Studying Inherited Immunity in a Caenorhabditis elegans Model of Microsporidia Infection
Summary
The infection of Caenorhabditis elegans by the microsporidian parasite Nematocida parisii enables the worms to produce offspring that are highly resistant to the same pathogen. This is an example of inherited immunity, a poorly understood epigenetic phenomenon. The present protocol describes the study of inherited immunity in a genetically tractable worm model.
Abstract
Inherited immunity describes how some animals can pass on the “memory” of a previous infection to their offspring. This can boost pathogen resistance in their progeny and promote survival. While inherited immunity has been reported in many invertebrates, the mechanisms underlying this epigenetic phenomenon are largely unknown. The infection of Caenorhabditis elegans by the natural microsporidian pathogen Nematocida parisii results in the worms producing offspring that are robustly resistant to microsporidia. The present protocol describes the study of intergenerational immunity in the simple and genetically tractable N. parisii –C. elegans infection model. The current article describes methods for infecting C. elegans and generating immune-primed offspring. Methods are also given for assaying resistance to microsporidia infection by staining for microsporidia and visualizing infection by microscopy. In particular, inherited immunity prevents host cell invasion by microsporidia, and fluorescence in situ hybridization (FISH) can be used to quantify invasion events. The relative amount of microsporidia spores produced in the immune-primed offspring can be quantified by staining the spores with a chitin-binding dye. To date, these methods have shed light on the kinetics and pathogen specificity of inherited immunity, as well as the molecular mechanisms underlying it. These techniques, alongside the extensive tools available for C. elegans research, will enable important discoveries in the field of inherited immunity.
Introduction
Inherited immunity is an epigenetic phenomenon whereby parental exposure to pathogens can enable the production of infection-resistant offspring. This type of immune memory has been shown in many invertebrates that lack adaptive immune systems and can protect against viral, bacterial, and fungal disease1. While inherited immunity has important implications for understanding both health and evolution, the molecular mechanisms underlying this protection are largely unknown. This is partly because many of the animals in which inherited immunity has been described are not established model organisms for research. In contrast, studies in the transparent nematode Caenorhabditis elegans benefit from an extensive genetic and biochemical toolkit2,3, a highly annotated genome4,5, and a short generation time. Indeed, research in C. elegans has enabled fundamental advances in the fields of epigenetics and innate immunity6,7, and it is now an established model for studying immune memory8,9.
Microsporidia are fungal pathogens that infect almost all animals and cause lethal infections in immunocompromised humans10. Infection begins when a microsporidia spore injects or "fires" its cellular contents (sporoplasm) into a host cell using a structure called a polar tube. Intracellular replication of the parasite results in the formation of meronts, which ultimately differentiate into mature spores that can exit the cell11,12. While these parasites are detrimental to both human health and food security, there is much still to learn about their infection biology12. Nematocida parisii is a natural microsporidian parasite that replicates exclusively in the intestinal cells of worms, resulting in reduced fecundity and, ultimately, death. The N. parisii –C. elegans infection model has been used to show: (1) the role of autophagy in pathogen clearance13, (2) how microsporidia can exit infected cells non-lytically14, (3) how pathogens can spread from cell-to-cell by forming syncytia15, (4) the proteins N. parisii use to interface with its host16, and (5) the regulation of the transcriptional intracellular pathogen response (IPR)17,18.
Protocols for the infection of C. elegans are described in the current work and can be used to reveal the unique microsporidia biology and dissect the host's response to infection. The microscopy of fixed worms stained with the chitin-binding dye Direct Yellow 96 (DY96) shows the infection spread of chitin-containing microsporidia spores throughout the intestine. DY96 staining also enables the visualization of chitin-containing worm embryos for the simultaneous assessment of worm gravidity (ability to produce embryos) as a readout of host fitness.
Recent work has revealed that C. elegans infected with N. parisii produce offspring that are robustly resistant to the same infection19. This inherited immunity lasts a single generation and is dose-dependent, as offspring from more heavily infected parents are more resistant to microsporidia. Interestingly, N. parisii-primed offspring are also more resistant to the bacterial gut pathogen Pseudomonas aeruginosa, though they are not protected against the natural pathogen Orsay virus19. The present work also shows that immune-primed offspring limit host cell invasion by microsporidia. The method also describes the collection of immune-primed offspring and how FISH can be used to detect N. parisii RNA in intestinal cells to assay host cell invasion and spore firing20.
Together, these protocols provide a solid foundation for studying microsporidia and inherited immunity in C. elegans. It is hoped that future work in this model system will enable important discoveries in the nascent field of inherited immunity. These techniques are also likely to be starting points for investigating microsporidia-induced inherited immunity in other host organisms.
Protocol
The present study uses wild-type C. elegans Bristol strain N2 grown at 21 °C.
1. Preparation of media
- Prepare M9 media as per previous report21,22.
- Prepare nematode growth medium (NGM) as per previous report21,22. Pour 12 mL of NGM per 6 cm plate or 30 mL per 10 cm plate.
- Prepare Escherichia coli strain OP50-1 as per a previous report23.
- Prepare OP50-1-seeded NGM plates following the steps below.
- Resuspend 10x concentrated OP50-1 by vortexing.
- Use a repeater pipette to seed the plates in the center. For 6 cm and 10 cm plates, seed with a volume of 200 µL or 500 µL, respectively.
- For 10 cm plates, use a glass spreader to spread the OP50-1 and create a lawn of bacteria. Do not spread the bacteria to the very edge of the plate.
NOTE: Ethanol dip and flame the spreader every five plates to ensure sterility. Allow the spreader to cool for a few seconds before spreading to prevent killing bacteria. - Leave plates at room temperature for 2 days to dry and to ensure the growth of the bacterial lawn.
NOTE: Seeded plates can be stored at room temperature for 2-3 weeks or at 4 °C for up to 1 month.
2. Maintenance of C. elegans
- Maintain worms on a constant bacterial food source on OP50-1-seeded NGM plates.
NOTE: Starvation may result in intergenerational phenotypes, which can affect results. A 10 cm OP50-1-seeded plate supports 2,500 L1s (the earliest larval stage of C. elegans) for up to 72 h. - If the worms starve, grow for three generations on OP50-1 before using the worms for experiments.
3. Synchronization of C. elegans populations using sodium hypochlorite (bleaching)
NOTE: This step is very time-sensitive, so ensure the centrifuge is available before beginning. Alternative, less rapid bleaching protocols are available in the literature and may be used if preferred. To prevent 6% sodium hypochlorite from losing activity over time, store the reagent in the dark at 4 °C and keep it for up to 1 year.
- Prepare 2 mL of bleach solution for each sample by mixing 400 µL of 1 M NaOH, 250 µL of 6% sodium hypochlorite (see Table of Materials), and 1350 µL of distilled water.
- Wash adult worms (~72 h post-hatch) off the plates into a microcentrifuge tube using 1 mL of M9 media.
NOTE: If many worms remain on the plates, pellet the worms by centrifugation at 1,400 x g for 30 s at room temperature, remove the supernatant using a 1 mL pipette tip, and perform additional plate washes. - Centrifuge at 1,400 x g for 30 s at room temperature to pellet the worms, then wash 2x with 1 mL of M9 media to remove bacteria.
- Remove the supernatant, add 1 mL of the prepared bleach solution (Step 3.1.), and set a timer for 1 min. Invert the tubes 3-4x.
NOTE: Under a light microscope, the worms can be seen to stop thrashing. - After 1 min, centrifuge at 3,000 x g for 30 s at room temperature to pellet the worms, then remove the supernatant and add 1 mL of fresh bleach solution.
- Monitor the tube(s) closely under a light microscope to visualize the worm carcasses splitting open and releasing embryos. Immediately advance to the next step when ~50%-80% of the carcasses have split open and many embryos have been released.
NOTE: Depending on the number of animals and the worm strain, this step can take 15 s to 4 min. For unknown reasons, N. parisii-infected worms often take longer to bleach than uninfected animals. - Centrifuge at 3,000 x g for 30 s at room temperature to pellet the embryos, then wash 3x in 1 mL of M9 media.
NOTE: Washes dilute the residual hypochlorite solution from the tubes and must be performed quickly to ensure the bleach solution does not damage the embryos. - Add 1 mL of M9 media to the final pellet and transfer to a 15 mL conical tube containing 4 mL of M9 media. Suspend the embryos in a final volume of 5 mL.
- Spin the tubes on a mechanical rotor (see Table of Materials) at 21 °C for 18-24 h to hatch the embryos into L1s.
NOTE: As N. parisii infects the worm intestine and does not invade the germline, infected parents do not pass the infection to their progeny by vertical transmission19. Bleaching of infected adults synchronizes F1 animals and destroys N. parisii spores, ensuring F1 progeny are not infected by spores carried over from the parents. - Centrifuge the tubes for 1 min at 1,800 x g at room temperature to pellet the L1s, and discard all but 1 mL of the supernatant.
- Pipette 1-5 µL of L1 mixture onto an unseeded NGM plate, and immediately count the number of L1s in the droplet by visualizing under a light microscope. Repeat 3x and average the counts to determine the concentration and total number of L1s.
NOTE: The majority of the embryos should be hatched, and the L1s should be rapidly moving in the liquid droplet. If a large proportion of unhatched embryos and lethargic (slow-moving) L1s remain, this indicates bleaching for too long.
4. Preparation of N. parisii spores
- Prepare N. parisii spores following previously published references19,24.
5. Infection of C. elegans with N. parisii to yield immune-primed offspring
- One day before planned infections, move the required number of 10 cm unseeded NGM plates from 4 °C to room temperature.
- Immediately before infection, centrifuge ~2,500 synchronized L1 worms at 1,400 x g for 30 s at room temperature in a microcentrifuge tube to pellet the worms. Remove supernatant with a pipette tip to leave worms in ~50 µL of M9 media.
- Add 1 mL of 10x OP50-1 to the L1s and N. parisii spores to the desired concentration (typically ~2.5 million spores). As an uninfected control, prepare an equivalent tube of L1s and OP50-1 with a volume of M9 media equivalent to the volume of spore preparation used.
NOTE: To obtain immune-primed offspring, use a spore dose high enough so that >90% of the animals are infected by 72 h (as measured by DY96 staining, Step 7) but low enough so that >80% of animals are still gravid. This ensures that most offspring come from infected parents and that bleaching of the parents yields sufficient embryos for F1 testing. Though spore preparations vary in concentration and infectivity, a suitable parental dose is typically between 5-15 µL of spore preparation (~2.5 million spores) per 10 cm plate. - Vortex briefly to mix and plate the above 1 mL of worms onto a 10 cm unseeded NGM plate. Swirl to ensure the liquid spreads over the whole plate.
- Dry the plates in a clean cabinet with the lids off for 10-20 min or until completely dry, before incubating at 21 °C for 72 h.
- At 72 h post-infection (hpi), wash the worms off the plates into a microcentrifuge tube using 1 mL of M9 media. If many worms remain on the plates, pellet the worms by centrifugation at 1,400 x g for 30 s, remove the supernatant, and perform additional plate washes.
- Centrifuge at 1,400 x g for 30 s at room temperature to pellet worms, wash 2x with 1 mL of M9 media or until the supernatant is clear. Resuspend in a final volume of 1 mL of M9 media.
- Pipette up and down to mix, then transfer 100 µL of the suspended worms to a fresh tube.
NOTE: This sample of ~250 adults can later be fixed and stained to determine parental worm fitness and infection status, as described in Step 7 and the beginning of Step 3. - To break open the adult worms and release F1 embryos for testing, bleach the remaining 900 µL of suspended worms, as described in Step 3.
NOTE: This step also destroys any N. parisii spores before subsequent infection assays.
6. Testing inherited immunity to N. parisii in C. elegans
- Perform infections as described in Steps 5.1.-5.6., with modifications as mentioned below.
NOTE: Infection assays on F1s can be scaled down and performed on 6 cm NGM plates using ~1,000 L1 worms and 400 µL of 10x OP50-1. A "high" dose of spores must also be used, such that ~100% of naïve F1s (i.e., worms from uninfected parents) are infected, and only ~10% of naïve F1s are gravid. Though spore preparations vary in concentration and infectivity, a suitable dose is typically between 5-15 µL (~2.5 million spores) per 6 cm plate. - At 72 hpi, fix and stain to determine worm fitness and infection status, as described in Step 7.
7. DY96 staining of C. elegans to visualize embryos and microsporidia spores
NOTE: DY96 is a green fluorescent chitin-binding dye that stains worm embryos and microsporidia spore walls19,15,25. This allows simultaneous monitoring of the fitness and infection status of the worms.
- Wash the worms off the plates into a microcentrifuge tube using 1 mL of M9 media containing 0.1% Tween-20. If many worms remain on the plates, pellet the worms by centrifugation at 1,400 x g for 30 s at room temperature, remove the supernatant using a pipette tip, and perform additional plate washes.
- Centrifuge at 1,400 x g for 30 s at room temperature to pellet the worms, and wash 2x with 1 mL of M9 media containing 0.1% Tween-20 or until the supernatant is clear.
- Remove the supernatant, add 700 µL of acetone and leave the worms to fix at room temperature for 10 min.
NOTE: At this point, the worms can be stored at -20 °C for processing up to several months later, if desired. - Centrifuge at 10,000 x g for 30 s at room temperature to pellet the fixed worms, and wash 2x with 1 mL of PBS containing 0.1% Tween-20 (PBST).
- Prepare 50 mL of DY96 working solution: PBST, 0.1% (v/v) sodium dodecyl sulfate (SDS), and 20 µg/mL DY96 (from a 5 mg/mL stock solution in distilled water) (see Table of Materials). Wrap the tube in foil and store it in a drawer to prevent exposure to light.
NOTE: DY96 stock and working solutions can be stored for >1 year at room temperature if kept in the dark. - Centrifuge at 10,000 x g for 30 s at room temperature to pellet the worms and remove supernatant. Add 500 µL of DY96 working solution to the pellet and rotate for 30 min at room temperature.
- Centrifuge at 10,000 x g for 30 s at room temperature to pellet the worms. Remove the supernatant and add 15 µL of the mounting medium with/without DAPI (see Table of Materials).
- Pipette 10 µL of the solution containing stained worms onto a microscope slide and place a coverslip over the top.
NOTE: Slides and additional samples can be stored at 4 °C in the dark for long-term storage.
8. Imaging and analysis of DY96-stained worms to assess worm fitness and infection status
- To analyze the DY96-stained worms (mounted on slides, as in Step 7.8.), perform imaging using the GFP channel of a fluorescence microscope.
- To determine the fitness of the worm populations, use a 5x or 10x objective to assay the gravidity of >100 worms per condition.
NOTE: Worms carrying ≥1 embryo are considered gravid. Mechanical cell counters can help to minimize human error when counting. Worm embryos are less stained (less fluorescent) than microsporidia spores19. Embryos are ovoid, ~50 µm x ~30 µm, and found in the midbody of healthy C. elegans adults. Healthy, uninfected adults carry 10-20 embryos organized in rows along their body length26. - To reveal more subtle differences in fitness, perform embryo counts. For this, manually count the number of embryos in 20-30 individual worms per condition.
- To determine how infected the worm populations are, use a 5x-40x objective to assess the infection status of >100 worms per condition.Worms comprising any number of intracellular gut spores are considered infected.
NOTE: Microsporidia spores are brighter than worm embryos. The bean-shaped spores of N. parisii are ~2.2 µm x ~0.8 µm and are produced in the worm's intestinal cells from ~48 hpi15. Worms in which spores are seen exclusively in the gut lumen and not along the intestinal walls are not considered infected19. This is because these spores likely represent newly ingested spores that do not result from infection of the individual. - Using image analysis software (see Table of Materials), quantify parasite burden and worm size following the steps below.
- Image 20-30 worms mounted on microscope slides using a fluorescent upright stereoscope for each sample. Capture images in Brightfield, DAPI, and GFP channels. Choose an appropriate exposure in the GFP channel such that the fluorescence in the most infected samples is not saturated.
NOTE: The infection can still be visualized in the least infected samples. Images are typically taken using a 5x objective. - Open files in FIJI/ImageJ. Depending on the file type, the Bio-Formats Importer plugin may be required to open images in ImageJ.
- Outline 20-30 individual worms in images using brightfield or DAPI images and the Polygon selections tool in the toolbar. Save each outline with Analyze > Tools > Regions of Interest (ROI) Manager in the dropdown menu by clicking on Add [t]. Outline animals that are fully visible in the frame.
NOTE: Worm outlines can be revisited later by saving the file with outlines as a Tiff. - Click on Deselect in the ROI manager to remove all outlines from the image and click on Image > Duplicate in the dropdown menu. Type the number corresponding to the channel of interest into the "Channels" box to duplicate the GFP channel for parasite burden (e.g., 2).
- Threshold this channel using Image > Adjust > Threshold to a level at which the bright parasite burden is captured but the dimmer fluorescence from worm embryos is not, and then click on Apply. Take note of the threshold values applied and use them consistently for all images of the same experiment.
NOTE: Check the threshold is an appropriate value for both the least and most infected samples before analyzing all the images. - Select Analyze > Set measurements and check the box for Area Fraction. Select single worm outlines in turn from the ROI manager and click on the Analyze > Measure function. A Results window will provide the measurement for %Area (i.e., % parasite burden).
NOTE: If the fluorescence from the embryos is so bright that it crosses the threshold, the Paintbrush tool in black in the toolbar can be used to manually erase these regions prior to measuring %Area. - To determine worm size as a readout of fitness, outline worms as described in Step 8.3. Select Analyze > Set measurements and check the box for Area. Select single worm outlines in turn from the ROI manager and click on the Analyze > Measure function. A Results window will provide the measurement for %Area.
- Image 20-30 worms mounted on microscope slides using a fluorescent upright stereoscope for each sample. Capture images in Brightfield, DAPI, and GFP channels. Choose an appropriate exposure in the GFP channel such that the fluorescence in the most infected samples is not saturated.
9. FISH assay to assess invasion of C. elegans by microsporidia and spore firing
NOTE: The MicroB FISH probe recognizes a conserved region of microsporidian 18s rRNA and can be used to label intracellular sporoplasms (i.e., invaded host cells) and the genetic material within spores.
- Infect 6,000 bleach synchronized L1 worms with 4 million spores of N. parisii and 10 µL of 10x OP50-1 in a final volume of 400 µL that is made up with M9 media.
- Plate the mixture on a 6 cm unseeded NGM plate, dry in a clean cabinet for 10-20 min, and place at 21 °C.
- After 30 min, wash the animals off the plates with 1 mL of M9 media containing 0.1% Tween-20.
- Pellet the worms by centrifuging at 1,400 x g for 1 min at room temperature.
- Remove the supernatant using a pipette tip, add 700 µL of acetone, and allow it to sit for 10 min at room temperature.
NOTE: At this point, the worms can be stored at -20 °C for processing at a later time. - Perform overnight FISH assay using the MicroB probe following previously published reports27,19.
NOTE: To assess spore firing, supplement the final 500 µL of wash buffer with 20 µg/mL of DY96 to the samples and rotate for 30 min at 21 °C before continuing with the protocol as normal. - Store the slides in a slide box at 4 °C. For long-term storage (i.e., several months), store additional samples in microcentrifuge tubes at 4 °C in darkness.
- To assess invasion in FISH-stained worms, look at the worms using the red channel of a fluorescence microscope. Visualize infection events at high magnification (63x objective) as L1 animals are small, and sporoplasms will be in the early stages of replication. To assess spore firing, use a 63x objective and both red and green fluorescence channels.
NOTE: Spores in which GFP and mCherry signal colocalize are considered unfired; empty GFP spore cases are considered fired. - To assay invasion or spore firing, manually count the number of sporoplasms (mCherry foci in the intestinal cells) or the percentage of spores fired in 20-30 worms per condition. Adjust the focus in the z-plane to capture all infection events and spores, which are often found in different planes.
NOTE: Sporoplasms are found exclusively within the intestinal cells. The highest concentration of sporoplasms is typically found immediately after the pharynx. If samples are also stained with DY96, look for the presence of sporoplasms that do not colocalize with the spore.
Representative Results
In the present study, parental populations of C. elegans (P0) were infected at the L1 stage with a low dose of N. parisii spores. These infection conditions are typically used to obtain high numbers of microsporidia-resistant F1 progeny through bleaching of the parents. Infected parental populations and uninfected controls were fixed at 72 hpi and stained with DY96 to visualize the worm embryos and microsporidia spores (Figure 1A). Infected animals are small, contain many microsporidia spores, and produce fewer embryos than healthy uninfected controls. Assessment of worm gravidity showed that ~95% of uninfected animals produced offspring, compared to less than 80% of the infected animals (Figure 1B). Quantifications revealed that ~90% of the microsporidia-treated population were infected, as determined by the number of worms containing DY96-stained spores (Figure 1C). To obtain immune-primed F1 progeny, it is important to use a dose of microsporidia that is high enough to ensure most parents are infected but low enough to ensure that the population can still produce progeny. A table of the microsporidia doses used to obtain the data in this study is provided (Table 1).
Uninfected and infected parent populations (Figure 1) were treated with sodium hypochlorite at 72 hpi to obtain naïve and immune-primed F1 progenies. F1 animals were exposed to a high dose of N. parisii spores at the L1 stage to test for inherited immunity to microsporidia. At 72 hpi, F1 animals were fixed and stained with DY96 to assess microsporidia resistance (Figure 2A). Quantifications of these fixed animals revealed that the primed worms contained significantly more embryos than their naïve counterparts, indicating greater fitness in the face of infection (Figure 2B). FIJI/ImageJ was used to determine the parasite burden of individual naïve and immune-primed worms (i.e., percentage of the body filled with fluorescent N. parisii spores) (Figure 2C). Quantifications revealed a dramatic reduction in the parasite burden of worms that came from infected parents (Figure 2D). Further, calculations of individual worm size revealed that primed worms had a significant growth advantage over naïve animals in the face of N. parisii infection (Figure 2E). These data demonstrate that N. parisii-infected parents produce offspring with high levels of microsporidia resistance.
Previous work has revealed that inherited immunity to microsporidia reduces invasion of intestinal cells by N. parisii19. To visualize differences in host cell invasion, naïve and immune-primed animals (obtained from uninfected or infected parents, as above) were exposed to a maximal dose of N. parisii at the L1 stage. At 30 dpi, the worms were fixed and co-stained, using a FISH probe to detect N. parisii RNA and DY96 to detect N. parisii spore walls. Imaging revealed that, while naïve animals typically contained multiple spores and several infected cells (sporoplasms), the primed animals had far fewer (or no) spores and typically no sporoplasms (Figure 3).
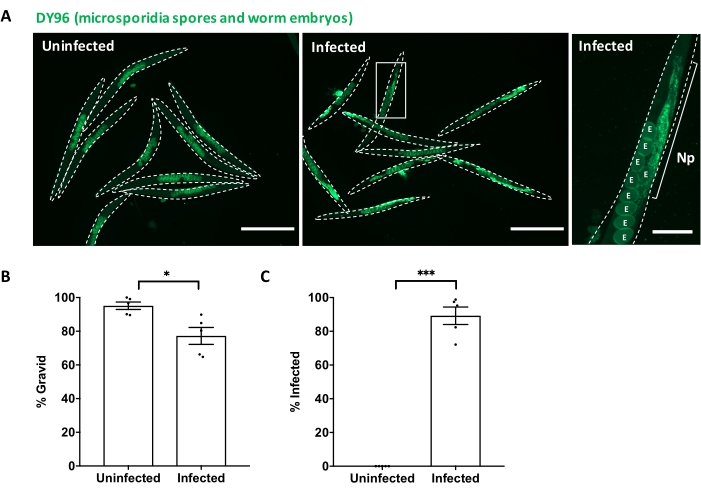
Figure 1: Direct Yellow 96 (DY96) staining of uninfected and N. parisii-infected C. elegans reveals worm gravidity and infection status. (A–C) N2 C. elegans were not infected or infected with a low dose of N. parisii at the L1 stage. At 72 hpi, the worms were fixed, stained with DY96, and imaged to assess worm gravidity and infection status. (A) Representative images are shown. DY96 enables visualization of worm embryos and microsporidia spores (chitin). An inset image of an infected worm is shown on the right-hand side. Embryos are labeled 'E'; N. parisii infection of the intestine is labeled 'Np'. Scale bar for left and middle images = 500 µm. Scale bar for the right image = 100 µm. (B) Worms carrying one or more embryos were scored as gravid and graphed.(C) Worms carrying any number of N. parisii spores in the intestinal cells were scored as infected and graphed. (B–C) Data pooled from 5 independent experiments using n = 100 worms per condition per experiment. Mean ± SEM is shown. The p values were determined by the unpaired two-tailed Student's t-test. Significance was defined as: *p < 0.05; ***p < 0.001. Please click here to view a larger version of this figure.
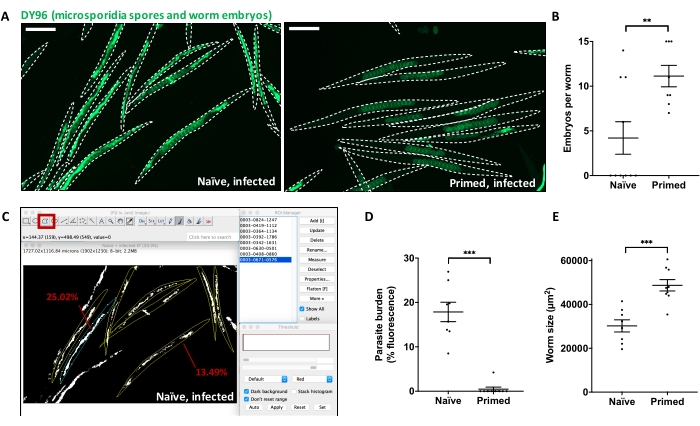
Figure 2: Direct Yellow 96 (DY96) staining of naïve and immune-primed C. elegans to determine embryos per worm, microsporidia burden, and worm size. (A) N2 C. elegans were infected or not infected with a low dose of N. parisii at the L1 stage. At 72 hpi, the worms were treated with sodium hypochlorite to release embryos. The resulting naïve and immune-primed offspring were infected with a high dose of N. parisii at the L1 stage. At 72 hpi, the worms were fixed, stained with DY96, and imaged to visualize worm embryos and parasite burden. Representative images are shown. Scale bar = 200 µm.(B) The number of embryos per worm was counted and graphed from (A). (C) A screenshot from FIJI/ImageJ shows naïve worms from panel A that have been thresholded to visualize parasite burden (shown in white) using the Thresholding window in the bottom right. Here, individual worms were outlined using the "Polygon selections" tool highlighted in red and defined as "Region of interests" (ROI) using the ROI window in the top right. The Analyze > Measure > Area function was used to quantify the parasite burden of two example worms, shown to be 25.02% and 13.49%. (D) The parasite burden per worm was determined and graphed from (A). (E) From the above images, individual worm size was calculated from animals outlined as in panel C using the Analyze > Measure > Area function. (B, D, and E) Mean ± SEM is shown. The p values were determined by unpaired two-tailed Student's t-test. Significance was defined as: *p < 0.05. **p < 0.01, ***p < 0.001. Please click here to view a larger version of this figure.
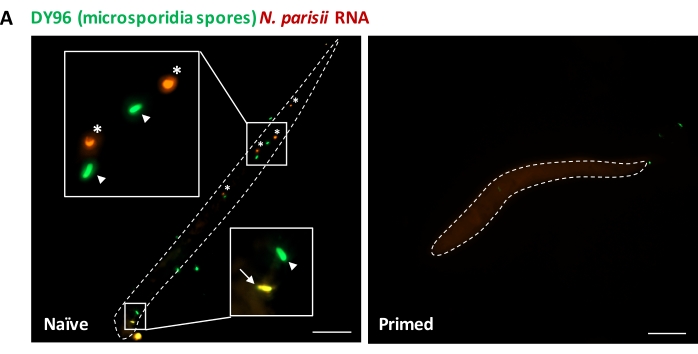
Figure 3: Fluorescence in situ hybridization (FISH) against N. parisii 18S rRNA reveals parasite invasion of C. elegans intestinal cells in naïve but not primed animals. N2 C. elegans were infected or not infected with a low dose of N. parisii at the L1 stage. At 72 hpi, the worms were treated with sodium hypochlorite to release embryos. The resulting naïve and immune-primed offspring were infected with a maximal dose of N. parisii at the L1 stage. At 30 mpi, the worms were fixed, subjected to FISH to detect sporoplasms, stained with DY96 to detect microsporidia spore walls, and imaged. Representative images are shown. Inset images for the naïve worm show sporoplasms (red, asterisks), fired spores (green, arrowheads), and an unfired spore (yellow, arrow). The naïve infected worm shown displays 4 sporoplasms. Scale bar = 20µm. Please click here to view a larger version of this figure.
| N. parisii dose | Plate concentration (spores/cm2) | Millions of spores per 6 cm plate | Millions of spores per 10 cm plate |
| Low | 35,400 | – | 2.7 |
| High | 88,400 | 2.5 | – |
| Maximal | 2,12,000 | 6 | – |
Table 1: N. parisii doses employed in the study.
Discussion
The present protocol describes the study of microsporidia and inherited immunity in a simple and genetically tractable N. parisii –C. elegans infection model.
Spore preparation is an intensive protocol that typically yields enough spores for 6 months of experiments, depending on productivity24. Importantly, infectivity must be determined for each new spore "lot" before using it for the experiments. Due to the variability in infectivity between spore preparations, a single lot must be used consistently for all repeats of an experiment. Individual aliquots should not be thawed and re-frozen, as thawing may cause spores to fire and reduce infectivity. As such, spores must only be removed from the -80 °C freezer immediately prior to infection and maintained on ice while on the bench.
In Steps 5-6, the infection assays were outlined. It is important during infection assays to avoid contamination or starvation of the animals, as this may result in additional intergenerational effects that confound immunity in F1s. An important limitation of the inherited immunity assay is that more infected parents produce fewer F1 offspring. Therefore, it is important to strike a careful balance between the parental generation being sufficiently infected to pass on immunity while being healthy enough to produce offspring for testing. Though the current protocol described infection assays for L1 animals, the protocol can be modified to test the impact of microsporidia infection on differently staged parents and the persistence of immunity in older offspring. The methods can also be modified to test the effects of multiple or distinct stresses (e.g., osmotic stress, heavy metal stress, coinfection with another pathogen) on inherited immunity. While inherited immunity to N. parisii in C. elegans is intergenerational (i.e., lasts a single generation)19, the protocol can be adapted to study further generations to test transgenerational effects. While the protocols provided are specific to N. parisii infection, many other nematode-infecting species of microsporidia exist28. The protocols can be adapted to study inherited immunity to other gut-infecting (e.g., Nematocida ausubeli) and epidermal- and muscle-infecting (e.g., Nematocida displodere) microsporidia29,30. C. elegans are also infected by many other natural and human pathogens (bacterial, fungal, and viral). The methods described here can be used to test inherited immunity to other pathogens in C. elegans and the pathogen-specificity of the inherited immune response to N. parisii. C. elegans is a well-established model organism. However, other members of the Caenorhabditis genus, including the hermaphroditic species C. tropicalis and C. briggsae and the male-female species C. kamaaina are also infected to varying degrees with N. parisii31. By making small modifications to the protocols provided, inherited immunity can also be tested in these animals.
In Step 8, quick and simple methods were given for determining differences in microsporidia susceptibility in DY96-stained worms (i.e., % animals infected, % animals gravid). However, sometimes these methods are not sensitive enough to detect small changes in immunity and fitness. This is because a worm with one embryo and many infected cells would be treated the same as a worm with 10 embryos and one infected cell. As such, methods with finer resolution have also been provided for determining infection outcomes in these animals (i.e., % parasite burden, number of embryos, worm size). While both methods can be used, phenotypic differences are typically more pronounced with the latter, making it easier to detect significant differences. Future adaptations to this method may include using machine learning to automate analysis.
In Step 9, techniques were provided for assaying host cell invasion by microsporidia and spore firing using FISH. While DY96 marks microsporidia with a strong fluorescent green signal, other dyes, including the lipophilic stain Nile Red and the chitin-binding Calcofluor White, can also be used to stain N. parisii30,32,33. The present protocol describes fixation using acetone and works well for detecting microsporidia with DY96 and FISH staining. However, fixation with 4% paraformaldehyde can help preserve tissue morphology and improve the signal in some imaging protocols.
Microsporidia is an understudied pathogen, and much of the cell biology of infection remains unknown. C. elegans is a simple model organism, and these protocols can serve as a platform to understand the key processes involved in infection and host defense against this parasite. Inherited immunity is an emerging field, and many questions remain outstanding1. How do infected parents transmit immunity to offspring (maternal provisioning or altered transcriptional regulation in the progeny)? What mediates immunity in offspring (antimicrobial peptides or other immune proteins)? How pathogen-specific are inherited immune responses, and how conserved is inherited immunity between species? Given the extensive tools available with C. elegans, studies building on the protocol described here are well poised to answer these fundamental questions of inherited immunity.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are grateful to Winnie Zhao and Yin Chen Wan for providing helpful comments on the manuscript. This work was supported by the Natural Sciences and Engineering Research Council of Canada (Grant #522691522691).
Materials
| 2.0 mm zirconia beads | Biospec Products Inc. | 11079124ZX | |
| 10 mL syringe | Fisher Scientific | 1482613 | |
| 5 μm filter | Millipore Sigma | SLSV025LS | |
| Axio Imager 2 | Zeiss | – | Fluorescent microscope for imaging of DY96- and FISH- stained worms on microscope slides |
| Axio Zoom V.16 Fluorescence Stereo Zoom Microscope | Zeiss | – | For live imaging of fluorescent transgenic animals to visualize the IPR |
| Baked EdgeGARD Horizontal Flow Clean Bench | Baker | – | |
| Bead disruptor, Genie SI-D238 Analog Disruptor Genie Cell Disruptor, 120 V | Global Industrial | T9FB893150 | |
| Cell-VU slide, Millennium Sciences Disposable Sperm Count Cytometers | Fisher Scientific | DRM600 | |
| Direct Yellow 96 | Sigma-Aldrich | S472409-1G | |
| EverBrite Mounting Medium with DAPI | Biotium | 23001 | |
| EverBrite Mounting Medium without DAPI | Biotium | 23002 | |
| Fiji/ImageJ software | ImageJ | https://imagej.net/software/fiji/downloads | |
| Mechanical rotor | Thermo Sceintific | 415110 / 1834090806873 | Used to spin tubes of bleached embryos for overnight hatching |
| MicroB FISH probe | Biosearch Technologies Inc. | – | Synthesized with a Quasar 570 (Cy3) 5' modification and HPLC purified, CTCTCGGCACTCCTTCCTG |
| N2 | Wild-type, Bristol strain | Default strain | Caenorhabditis Genetics Center (CGC) |
| Sodium dodecyl sulfate (SDS) | Sigma-Aldrich | L3771-100G | |
| Sodium hydroxide solution (5 N) | Fisher Chemical | FLSS256500 | |
| Sodium hypochlorite solution (6%) | Fisher Chemical | SS290-1 | |
| Stemi 508 Stereo Microscope | Zeiss | – | For daily maintenance of worms and counting of L1 worms for assay set ups |
| Tween-20 | Sigma-Aldrich | P1379-100ML | |
| Vectashield + A16 | Biolynx | VECTH1500 |
References
- Tetreau, G., Dhinaut, J., Gourbal, B., Moret, Y. Trans-generational immune priming in invertebrates: current knowledge and future prospects. Frontiers in Immunology. 10, 1938 (2019).
- Au, V., et al. CRISPR/Cas9 methodology for the generation of knockout deletions in Caenorhabditis elegans. G3 Genes|Genomes|Genetics. 9 (1), 135-144 (2019).
- Kamath, R. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 30 (4), 313-321 (2003).
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 282 (5396), 2012-2018 (1998).
- Yoshimura, J., et al. Recompleting the Caenorhabditis elegans genome. Genome Research. 29, 1009-1022 (2019).
- Weinhouse, C., Truong, L., Meyer, J. N., Allard, P. Caenorhabditis elegans as an emerging model system in environmental epigenetics: C. elegans as an environmental epigenetics model. Environmental and Molecular Mutagenesis. 59 (7), 560-575 (2018).
- Ermolaeva, M. A., Schumacher, B. Insights from the worm: the C. elegans model for innate immunity. Seminars in Immunology. 26 (4), 303-309 (2014).
- Willis, A. R., Sukhdeo, R., Reinke, A. W. Remembering your enemies: mechanisms of within-generation and multigenerational immune priming in Caenorhabditis elegans. TheFEBS Journal. 288 (6), 1759-1770 (2020).
- Burton, N. O., et al. Cysteine synthases CYSL-1 and CYSL-2 mediate C. elegans heritable adaptation to P. vranovensis infection. Nature Communications. 11, 1741 (2020).
- Wadi, L., Reinke, A. W. Evolution of microsporidia: an extremely successful group of eukaryotic intracellular parasites. PLoS Pathogens. 16, 1008276 (2020).
- Han, B., Takvorian, P. M., Weiss, L. M. Invasion of host cells by microsporidia. Frontiers in Microbiology. 11, 172 (2020).
- Tamim El Jarkass, H., Reinke, A. W. The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cellular Microbiology. 22 (11), 13247 (2020).
- Balla, K. M., Lažetić, V., Troemel, E. R. Natural variation in the roles of C. elegans autophagy components during microsporidia infection. PLoS ONE. 14, 0216011 (2019).
- Szumowski, S. C., Estes, K. A., Troemel, E. R. Preparing a discreet escape: Microsporidia reorganize host cytoskeleton prior to non-lytic exit from C. elegans intestinal cells. Worm. 1 (4), 207-211 (2012).
- Balla, K. M., Luallen, R. J., Bakowski, M. A., Troemel, E. R. Cell-to-cell spread of microsporidia causes Caenorhabditis elegans organs to form syncytia. Nature Microbiology. 1 (11), 1-6 (2016).
- Reinke, A. W., Balla, K. M., Bennett, E. J., Troemel, E. R. Identification of microsporidia host-exposed proteins reveals a repertoire of rapidly evolving proteins. Nature Communications. 8, 14023 (2017).
- Bakowski, M. A., et al. Ubiquitin-mediated response to microsporidia and virus infection in C. elegans. PLoS Pathogen. 10, 1004200 (2014).
- Reddy, K. C., et al. An intracellular pathogen response pathway promotes proteostasis in C. elegans. Current Biology. 27 (22), 3544-3553 (2017).
- Willis, A. R., et al. A parental transcriptional response to microsporidia infection induces inherited immunity in offspring. Science Advances. 7 (19), (2021).
- Tamim El Jarkass, H., et al. An intestinally secreted host factor promotes microsporidia invasion of C. elegans. eLife. 11, 72458 (2022).
- Solis, G. M., Petrascheck, M. Measuring Caenorhabditis elegans life span in 96 well microtiter plates. Journal of Visualized Experiments. 49, 2496 (2011).
- Stiernagle, T. Maintenance of C. elegans. WormBook. , (2006).
- Sutphin, G. L., Kaeberlein, M. Measuring Caenorhabditis elegans life span on solid media. Journal of Visualized Experiments. (27), e1152 (2009).
- Estes, K. A., Szumowski, S. C., Troemel, E. R. Non-lytic, actin-based exit of intracellular parasites from C. elegans intestinal cells. PLOS Pathogens. 7, 1002227 (2011).
- Botts, M. R., Cohen, L. B., Probert, C. S., Wu, F., Troemel, E. R. Microsporidia intracellular development relies on myc interaction network transcription factors in the host. G3 Genes|Genomes|Genetics. 6 (9), 2707-2716 (2016).
- Corsi, A. K. A Transparent window into biology: A primer on Caenorhabditis elegans. WormBook. , 1-31 (2015).
- Rivera, D. E., Lažetić, V., Troemel, E. R., Luallen, R. J. RNA fluorescence in situ hybridization (FISH) to visualize microbial colonization and infection in the Caenorhabditis elegans intestines. bioRxiv. , (2022).
- Zhang, G., et al. A large collection of novel nematode-infecting microsporidia and their diverse interactions with Caenorhabditis elegans and other related nematodes. PLoS Pathogens. 12, 1006093 (2016).
- Luallen, R. J., et al. Discovery of a natural microsporidian pathogen with a broad tissue tropism in Caenorhabditis elegans. PLoS Pathogens. 12, 1005724 (2016).
- Troemel, E. R., Félix, M. -. A., Whiteman, N. K., Barrière, A., Ausubel, F. M. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biology. 6, 309 (2008).
- Burton, N. O., et al. Intergenerational adaptations to stress are evolutionarily conserved, stress-specific, and have deleterious trade-offs. eLife. 10, 73425 (2021).
- Jaroenlak, P., et al. 3-Dimensional organization and dynamics of the microsporidian polar tube invasion machinery. PLoS Pathogens. 16, 1008738 (2020).
- Weidner, E., Manale, S. B., Halonen, S. K., Lynn, J. W. Protein-membrane interaction is essential to normal assembly of the microsporidian spore invasion tube. The Biological Bulletin. 188 (2), 128-135 (1995).

