Magnetic-, Acoustic-, and Optical-Triple-Responsive Microbubbles for Magnetic Hyperthermia and Pothotothermal Combination Cancer Therapy
Summary
Presented here is a protocol for the fabrication of iron oxide nanoparticle-shelled microbubbles (NSMs) through self-assembly, synergizing magnetic, acoustic, and optical responsiveness in one nanotherapeutic platform for magnetic hyperthermia and photothermal combination cancer therapy.
Abstract
The precision delivery of anti-cancer agents which aim for targeted and deep-penetrated delivery as well as a controlled release at the tumor site has been challenged. Here, we fabricate iron oxide nanoparticle shelled microbubbles (NSMs) through self-assembly, synergizing magnetic, acoustic, and optical responsiveness in one nanotherapeutic platform. Iron oxide nanoparticles serve as both magnetic and photothermal agents. Once intravenously injected, NSMs can be magnetically guided to the tumor site. Ultrasound triggers the release of iron oxide nanoparticles, facilitating the penetration of nanoparticles deep into the tumor due to the cavitation effect of microbubbles. Thereafter, magnetic hyperthermia and photothermal therapy can be performed on the tumor for combinational cancer therapy, a solution for cancer resistance due to the tumor heterogeneity. In this protocol, the synthesis and characterization of NSMs including structural, chemical, magnetic and acoustic properties were performed. In addition, the anti-cancer efficacy by thermal therapy was investigated using in vitro cell cultures. The proposed delivery strategy and combination therapy holds great promise in cancer treatment to improve both delivery and anticancer efficacies.
Introduction
Cancer is one of the deadliest diseases, causing millions of deaths every year worldwide and huge economic losses1. In clinics, conventional anticancer therapies, such as surgical resection, radiotherapy, and chemotherapy still cannot provide a satisfactory therapeutic efficacy2. Limitations of these therapies are high toxic side effects, high recurrence rate and high metastasis rate3. For example, chemotherapy is suffered from the low delivery efficiency of chemo drugs precisely to the tumor site4. The inability of drugs to penetrate deep into the tumor tissue across the biological barriers, including extracellular matrix and high tumor interstitial fluid pressure, is also responsible for the low therapeutic efficacy5. Besides, the tumor resistance usually happens in the patients who received treatment by single chemotherapy6. Therefore, techniques where thermal ablation of tumor occurs, such as photothermal therapy (PTT) and magnetic hyperthermia therapy (MHT), have shown promising results to reduce tumor resistance and have been emerging in clinical trials7,8,9.
PTT triggers thermal ablation of cancer cells by the action of photothermal conversion agents under the irradiation of the laser energy. The generated high temperature (above 50 ˚C) induces complete cell necrosis10. Very recently, iron oxide nanoparticles (IONPs) were demonstrated to be a photothermal conversion agent that can be activated by near-infrared (NIR) light11. Despite the low molar absorption coefficient in the near infrared region, IONPs are candidates for low-temperature (43 ˚C) photothermal therapy, a modified therapy to reduce the damage caused by heat exposure to normal tissues and to initiate antitumor immunity against tumor metastasis12. One of the limitations of PTT is the low penetration depth of the laser. For deep seated tumors, alternating magnetic field (AFM) induced heating of iron oxide nanoparticles, also called magnetic hyperthermia, is an alternative therapy for PTT13,14. The main advantage of MHT is the high penetration of magnetic field15. However, the required relatively high concentration of IONPs remains a major disadvantage for its clinical application. The delivery efficiency of nanomedicine (or nanoparticles) to solid tumors in animals has been 1-10% due to a series of obstacles including circulation, accumulation, and penetration16,17. Therefore, a controlled and targeted IONPs delivery strategy with the ability to achieve high tissue penetration is of great interest in cancer treatment.
Ultrasound mediated nanoparticle delivery has shown its ability to facilitate the penetration of nanoparticles deep into the tumor tissue, due to the phenomenon called microbubble cavitation18,19. In the present study, we fabricate IONPs shelled microbubbles (NSMs) through self-assembly, synergizing magnetic, acoustic, and optical responsiveness in one nanotherapeutic platform. The NSM contains an air core and a shell of iron oxide nanoparticles, with a diameter of approximately 5.4 µm. The NSMs can be magnetically guided to the tumor site. Then the release of IONPs is triggered by ultrasound, accompanied by microbubble cavitation and microstreaming. The momentum received from the microstreaming facilitates the penetration of IONPs into the tumor tissue. The PTT and MHT can be achieved by NIR laser irradiation or AFM application, or with the combination of both.
Protocol
All animal experiments were performed in accordance with the protocols approved by the OG Pharmaceutical guidelines for Animal Care and Use of Laboratory Animals. The protocols followed the guidelines of Ethics Committee for laboratory animals of OG Pharmaceutical.
1. Nanoparticle shelled microbubbles (NSMs) synthesis
- Disperse magnetic nanoparticles (Fe3O4, iron oxide) in deionized water to form a 10 mg/mL stock solution.
- Place the tube containing the IONPs solution in an ultrasonic cleaning machine for 20 min. Obtain a uniformly dispersed IONPs solution before use.
- Add 150 μL of deionized water, 150 μL of 10 mM sodium dodecyl sulfate (SDS), and 400 μL of a stock solution of IONPs from step 1.1. in 1.5 mL centrifuge tube.
- Fix the homogenizer with a scaffold in an ice bath.
- Place the tube of the mixture in an ice bath and position the homogenizer probe precisely to be immersed in the mixture solution.
- Adjust the homogenizer speed to 20,000 rpm and turn on the homogenizer for 3 min.
- Turn off the homogenizer and remove the tube from the ice bath.
- Place the tube to the tube rack for 12 h stabilization at room temperature.
- Adsorb the resulted NSMs to the tube wall by a magnet and remove the supernatant. Then replenish with 1 mL of fresh deionized water.
- Repeat the wash process for three times and re-suspend NSMs in 1 mL of fresh distilled water.
- Transfer 10 μL of NSMs suspension to a clean glass slide after slightly shaking.
- Use a fluorescence microscope at 20x magnification to visualize NSMs morphology. Ensure taking photos at random area.
- Measure the diameter of the NSMs from the photos using an open access Nano Measurer 1.2 software. Count at least 200 microbubbles.
- Click “File” and “Open” to select the image file to be processed. After the image is imported, press the “ruler”. Draw a red line with the same length as the ruler.
- Then press “Settings” | “Ruler” and enter the length of the ruler. Draw lines of the same lengths as the diameters of the individual microbubbles in the image. Complete all the measurements, then click on “Report” | “View Report”.
2. Acoustic response of NSMs
- Dilute 200 μL of NSMs with 800 μL deionized water, then transfer into a 1.5 mL centrifuge tube to form a stock solution.
- Connect the function generator, amplifier, impedance matching, and homemade focused transducer. Place the transducer in the center at the bottom of the artificial cuboid sink and connect the hydrophone with an oscilloscope to monitor the output ultrasound intensity (Figure 1). Add deionized water into the sink to immerse the transducer.
- Adjust the function generator to the sweep mode, tune the frequency range from 10 kHz to 900 kHz and set the amplitude to 20 Vpp (Voltage peak-peak). Adjust the power of ultrasound to 0.1% by the amplifier. Each cycle’s duration is 4 s with a time interval of 1 s.
- Prepare 1 mL NSMs stock solution samples in the tube and fix the tube with a scaffold on the top of the homemade focused transducer. Attach the magnet to the bottom of the tube and attract the NSMs.
- Turn on the power of the function generator and the amplifier. Switch off the function generator after application of 5 cycles (25 s) of ultrasound. Remove the magnet and collect 1 mL of the solution containing the released IONPs. Add 1 mL of deionized water to the centrifuge tube.
- Repeat step 2.5. until NSMs in the tube collapse completely.
- Quantify all the released IONPs by the inductively coupled plasma optical emission spectrometry (ICP-OES) as described previously13.
3. Optical response of NSMs
NOTE: In this work, a laser system containing 808 nm laser power and an infrared thermal imaging camera previously described by Xu et al. is utilized20.
- Laser system preparation
- Turn on the power supply of the laser and allow it to warm for several minutes. Fix a fiber coupled 808 nm laser diode on a retort stand.
- Direct the laser beam to the sample stage through an optical fiber and focus on the sample stage to achieve a 6 mm light spot (in diameter) by a convex lens.
- Measure the power output with a laser power meter and adjust the power to 1 W/cm2.
- Fix the infrared thermal imaging camera on the tripod. Turn on the camera and check the if working (e.g., monitor the focused region of interest (ROI) temperature). Turn off the power supply and the infrared thermal imaging camera.
- Photothermal measurement in aqueous solution
- Prepare the 1 mL sample at different IONPs concentration (1.05 mg/mL, 1.35 mg/mL, 3.65 mg/mL, 5 mg/mL) in a 1.5 mL centrifuge tube.
- Place the tube of interest at the focused region of the laser beam and record the baseline temperature of the sample.
- Turn on the laser power and the infrared thermal imaging camera and irradiate the sample for 10 min continuously. At the same time, record the temperature in real time.
- Turn off the laser power and infrared thermal imaging camera after 10 min of irradiation. Wait for the temperature of the region to return to the baseline.
- Repeat 3.2.2 to 3.2.4 for the measurement of other samples.
NOTE: Use deionized water at 20 °C as a control for the photothermal measurement.
- Photothermal measurement in cultured cells
NOTE: Murine breast cancer cells (4T1) were selected as a model to investigate the inhibition effect by photothermal treatment.- Feed the cells with Roswell Park Memorial Institute-1640 (RPMI-1640) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin. Set the culturing environment as 37 °C and 5% CO2.
- Culture 4T1 cells in T25 flasks and passage the cells in a 1:2 ratio when 90% confluence is reached.
NOTE: The subculture ratio can be adjusted according to the specific cell conditions in different labs. - Remove and discard the culture medium. Rinse the cell layer with 1x PBS solution to remove the residual serum containing trypsin inhibitor.
- Add 2 mL of Trypsin-EDTA solution (0.25%) to the flask for detachment. Then add 3 mL of 1640 medium and aspirate cells by gently pipetting.
- Collect 5 mL of the cell suspension and centrifuge at 500 x g for 3 min.
- Remove the supernatant and add 1 mL of fresh 1640 medium to form a cell suspension.
- Add 100 μL of the cell suspension to a confocal dish containing 1 mL of the culture medium. Ensure that the concentration of cell suspension is 9 x 105/mL, and the final cell concentration in cell culture confocal dish is 8.1 x 104/mL.
- Place the inoculated cell culture confocal dish in an incubator for 24 h.
- Dilute different concentration (1.05 mg/mL, 1.35 mg/mL, 3.65 mg/mL, 5 mg/mL) of the IONPs sample to make 1 mL solution with serum-free 1640 medium.
- Aspirate the culture medium from the confocal dish and add the prepared sample solution.
- Turn on the laser power. Focus the laser beam at the center of the dish and adjust the output power to 1 W/cm2. Turn on the infrared thermal imaging camera, irradiate the cells in the confocal dish for 10 min continuously. Record the temperature in real time for the focused region.
- Turn off the laser and infrared thermal imaging camera. Transfer the irradiated dish to the incubator for another 24 h.
- Remove and discard the culture medium, add 1 mL of the fresh culture medium in the confocal dish. Add 5 μL of Calcein-AM solution (1 mg/mL) into the dish.
- Incubate the confocal dish at 37 °C and 5% CO2 for 15 min. Rinse the cell layer with 1x PBS solution twice.
- Observe and image the cells by a confocal fluorescence microscope with an excitation wavelength of 488 nm and an emission wavelength of 500-540 nm.
- Choose 5 areas in the confocal images randomly and count the number of live 4T1 cells in each area manually. Quantify the viability of 4T1 cells by comparing the number of live cells in all experimental groups with the control group.
NOTE: Use the serum-free 1640 medium at 20 °C as a control for the photothermal measure. Use the sample without laser irradiation as cell viability control group.
- Photothermal measurement in vivo
- Prepare 3 of 8-week-old ICR male mice with a mean weight of 25 ± 2 g.
- Add 2 g of gelatin powder to 20 mL of deionized water. Heat the solution to 40 – 50 °C, dissolve the gelatin gel completely to form a transparent and clear solution.
- Add 100 mg IONPs to the solution from 3.4.2.
- Heat the gel to 40 – 50 °C, immediately inject 500 μL of the gelatin solution into the right breast pad of the mouse.
- Turn on the laser power and focus the laser beam at the interest region (right breast pad of the mice). Adjust the output power to 1 W/cm2. Turn on the infrared thermal imaging camera, irradiate the interest region for 10 min continuously. Record the temperature of the region of interest in real time.
- Turn off the laser and infrared thermal imaging camera.
- Euthanize mice by CO2 asphyxiation and cervical vertebra dislocation or any method approved by the Institute’s animal research committee.
4. Magnetic hyperthermia measurement
NOTE: Here, a magnetic hyperthermia system previously described by Wu et al. is utilized (21).
- Prepare a magnetic hyperthermia system include an alternating magnetic field (AFM) generator and an infrared thermal imaging camera.
- Turn on the chiller for 10 min and then power on the moderate radio frequency heating machine (i.e., AFM generator).
- Set the parameters of the machine as follows: frequency (f) = 415 kHz, magnetic field intensity = 1.8 kA/m.
- Magnetic hyperthermia measurement in aqueous solution
- Prepare 1 mL of the sample at different IONPs concentration (1.05 mg/mL, 1.35 mg/mL, 3.65 mg/mL, 5 mg/mL) in a 1.5 mL centrifuge tube.
- Place the tube in the center of a water-cooled magnetic induction copper coil.
- Turn on the alternating magnetic field (AFM) and the infrared thermal imaging camera. Induce the sample for 10 min continuously and record the temperature in real time.
NOTE: The camera is located at the top of the sample, supplying a cross-sectional view of the sample. - Turn off the AFM and the infrared thermal imaging camera. Wait for the temperature of the copper coil to come back to the baseline for 10 min.
NOTE: Be careful of high temperature, avoid direct contact with hands and wait for cooling before removing samples. - Repeat 4.2.2 to 4.2.4 for the measurement of the other samples.
- Power off the moderate radio frequency heating machine (AFM) and chiller.
NOTE: Use the deionized water at 20 °C as a control for magnetic hyperthermia measurement.
- Magnetic hyperthermia measurement in vivo
- Prepare 3 of 8-week-old ICR male mice with a mean weight of 25 ± 2 g.
- Prepare 20 mL of 10 % gelatin gel containing 5 mg/mL IONPs solution.
- Heat the gelatin gel to 40 – 50 °C, immediately inject 500 μL of the gelatin solution into the right breast pad of the animal.
NOTE: Magnetic-induced hyperthermia experiments were conducted with the heating machine using the same parameters as the in vitro test. - Turn on the alternating magnetic field (AFM) and the infrared thermal imaging camera. Place the right breast pad of the mice in the center of a water-cooled magnetic induction copper coil.
- Turn on the infrared thermal imaging camera, image the interest region (right breast pad of the mice) for 10 min continuously and record the temperature of the interest region in real time.
- Turn off the power switch of the machine and infrared thermal imaging camera after 10 min of induction. Wait for the temperature of the copper coil to return to the baseline for 10 min.
- Repeat 4.3.4 to 4.3.6 for the measurement of other samples.
- Power off the moderate radio frequency heating machine (AFM) and chiller.
NOTE: Be careful of high temperature, avoid direct contact with hands and wait for cooling before removing samples. - Euthanize mice by CO2 asphyxiation and cervical vertebra dislocation or any method approved by the Institute’s animal research committee.
Representative Results
The triple-responsive nanoparticle-shelled microbubbles (NSMs) used in this study were prepared by agitating the mixture of the surfactant and IONPs. The IONPs (50 nm) self-assembled at the interface of liquid and gas core, to form a densely packed magnetic shell. The morphology of NSMs is shown in Figure. 1A. The resulted NSMs presented a spherical shape and with an average diameter of 5.41 ± 1.78 μm (Figure 1B). The results indicated the NSMs were prepared successfully. When stored in water, the microbubbles remained intact for more than 1 year, and were stable in buffers and cell culture medium for at least 10 days19. As shown in Figure 1D, a stepwise release of Fe was achieved with increasing the number of cycles of applied ultrasound. After 10 cycles, approximately 20% of Fe were released. Until 50 ultrasound cycles, the amount of released Fe reached a plateau to around 80%. These results suggested the on-demand release of IONPs by an external ultrasound trigger.
IONPs-mediated photothermal measurement in aqueous solution is shown in Figure 2. The temperature of IONPs increased rapidly with increasing irradiation time as shown in Figure 2A,B. A 30 °C increase of temperature could be achieved when being exposed to a NIR laser (808 nm,1 W/cm2) for 10 min at the Fe concentration of 5 mg/mL.
The heat generated by PTT could kill the cancer cells. The viability of 4T1 cell by PTT was evaluated by NIR laser (808 nm, 1 W/cm2) treatment for 10 min. As shown in Figure 3A,B, in comparison with the control group, there was no difference in the morphology and live cell number when incubated with a high concentration of Fe (5 mg/mL), suggesting the good bioavailability of IONPs. Once irradiated by NIR laser, cells became round shape, indicating apoptosis. The quantification of the live cell number, i.e., the viability of cells is shown in Figure 3C. The cells incubated with high IONPs concentration (3.65 mg/mL and 5 mg/mL) under NIR irradiation had the highest death rate, which is around 80% and 100% respectively. The low IONPs concentration (1.025 mg/mL and 1.35 mg/mL) treated group showed a similar killing efficiency as approximately 40%. The results demonstrate that the photothermal effect of NSMS can effectively treat cancer.
As shown in Figure 4A,B, the temperature of the gelatin injection area rapidly increased by about 20 °C after 5 min of NIR irradiation. The real surface temperature of the area of interest in mice could be reached to around 57 °C. As shown in Figure 5, when exposed to the AFM, the thermal imaging of different concentrations of IONPs (1.05 mg/mL, 1.35 mg/mL, 3.65 mg/mL, 5 mg/mL) were monitored by an infrared thermal camera (Figure 5A), and the elevated temperature curves were recorded and plotted at different time intervals (Figure 5B). Among them, the 1.35 mg/mL, 3.65 mg/mL and 5 mg/mL IONPs could quickly heat the solution and increase the temperature (20 °C, 30 °C, 40 °C, respectively) after 10 min of induction. The results demonstrate an alternating magnetic field response characteristic of NSMS.
In the in vivo magnetic hyperthermia experiment, mice were exposed to AFM at the frequency of 415 kHz and the magnetic amplitude of 1.8 kA/m for 10 min. The heating process was monitored by an infrared thermal imaging camera in real time (Figure 6A, 6B). Significant temperature changes of the area of interest were observed (Figure 6). The temperature increased rapidly with time, with an increment of 50 °C for 10 min of induction.
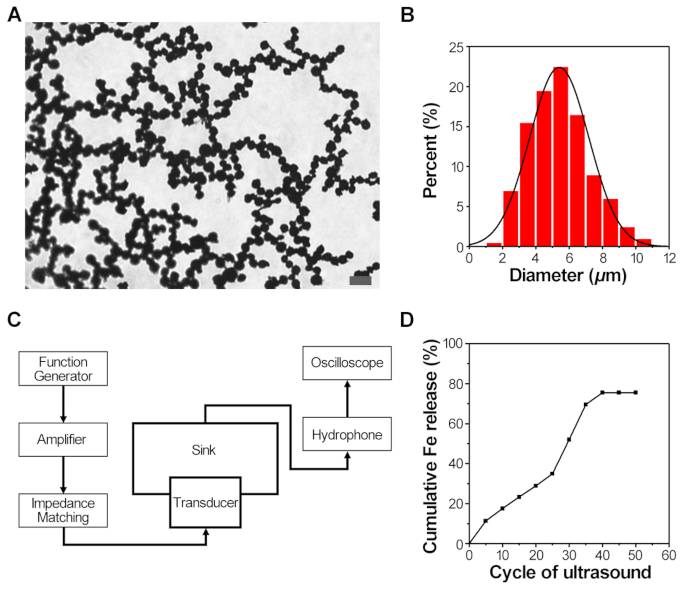
Figure 1: Characterization and controlled IONPs release of the NSMs. (A) Representative bright-field microscopy image of NSMs. Scale bar: 20 μm. (B) The diameter distribution of the NSMs, n = 200. (C) The diagram of ultrasonic equipment used in the experiment. (D) Cumulative release profiles of IONPs from NSMs under ultrasound stimulation. Please click here to view a larger version of this figure.
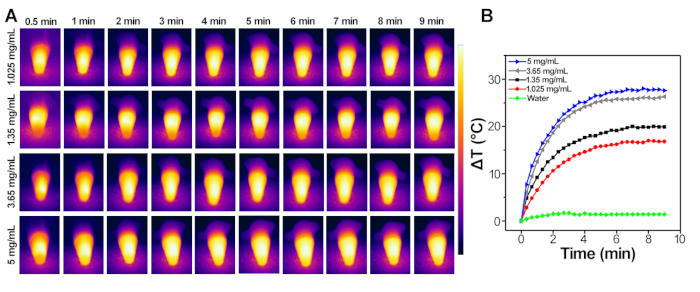
Figure 2: IONPs-mediated photothermal measurement in aqueous. (A) Infrared thermal images of different concentrations of IONPs after 10 min of laser irradiation at 808 nm for 1 W/cm2. (B) Typical temperature elevation curves of different concentrations of IONPs (808 nm, 1 W/cm2, 10 min). Please click here to view a larger version of this figure.
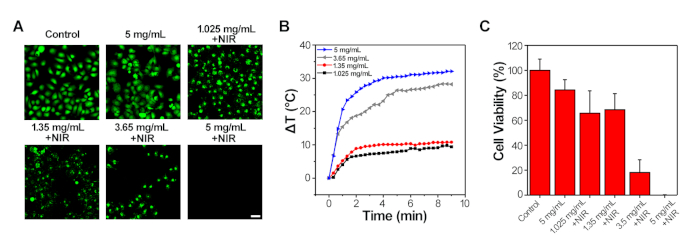
Figure 3: IONPs-mediated photothermal measurement in 4T1 cells. (A) Confocal fluorescence microscopy images of live 4T1 cells after 24h incubation with different concentrations of IONPs (stained with Calcein-AM, green). NIR treated cells were exposed to the 808 nm laser for 10 min (1 W/cm2). Scale bar: 50 μm. (B) Typical temperature elevation curves of different concentrations of IONPs treated 4T1 cells for 10 min of NIR irradiation (1 W/cm2). (C) Quantification of the viability of 4T1 cells incubated with IONPs at different concentrations with or without NIR treatment. Please click here to view a larger version of this figure.
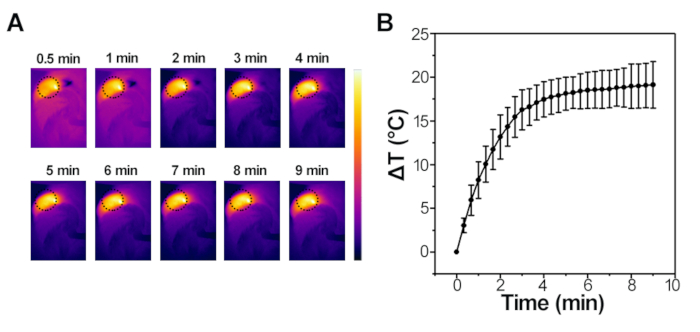
Figure 4: IONPs-mediated photothermal measurement in vivo. (A) Infrared thermal images of the interest region of the mouse exposed to the NIR laser (808 nm, 1 W/cm2, 10 min) captured at different time intervals. (B) Elevated temperature curves at different time intervals after NIR laser (808 nm, 1 W/cm2, 10 min) treatment. Please click here to view a larger version of this figure.
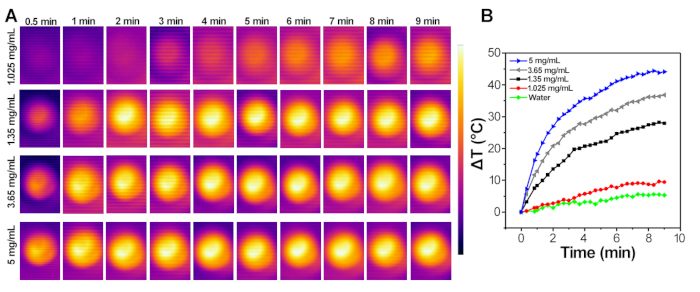
Figure 5: IONPs-mediated magnetic hyperthermia measurement in aqueous. (A) Infrared thermal images of different concentrations of IONPs solution under the AFM at the frequency of 415 kHz and the magnetic amplitude of 1.8 kA/m for 10 min. (B) Typical temperature curves of different concentrations of IONPs solution under the AFM at the frequency of 415 kHz and the magnetic amplitude of 1.8 kA/m. Please click here to view a larger version of this figure.
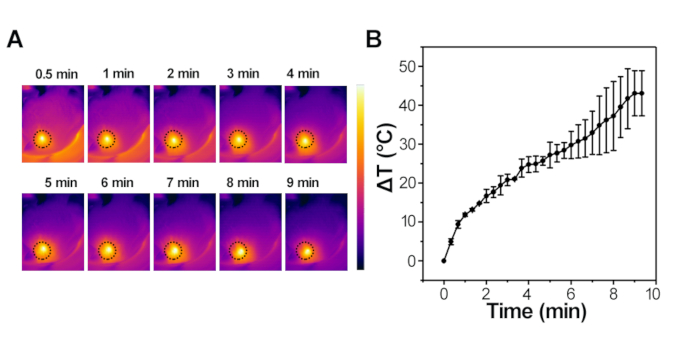
Figure 6: IONPs-mediated magnetic hyperthermia in vivo. (A) Infrared thermal images of the interest region of the mice captured at different time intervals under the AFM at the frequency of 415 kHz and the magnetic amplitude of 1.8 kA/m for 10 min. (B) Elevated temperature curves at different time intervals under AFM at the frequency of 415 kHz and the magnetic amplitude of 1.8 kA/m. Please click here to view a larger version of this figure.
Discussion
Here, we presented a protocol of fabricating iron oxide nanoparticle shelled microbubbles (NSMs) through self-assembly, synergizing magnetic, acoustic, and optical responsiveness in one nanotherapeutic platform. The IONPs were densely packed around the air core to form a magnetic shell, which can be controlled by the external magnetic field for targeting. Once delivered, the release of IONPs can be achieved by ultrasound trigger. The released IONPs can be activated by both NIR light and AFM for PTT and MHT, or the combination of both.
During the whole protocol, the synthesis steps of NSMs play an important role, which is the basis of the whole protocol. At the same time, the controlled release of IONPs in vitro validated the acoustic response of NSMs. The protocol of photothermal measurement in aqueous solution and magnetic hyperthermia measurement in aqueous solution also validated the magnetic and optical response of NSMs respectively.
In order to prepare the NSMs successfully, the solution of IONPs must be sonicated for 20 min before use to ensure the even dispersion of IONPs in the water. When the agitation was performed, the homogenizer probe must be immersed in the solution completely. When studying acoustic response of NSMs, the sample tube must be placed on the top of the transducer directly and attract the NSMs to the bottom of the tube by the magnet to prevent the attenuation of the ultrasound intensity. Besides, if the temperature increase was not significant during the photothermal measurement or magnetic hyperthermia measurement, it may because the sample was neither in the focus of the laser beam nor in the center of a water-cooled magnetic induction copper coil.
It should be noted that the protocol still has some limitations. For example, although the mean diameter of the prepared NSMs was similar to the diameter of some clinically used microbubbles22 (for ultrasound imaging), however, the uniformity of NSMs size needs to be improved. In addition, the circulation of NSMs in blood should be improved by modification of the microbubble surface. Apart from this, synergizing therapy has not been verified in vivo, and the therapeutic efficacy in vivo is still unknown.
The proposed IONPs delivery strategy not only achieve the on-demand release of IONPs but also promote the penetration of IONPs into the tumor tissue. Currently, the increased interstitial fluid pressure in the tumor and the dense tumor stroma greatly limit the nanoparticle delivery efficiency5. Therefore, this protocol provides a tissue penetrating strategy for nanomedicine delivery and is of great interest in cancer treatment.
We also demonstrated that IONPs-mediated PTT and MHT are effective both in vitro and in vivo. The results showed that the IONPs had a good photothermal conversion and magnetic thermal conversion abilities and might ablate tumor efficiently. The combination of the both PTT and MHT were sufficient to ensure complete cancer cell death and improve anticancer efficacies. In the future, dual thermal therapy (i.e., PTT and MHT) by NSMs will provide a new option for the treatment of deep-seated solid tumor in clinics.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81601608) and NUPTSF (NY216024).
Materials
| 808 nm laser power | Changchun New Industries Optoelectronics Tech | MDL-F-808-5W-18017023 | |
| Calcein-AM | Thermo Fisher SCIENTIFIC | C3099 | |
| Fetal bovine serum | Invitrogen | 16000-044 | |
| Fluorescence Microscope | Olympus | IX71 | |
| Function generator | Keysight | 33500B series | 20 MHz, 2 channels with arbitrary waveform generation capability |
| Gelatin gel | Sigma | 9000-70-8 | |
| Heating machine | Shuangping | SPG-06- II | |
| Homemade focused transducer | Frequency=855, R-X=36.2W+5.8W, |Z|-θ=37W+8° | ||
| Homogenizer | SCILOGEX | D-160 | 8000-30000 rpm |
| Hydrophone | T&C | NH1000 | |
| ICR male mice | OG Pharmaceutical. Co. Ltd | 8-week-old | |
| Inductively coupled plasma optical emission spectrometry | PerkinElmer | ||
| Infrared thermal imaging camera. | FLIR | E50 | |
| Iron(II,III) oxide | Alfa Aesar | 1317-61-9 | 50-100nm APS Powder |
| Laser power meter | Changchun New Industries Optoelectronics Tech | ||
| Oscilloscope | Keysight | DSOX3054T | Bandwidth 500 MHz, Sampling Rate 5 GS/S, 4 channels |
| RF Power Amplifier | T&C | AG1020 | The signal source can also be connected to an external signal source. The gain can be adjusted from 0 to 100%. It has multiple functions such as frequency sweep, pulse, and triangle. |
| Roswell Park Memorial Institute-1640 | KeyGEN BioTECH | KGM31800 | |
| Sodium dodecyl sulfate | Sigma | 151-21-3 | |
References
- Kievit, F. M., Zhang, M. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Advanced Materials. 23 (36), 217-247 (2011).
- Wu, H., et al. Fe3O4-Based Multifunctional Nanospheres for Amplified Magnetic Targeting Photothermal Therapy and Fenton Reaction. ACS Biomaterials Science & Engineering. 5 (2), 1045-1056 (2018).
- Thorat, N. D., et al. Physically stimulated nanotheranostics for next generation cancer therapy: Focus on magnetic and light stimulations. Applied Physics Reviews. 6 (4), 041306 (2019).
- Sun, Q., Zhou, Z., Qiu, N., Shen, Y. Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Advanced Materials. 29 (14), 1606628 (2017).
- Minchinton, A. I., Tannock, I. F. Drug penetration in solid tumours. Nature Reviews Cancer. 6 (8), 583-592 (2006).
- Anchordoquy, T. J., et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano. 11 (1), 12-18 (2017).
- Cazares-Cortes, E., et al. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Advanced Drug Delivery Reviews. 138, 233-246 (2019).
- Espinosa, A., et al. of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano. 10 (2), 2436-2446 (2016).
- Rastinehad, A. R., et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proceedings of the National Academy of Sciences of the United States of America. 116 (37), 18590-18596 (2019).
- Sharma, S. K., Shrivastava, N., Rossi, F., Tung, L. D., Thanh, N. T. K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today. 29, 100795 (2019).
- Das, R., Rinaldi-Montes, N. Boosted Hyperthermia Therapy by Combined AC Magnetic and Photothermal Exposures in Ag/Fe3O4 Nanoflowers. ACS Applied Materials & Interfaces. 8 (38), 25162-25169 (2016).
- Yang, Y., et al. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Advanced Materials. 29 (40), 1703588 (2017).
- Curcio, A., et al. Iron Oxide Nanoflowers CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics. 9 (5), 1288-1302 (2019).
- Espinosa, A., et al. Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Advanced Functional Materials. 28 (37), 1803660 (2018).
- Xu, C., et al. Magnetic Hyperthermia Ablation of Tumors Using Injectable Fe(3)O(4)/Calcium Phosphate Cement. ACS Applied Materials & Interfaces. 7 (3), 13866-13875 (2015).
- Wilhelm, S., et al. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 1, 16014 (2016).
- Chen, H., Zhang, W., Zhu, G., Xie, J., Chen, X. Rethinking cancer nanotheranostics. Nature Reviews Materials. 2, (2017).
- Rapoport, N. Y., Kennedy, A. M., Shea, J. E., Scaife, C. L., Nam, K. H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. Journal of Controlled Release : The Official Journal of the Controlled Release Society. 138 (3), 268-276 (2009).
- Gao, Y., et al. Controlled nanoparticle release from stable magnetic microbubble oscillations. NPG Asia Materials. 8 (4), 260 (2016).
- Bao, B., et al. Mussel-inspired functionalization of semiconducting polymer nanoparticles for amplified photoacoustic imaging and photothermal therapy. Nanoscale. 11 (31), 14727-14733 (2019).
- Wu, H., et al. Enhanced Tumor Synergistic Therapy by Injectable Magnetic Hydrogel Mediated Generation of Hyperthermia and Highly Toxic Reactive Oxygen Species. ACS Nano. 13 (12), 14013-14023 (2019).
- Alzaraa, A., et al. Targeted microbubbles in the experimental and clinical setting. American Journal of Surgery. 204 (3), 355-366 (2012).

