Visualizing Shifts on Neuron-Glia Circuit with the Calcium Imaging Technique
Summary
Cell calcium imaging is a versatile methodology to study dynamic signaling of individual cells, on mixed populations in culture or even on awakened animals, based on the expression of calcium-permeable channels/receptors that gives unique functional signatures.
Abstract
Here, we report on selective in vitro models of circuits based on glia (astrocytes, oligodendrocytes, and microglia) and/or neurons from peripheral (dorsal root ganglia) and central tissues (cortex, subventricular zone, organoid) that are dynamically studied in terms of calcium shifts. The model chosen to illustrate the results is the retina, a simple tissue with complex cellular interactions. Calcium is a universal messenger involved in most of the important cellular roles. We explain in a step-by-step protocol how retinal neuron-glial cells in culture can be prepared and evaluated, envisioning calcium shifts. In this model, we differentiate neurons from glia based on their selective response to KCl and ATP. Calcium permeable receptors and channels are selectively expressed in different compartments. To analyze calcium responses, we use ratiometric fluorescent dies such as Fura-2. This probe quantifies free Ca2+ concentration based on Ca2+-free and Ca2+-bound forms, presenting two different peaks, founded on the fluorescence intensity perceived on two wavelengths.
Introduction
Due to the universal properties of calcium as a second messenger, this ion is involved in a vast number of signaling activities: gene transcription, birth and death, proliferation, migration and differentiation, synaptic transmission, and plasticity. Hence, a method capable of tracking calcium activation dynamics with fidelity and agility would provide a way to observe unique spatial-temporal responses. Such a method is the cellular calcium imaging technique, which correlates calcium shifts functional data with specific cell phenotypes based on their distinct responses.
Ca2+ probes were first developed in the 1980's, with later improvements allowing these molecules to be used in live cell assays1. As a chemical indicator, Fura-2 is considered to be the standard for quantitative [Ca2+]i measurements. The acetoxymethyl (AM) ester of this indicator (i.e., Fura-2 AM) easily permeates the cell membrane and can reach intracellular concentrations 20-fold greater than the incubation dilution (e.g., [5 µM]o/[100 µM]i). Another advantage of Fura-2 is that it has good photobleaching resistance; thus, imaging this indicator for longer periods of time will not greatly affect its fluorescence capabilities. Finally, Fura-2 is sensitive to a wide range of calcium levels, from ~100 nM to ~100 µM, and has a Kd of ~145 nM, which is comparable to the resting [Ca2+]i2. Later, cell calcium imaging was developed with better fluorescent microscopes and computation methods, together with ratiometric probes that are not affected by dye loading.
Every cell expresses different calcium devices (pumps, transporters, receptors, and channels) that contribute to the final response as a particular signature. The important tip is to find selective responses of different types of cells correlated with their phenotypic expression. Accordingly, there are at least two different receptors that operate through calcium shifts: ionotropic receptors that permeate Ca2+ in a fast mode and slow metabotropic receptors coupled to signaling pathways and intracellular stocks that release Ca2+ activated by second messengers, such as inositol triphosphate and cyclic ADP-ribose3.
For example, progenitor cells express nestin in the immature retina and show GABAA receptors depolarized by GABA (or muscimol)4. This happens due to the Cl– electrochemical gradient with high intracellular Cl− levels; as the tissue develops, KCC2 transporters switch from excitation on progenitors to inhibition on mature GABAergic neurons5. On the other hand, stem cells that express sox-2 at the immature subventricular zone (SVZ) of postnatal rodents also present metabotropic H1 receptors activated by histamine increasing Ca2+ in a slow manner6. A second metabotropic receptor from the protease-activated receptor-1 (PAR-1) family, activated by thrombin and downstream to G(q/11) and phospholipase C (PLC), gives slow Ca2+ shifts in oligodendrocytes (that express O4 and PLP) generated from multipotent SVZ neural stem cells7.
In general, neurons express voltage-dependent calcium channels as well as major neurotransmitter receptors permeable to Ca2+, as glutamatergic (AMPA, NMDA, kainate) and peripheral and central nicotinic receptors. Potassium chloride is usually used as a depolarizing agent to activate peripheral neurons, as the dorsal root ganglion neurons8 or central neurons, as from subventricular zone9 or retina10. On the other hand, ATP is acknowledged as the major gliotransmitter (in addition to D-serine), which activates selective Ca2+ permeable P2X members, as P2X7 and P2X4. Both receptors present equivalent Ca2+ currents, similar to the ones shown by NMDA receptors acknowledged as the largest Ca2+ currents activated by transmitters11. P2X7 receptors are highly expressed on microglia, but at a lower density on astrocytes and oligodendrocytes, having a role in the release of proinflammatory cytokines12. P2X7 receptors are also expressed on Schwann cells13 and Müller glia in the retina14,15.
The retina is known to show almost all transmitters seen in the brain. For instance, the vertical axis (photoreceptors, bipolar and retinal ganglion cells) is mainly glutamatergic, with calcium-permeable AMPA or kainate receptors expressed in OFF-bipolar cells and mgluR6 expressed in ON-bipolar cells16. Curiously, all three receptors are also found in Müller glia, which are coupled to calcium and inositol triphosphate pathways17,18. The horizontal inhibitory axis, made by horizontal and amacrine cells, secrete not only GABA, but also dopamine, acetylcholine, and other classical neurotransmitters. Amacrine cells are the main types of cells found in the avian retinal cultures, showing several types of calcium operated channels, as glutamatergic, purinergic, nicotinic and voltage dependent calcium channels. For this reason, this is an excellent model to evaluate different properties of calcium shifts among neurons and glia.
Therefore, the combination of different receptors and channels summed to selective phenotypic markers during development with distinct agonist response patterns allows unique signatures in stem, progenitor, neuron, astrocyte, oligodendrocyte, and microglia that operate through selective signaling devices.
Protocol
All experiments involving animals were approved by and carried out following the guidelines of the Institutional Animal Care and Use Committee of the Federal University of Rio de Janeiro, following the "Principles of Laboratory Animal Care" (NIH, Bethesda, USA); permit number IBCCF-035 for fertilized White Leghorn chicken eggs.
1. Preparation of solutions
- Prepare Krebs solution: (132 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 2.5 mM CaCl2, 6 mM glucose, 10 mM HEPES, pH 7.4, 373 mOsm).
- Prepare saline buffer (Ca2+ and Mg2+ free solution – CMF): 76.55 g/L NaCl, 3.05 g/L KCl, 1.65 g/L Na2HPO4, 0.610 g/L KH2PO4, 21.95 g/L glucose, and 7.90 g/L NaHCO3.
- Prepare incubating solution (with Fura-2 in Krebs solution:) 5 µM Fura-2-acetoxymethyl ester (Fura-2), 0.1% fatty acid-free bovine serum albumin (BSA), and 0.02% Poloxamer 407.
- For mixed population or glia culture, prepare DMEM/F12 with 10% fetal calf serum (FCS) and 40 mg/L gentamicin as the medium.
- For neuron enriched culture, prepare DMEM/F12 with 1% FCS, neuronal cell culture supplement, 40 mg/L and gentamicin as the medium.
2. Retina dissection and cell culture preparation
- Open the 8-day old chick embryo (E8) egg where the air cell is located.
- Remove the egg contents to a Petri dish and proceed with the embryo euthanasia by decapitation with a pair of tweezers.
- Bring the head to a clean Petri dish and pour some calcium and magnesium free solution (CMF) to wash it.
- Remove the eyes, taking care not to damage it in the process.
NOTE: Try to avoid cutting or shredding eye's layers before removing it from the eye cavity. - Bring the eyes to a clean Petri dish containing CMF.
- Begin the eye dissection by removing the lens.
- Make 3 or 4 longitudinal cuts in the eye, starting by the hole left by the lens. Make cuts by pulling the tweezers, holding the eye sclera in opposite directions.
- Remove the transparent vitreous body with caution, making sure that the retina is not bound to it.
- Detach the retina from the pigmented epithelium and remove any remaining tissue.
- Cut the clear retina in small pieces.
- Transfer the retina to another recipient and briefly centrifuge (~1,800 x g for 1 min) it to remove the CMF.
- Enzymatically dissociate the retina with 1 mL of 0.25% trypsin, by incubating it at 37 °C for 10 min.
- Stop the reaction by adding 1 mL of medium containing 10% FCS.
- Wash the retina 2 or 3 times with medium. The wash consists of cycles of adding medium and removing it by centrifugation (~1,800 x g for 1 min).
- Add 2 mL of complete medium per retina and gently mechanically dissociate the retina by pipetting it up and down.
NOTE: Here, investigators can choose which type of culture will be prepared. For an enriched neuronal culture, use DMEM-F12 + neuronal supplement + 1% FCS + antibiotics. For mixed population or purified glia, use DMEM-F12 + 10% FCS + antibiotics. - Count the cells and dilute them to the desired density.
NOTE: Ideally, this should be a low-density culture with ~ 2 x 106 cells or less. - Add 50 µL of the cell suspension to each coverslip.
- Coverslip treatment
- Incubate coverslips for at least 1 h (ideally overnight) in 1 mL of 10-50 µg/mL poly-L-lysine in sterilized purified water at 37 °C.
- Remove the poly-L-lysine solution and wash coverslips with sterilized purified water 2-3 times.
- Let coverslips dry in a safety cabinet with UV lights turned on. At this point, store them in a sealed container at 4 °C for up to 1 month.
- Optional step: For neuron enriched cultures, add 50 µL of 10-20 ng/mL laminin in PBS or medium to each coverslip for 2 h at 37 °C. After incubation, remove laminin solution excess and the coverslip is ready to use.
- Incubate cells at 37 °C at a 5% CO2 atmosphere until they attach to the glass. This should take about 1-2 h.
- Add 1 mL of complete medium to each well and return the plate to the incubator until the day of the experiment. If needed, change the medium every 2-3 days.
3. Loading cells with Fura-2 AM
- Reconstitute a 50 µg vial of Fura-2 AM with 50 µL of DMSO.
- To prepare working Fura-2 AM solution, add 3 µL of 10% Poloxamer 407, 7.5 µL of Fura-2 AM in DMSO and Krebs solution q.s.p. to 1.5 mL.
- Sonicate the mixture for 7 min in a water bath.
- Wash the coverslip with the cell culture 3x with Krebs solution before incubating it in Fura-2.
- Incubate at 37 °C and 5% CO2 for 30 min.
- After incubation, wash it 3x again and transfer it to another recipient containing Krebs and protected from the light.
NOTE: Working Fura-2 AM solution remains stable for 24 hours if protected from the light.
4. Calcium imaging of cells
- Before each run, add silicon to the coverslip support and chamber to avoid leakage during the experiment.
- Wash the bottom of the coverslip with distilled water to avoid salt crystallization on the microscope lens.
- Put the coverslip on the support, gently pressing the borders.
- Attach the support and chamber to the microscope and start perfusing cells with Krebs solution.
NOTE: Cell perfusion during single cell calcium imaging experiments is calibrated to a flow rate of 0.5 mL/min, and it takes 8-10 s for the platform solution to be fully replaced. - Have a look at the cells and select an appropriate field of view.
- Manually select the cell bodies based on their distinct morphology.
- Before applying any stimulus, wait for baseline stabilization. Each stimulus should take about 30 seconds.
NOTE: Prepare all the solutions immediately before each experiment. - Evaluate the variations in [Ca2+]i by quantifying the ratio of the fluorescence emitted at 510 nm following alternate excitation (750 milliseconds) at 340 and 380 nm.
- Process acquired values using a fluorescence analysis software.
- Express experimental results in a table where each row represents an individual cell, and each line a time point.
5. Data processing
- Using a spreadsheet software, plot the variation of Fura-2 fluorescence ratio values of singular cells separately or of all of them at the same time.
- To quantify the number of reactive cells to determined stimulus, set a cutoff of 30% increase in calcium baseline levels
Representative Results
Here, we used retina cells in culture from embryonic day 8 chicks to investigate how neurons and glia signal in terms of calcium shifts. Cultures were prepared essentially as described15,19 as mixed neuron-glial cells (at a density of ≥ 1 x 106 cell/dish) at a stage of 7 days in vitro (Figure 1A). Alternatively, enriched neuronal cells prepared in low density (5 x 105 cell/dish), seeded on treated poly-L-lysine (10 µg/mL) coverslips at a stage of 3 days in vitro (Figure 1B). In addition, purified Müller glia were maintained for 10 days in DMEM containing 10% FCS, when neurons were removed. In order to functionally quantify the responses of neurons and glia, cells were stimulated with 50 mM KCl or 1 mM ATP. As shown (Figure 1A), out of 302 cells, 50% responded to KCl while 53% signaled to ATP. In this sense, enriched neuronal cell culture had an 89% of calcium responses to KCl compared to 17% that responded to ATP (Figure 1B). Indeed, a purified Müller glia culture, where neurons are removed after 10 days in culture, were solely activated by ATP (Figure 1C).
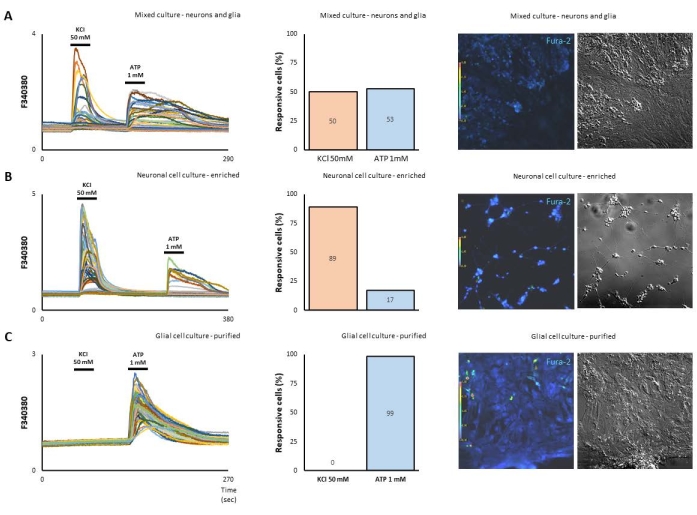
Figure 1. Retinal cells prepared as mixed, neuronal-enriched or glia-purified cultures show different response patterns on calcium imaging. (A) Bright and fluorescence fields of mixed embryonic retinal cells in culture. The same microscope field shown under 5 µM fura-2 AM fluorescence. 50 mM KCl activates half of the cells (neuronal phenotype), while 1 mM ATP activates the other half (glial phenotype), with high F340/380 ratios corresponding to increases in intracellular calcium ([Ca2+]i) levels. (B) The enriched neuronal cell culture had an 89% calcium response to KCl compared to 17% that responded to ATP. (C) On the other hand, a purified Müller glia culture, where neurons are removed after 10 days in culture, was solely activated by ATP. Please click here to view a larger version of this figure.
Discussion
We have used the retina tissue to show that calcium responses mediated by KCl or ATP are clearly compartmentalized into neuronal and glial responses, respectively (Figure 1). Although some data in the literature imply that P2X7 receptors are expressed in neurons, which regulate neuronal activity and synaptic neurotransmitter release20, other authors question the existence of neuronal P2X7 receptors. Indeed, current results sustain the idea that primary glial P2X7 receptors mediate neuronal effects summed to high extracellular ATP concentrations found in unhealthy tissues21.
We have previously linked the functional differentiation of retinal cells with their phenotypic display, in a way that variations of [Ca2+]i shifts that are activated by KCl or AMPA (a glutamate agonist) express microtubule associated protein (MAP-2), marker of a mature neuron1. Alternatively, cells activated by ATP express glutamine synthetase, a typical Muller glia marker.
We have been using different types of cell cultures as shown here (mixed, neuronal enriched or purified glial cells), in addition to neurospheres derived4 to answer many questions related to different neurochemical systems as glutamatergic22, dopaminergic19, GABAergic23, cannabinoid9,10, purinergic14,24, serotoninergic25, among others to understand the neuro-glial retinal communication. Müller glia express a great number of neurotransmitter receptors26 and secrete gliotransmitters as D-serine, ATP and glutamate, which may be released in a vesicular, Ca2+ dependent manner27.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Grants, sponsors, and funding sources: MH is recipient of a PhD CNPq fellowship. HRF is recipient of a postdoc fellowship supported by CNPq (HRF grant number 152071/2020-2). RAMR is supported by CNPq and FAPERJ (grant numbers E-26/202.668/2018, E-26/010.002215/2019, 426342/2018-6 and 312157/2016-9 and INCT-INNT (National Institute for Translational Neuroscience).
Materials
| 15 mm coverslip | Paul Marienfeld GmbH & Co. KG | 111550 | Cell suport |
| 510 nm long-pass filter | Carl Zeiss | ||
| ATP | Sigma | A1852 | |
| B-27 Supplement | Gibco | 17504044 | Suplement |
| CaCl2 | Sigma-Aldrich | C8106 | |
| CoolSNAP digital camera | Roper Scientific, Trenton, NJ | ||
| D-(+)-Glucose | Neon | 1466 | |
| DMEM/ F-12 | Gibco | 12400-24 | Cell culture medium |
| Excel Software | Microsoft | ||
| Fetal Calf Serum | Sigma-Aldrich | F9665 | Suplement |
| Fluorescence Microscope | Axiovert 200; Carl Zeiss | B 40-080 | |
| Fura-2 AM | Molecular Probes | F1221 | Ratiometric Ca2+ indicator |
| Gentamicin Sulfate | Calbiochem | 1405-41-0 | antibiotics |
| HEPES | Sigma | H4034 | |
| KCl | Sigma | P5405 | |
| KH2PO4 | Sigma | P5655 | |
| Lambda DG-4 apparatus | Sutter Instrument, Novato, CA | DG-4PLUS/OF30 | |
| Laminin | Gibco | 23017-015 | Help cell adhesion |
| Metafluor software | Universal Imaging Corp. West Chester, PA | ||
| MgCl2 | Sigma | M4880 | |
| Na2HPO4 | Vetec | 129 | |
| NaCl | Isofar | 310 | |
| NaHCO3 | Vetec | 306 | |
| PH3 platform | Warner Intruments, Hamden, CT | 64-0286 | |
| Pluronic F-127 | Molecular Probes | P6866 | nonionic, surfactant |
| Poly-L-lysine | Sigma-Aldrich | P8920 | Help cell adhesion |
| Trypsin-EDTA 0.25% | Gibco | 25200056 | Dissociation enzyme |
References
- Tsien, R. Y., Pozzan, T., Rink, T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. Journal of Cell Biology. 94 (2), 325-334 (1982).
- Paredes, R. M., Etzler, J. C., Watts, L. T., Zheng, W., Lechleiter, J. D. Chemical calcium indicators. Methods. 46 (3), 143-151 (2008).
- Islam, M. S. Calcium Signaling: From Basic to Bedside. Advances in Experimental Medicine and Biology. 1131, 1-6 (2020).
- De Melo Reis, R. A., et al. Functional identification of cell phenotypes differentiating from mice retinal neurospheres using single cell calcium imaging. Cellular and Molecular Neurobiology. 31 (6), 835-846 (2011).
- Ganguly, K., Schinder, A. F., Wong, S. T., Poo, M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 105 (4), 521-532 (2001).
- Schitine, C., et al. Ampakine CX546 increases proliferation and neuronal differentiation in subventricular zone stem/progenitor cell cultures. European Journal of Neuroscience. 35 (11), 1672-1683 (2012).
- Xapelli, S., et al. Modulation of subventricular zone oligodendrogenesis: a role for hemopressin. Frontiers in Cellular Neuroscience. 8, 59 (2014).
- Ribeiro-Resende, V. T., et al. Mice lacking GD3 synthase display morphological abnormalities in the sciatic nerve and neuronal disturbances during peripheral nerve regeneration. PLoS One. 9 (10), 108919 (2014).
- Xapelli, S., et al. Activation of type 1 cannabinoid receptor (CB1R) promotes neurogenesis in murine subventricular zone cell cultures. PLoS One. 8 (5), 63529 (2013).
- Kubrusly, R. C. C., et al. Neuro-glial cannabinoid receptors modulate signaling in the embryonic avian retina. Neurochemistry International. 112, 27-37 (2018).
- Egan, T. M., Khakh, B. S. Contribution of calcium ions to P2X channel responses. Journal of Neuroscience. 24 (13), 3413-3420 (2004).
- Illes, P. P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases. International Journal of Molecular Sciences. 21 (17), (2020).
- Faroni, A., et al. Purinergic signaling mediated by P2X7 receptors controls myelination in sciatic nerves. Journal of Neuroscience Research. 92 (10), 1259-1269 (2014).
- Freitas, H. R., et al. Cannabinoids Induce Cell Death and Promote P2X7 Receptor Signaling in Retinal Glial Progenitors in Culture. Molecular Neurobiology. 56 (9), 6472-6486 (2019).
- Freitas, H. R., et al. Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells. PLoS One. 11 (4), 0153677 (2016).
- Yang, X. L. Characterization of receptors for glutamate and GABA in retinal neurons. Progress in Neurobiology. 73 (2), 127-150 (2004).
- Reis, R. A., Kubrusly, R. C., de Mello, M. C., de Mello, F. G. Transient coupling of NMDA receptor with ip3 production in cultured cells of the avian retina. Neurochemistry International. 26 (4), 375-380 (1995).
- López-Colomé, A. M., Ortega, A., Romo-de-Vivar, M. Excitatory amino acid-induced phosphoinositide hydrolysis in Müller glia. Glia. 9 (2), 127-135 (1993).
- Ventura, A. L., de Mello, F. G., de Melo Reis, R. A. Methods of dopamine research in retina cells. Methods in Molecular Biology. 964, 25-42 (2013).
- Miras-Portugal, M. T., Sebastián-Serrano, &. #. 1. 9. 3. ;., de Diego García, L., Díaz-Hernández, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. Journal of Neuroscience. 37 (30), 7063-7072 (2017).
- Illes, P., Khan, T. M., Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist. Journal of Neuroscience. 37 (30), 7049-7062 (2017).
- Schitine, C. S., et al. Functional plasticity of GAT-3 in avian Müller cells is regulated by neurons via a glutamatergic input. Neurochemistry International. 82, 42-51 (2015).
- Ferreira, D. D., Stutz, B., de Mello, F. G., Reis, R. A., Kubrusly, R. C. Caffeine potentiates the release of GABA mediated by NMDA receptor activation: Involvement of A1 adenosine receptors. 神经科学. 281, 208-215 (2014).
- Faria, R. X., Freitas, H. R., Reis, R. A. M. P2X7 receptor large pore signaling in avian Müller glial cells. Journal of Bioenergetics and Biomembranes. 49 (3), 215-229 (2017).
- Passos, A., et al. Regulation of the Serotonergic System by Kainate in the Avian Retina. Cellular and Molecular Neurobiology. 39 (7), 1039-1049 (2019).
- de Melo Reis, R. A., Ventura, A. L., Schitine, C. S., de Mello, M. C., de Mello, F. G. Müller glia as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochemical Research. 33 (8), 1466-1474 (2008).
- Harada, K., Kamiya, T., Tsuboi, T. Gliotransmitter Release from Astrocytes: Functional Developmental, and Pathological Implications in the Brain. Frontiers in Neuroscience. 9, 499. 9, 499 (2015).

