dll1 expression analyzed by Western blotting analysis. Dll1 proteins were induced by periodic light illumination with a 2.5 h period, 2 min duration and the intensity of 24.7 W/m2. (D) Fluorescence image of co-culture system of sender and receiver cells. (E, F) Representative results of the dynamic sender-receiver assay. (E) Temporal patterns of receiver responses recorded by the live-cell monitoring system equipped with the PMT. The total cell number was fixed to 1.5 x 105 cells, but the ratios between the senders and the receivers were distributed from 5:1 to 1:2. (F) Single-cell imaging revealed that oscillatory information, which was induced by periodic light illumination (2 min duration, 2.75 h intervals), was transferred from sender to receiver cells. 1.5 x 105 cells with the sender-to-receiver ratio of 5:1 were used. Adapted from Ref. 10. Please click here to view a larger version of this figure.
Discussion
We showed a method to control gene expression dynamics with a periodicity of 2 to 3 h. This time-scale is much shorter than the ones in other conventional systems, including the Tet-On system and the original LightOn system. Key parameters to reach the ultradian time-scales are half-lives of photo-induced molecular products, mRNAs, and proteins. These kinetic parameters may depend on cell types and species. For tuning the kinetics, replacing Hes1 3'UTR sequences with others is a straight-forward way, because it does not alter the sequences and functions of target proteins. For seeking other 3'UTRs to obtain desirable pulsatile patterns in particular cells of interest, live-cell monitoring of dLuc by light induction is a good starting point and used for screening and validation.
One of the most frequently asked questions is about the daily handling of light-sensitive cells. In the case of the dynamic sender-receiver assay of the Notch signaling, we were able to do daily cell passages under room lights, because our systems (photo-inducible dLuc or Dll1 cells and receiver cells) do not alter cell proliferation and quickly return to resting states within 6 hours after the cells are moved to a dark condition. If genes of interest are fate-determination factors, leaky expression can induce cellular differentiation or prevent expansions of the cells, and therefore it is essential to set up a red-light environment to avoid undesirable optogenetic perturbation during cell handling.
In this protocol, we presented procedures to generate stable cell lines with photo-inducible gene expression cassettes. This is a critical step to obtain reliable results in the dynamic sender-receiver assay. One may consider transient transfection for the introduction of optogenetic modules into cells, but it does not work well in our hands for quantitative measurements of ultradian pulses. We found that transient transfection of plasmids carrying UAS-promoter cassettes leads to leaky expression at relatively high levels even under a dark condition. This leaky expression causes a high background without any light stimulation and hampers a reliable measurement of time-course. An alternative method to establish stable cells is a lentivirus system, which is useful for cell types that are not suitable for transfection12. Clonal cell lines present more homogeneous responses than heterogeneous cell lines, but even in a clonal population, cell responses are not always uniform: some cells do not respond to light stimulation and therefore present a distributed levels of light-induced protein expression. This might be due to variable expression levels of hGAVPO protein and/or variable endogenous abundance of flavin, a chromophore required for light sensing of hGAVPO.
The protocol presented here provides a powerful tool for the analysis of gene expression dynamics, but there are some limitations. One drawback is that some fluorescence channels for live-cell microscopy are not compatible with blue-light stimulation. Blue-light illumination activates not only the blue-light-sensitive proteins but also fluorescent proteins, including green and yellow fluorescent proteins (GFPs and YFPs), which are susceptible to photo-bleaching. On the contrary, visualization of GFP requires blue-light excitation that may trigger undesirable activation of photo-sensitive cells during capturing fluorescent images. Bright-field (phase-contrast) imaging by the ambient light source is also incompatible with blue-light optogenetic experiments, but one can circumvent this problem by using green or longer wave-length light sources for the imaging.
The dynamic sender-receiver assay would be useful for investigating other signaling pathways. One possibility is an application to secreting signaling systems. For such analyses, applying purified ligands in micro-fluidic devices is an alternative way to stimulate receiver cells in a precise temporal manner16,17. However, those approaches are inaccessible to dynamic production processes of ligand molecules in sender cells. Employing the dynamic sender-receiver assay together with photo-inducible ligand expression would allow further dissection of signal transmission from sender to receiver cells.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by JST, PRESTO (A.I.), Core Research for Evolutional Science and Technology (JPMJCR12W2 (R.K.)), Grant-in-Aid for Scientific Research on Innovative Areas (Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan 26119708 (A.I.) and 16H06480 (R.K.)), Scientific Research (A) (Japan Society for the Promotion of Science (JSPS) 24240049 (R.K.)), and Young Scientists (A) (JSPS 15H05326 (A.I.)), and a Grant-in-Aid for Scientific Research on Innovative Areas "Fluorescence Live imaging" of the MEXT, Japan, and Platform for Dynamic Approaches to Living System from the MEXT, Japan.
Materials
| FACS | Becton, Dickinson and Company | FACSAriaII SORP | |
| Camera | Andor | iKon M-934 | |
| Microscope | Olympus | IX-81 ZDC | |
| PMT device | Churitsu eletric corp. | CL24B-LIC/B | |
| Blue LED illuminator | OptoCode | LEDB-SBOXH | |
| DMEM | Nacalai | 08459-35 | |
| Penicillin-streptomycin | Nacalai | 26253-84 | |
| Fetal bovine serum | Sigma | 172012 | |
| KRYSTAL24 (black 24 well plate ) | Hi-tech | 303012 | |
| D-Luciferin Potassium Salt | Nacalai | 20028-24 | |
| Light meter | LI-COR Biosciences | LI-250A | |
| anti-HA-Peroxidase antibody | Roche | clone 3F10 | |
| anti-Actin-Peroxidase antibody | Wako | clone 2F3 |
References
- Hubaud, A., Pourquie, O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 15, 709-721 (2014).
- Maroto, M., Dale, J. K., Dequeant, M. L., Petit, A. C., Pourquié, O. Synchronised cycling gene oscillations in presomitic mesoderm cells require cell-cell contact. Int. J. Dev. Biol. 49, 309-315 (2005).
- Masamizu, Y., Ohtsuka, T., Takashima, Y., Nagahara, H., Takenaka, Y., Yoshikawa, K., Okamura, H., Kageyama, R. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cell. Proc. Natl. Acad. Sci. USA. 103, 1313-1318 (2006).
- Tsiairis, C., Aulehla, A. Self-Organization of Embryonic Genetic Oscillators into Spatiotemporal Wave Patterns. Cell. 164, 656-667 (2016).
- Jiang, Y. J., Aerne, B. L., Smithers, L., Haddon, C., Ish-Horowicz, D., Lewis, J. Notch signalling and the synchronization of the somite segmentation clock. Nature. 408, 475-479 (2000).
- Delaune, E. A., François, P., Shih, N. P., Amacher, S. L. Single-cell-resolution imaging of the impact of Notch signaling and mitosis on segmentation clock dynamics. Dev. Cell. 23, 995-1005 (2012).
- Dale, J. K., Maroto, M., Dequeant, M. L., Malapert, P., McGrew, M., Pourquié, O. Periodic inhibition by Lunatic Fringe underlies the chick Segmentation Clock. Nature. 421, 275-278 (2003).
- Okubo, Y., Sugawara, T., Abe-Koduka, N., Kanno, J., Kimura, A., Saga, Y. Lfng regulates the synchronized oscillation of the mouse segmentation clock via trans-repression of Notch signalling. Nat. Commun. 3, 1141 (2012).
- Shimojo, H., Isomura, A., Ohtsuka, T., Kori, H., Miyachi, H., Kageyama, R. Oscillatory control of Delta-like1 in cell interactions regulates dynamic gene expression and tissue morphogenesis. Genes Dev. 30, 102-116 (2016).
- Isomura, A., Ogushi, F., Kori, H., Kageyama, R. Optogenetic perturbation and bioluminescence imaging to analyze cell-to-cell transfer of oscillatory information. Genes Dev. 31, 524-535 (2017).
- Wang, X., Chen, X., Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Meth. 9, 266-269 (2012).
- Imayoshi, I., Isomura, A., Harima, Y., Kawaguchi, K., Kori, H., Miyachi, H., Fujiwara, T. K., Ishidate, F., Kageyama, R. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 342, 1203-1208 (2013).
- Kawakami, K. Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. 8, S7 (2007).
- Yagita, K., Yamanaka, I., Emoto, N., Kawakami, K., Shimada, S. Real-time monitoring of circadian clock oscillations in primary cultures of mammalian cells using Tol2 transposon-mediated gene transfer strategy. BMC Biotechnology. 10, 3 (2010).
- Filonov, G. S., Piatkevich, K. D., Ting, L. -. M., Zhang, J., Kim, K., Verkhusha, V. V. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 29, 757-761 (2011).
- Gregor, T., Fujimoto, K., Masaki, N., Sawai, S. The onset of collective behavior in social amoebae. Science. 328, 1021-1025 (2010).
- Kellogg, R. A., Tay, S. Noise facilitates transcriptional control under dynamic inputs. Cell. 160, 381-392 (2015).

